Abstract
Recent reports have demonstrated the role of phyto-constituents in modulating inflammatory responses. Mangiferin isolated from Mangifera indica is known to induce potent anti-oxidative, anti-diabetic and anti-inflammatory activity. However, the molecular mechanism of its anti-inflammatory activity is not properly understood. In this study we have isolated Mangiferin from the tubers of Pueraria tuberosa (PT-Mangiferin) and analysed the mechanism of its potent anti-inflammatory effects in LPS stimulated RAW 264.7 mouse macrophage cell line and in a carrageenan induced air pouch model. PT-Mangiferin was non-toxic to primary cells but showed significant toxicity and apoptotic effect on cancerous cells. It significantly reduced the production of pro-inflammatory mediators (COX-2, iNOS and TNF-α) in LPS stimulated RAW 264.7 cells. Further, it has also reduced the generation of ROS and inhibited LPS induced NF-kB translocation in these cells. Additionally, PT-Mangiferin significantly reduced inflammation in a mouse air pouch model by inhibiting the infiltration of monocytes and neutrophils and reducing the production of cytokines. These effects were mediated via inactivation of NLRP3 inflammasome complex and its downstream signalling molecules. Taken together these results suggest that PT-Mangiferin is potent anti-inflammatory compound that reduces inflammation and holds promise in development of herbal based anti-inflammatory therapeutics in future.
Similar content being viewed by others
Introduction
Inflammation is a protective response after infection or injury. It is a biological process which eliminates the inciting stimulus, promotes tissue repair/wound healing and establishes memory so that host shows faster and specific response upon future encounter. Inflammation is associated with many disorders such as atherosclerosis, thrombosis, arthritis, asthma, neurodegenerative disorders and even cancer1.
NLRs (nucleotide binding domain, leucine rich repeat containing receptors) are associated with inflammation and their dysregulated activation is responsible to several inflammatory diseases, metabolic disorders and auto-inflammatory immune disorders. NLRs upon activation form multi protein complex called ‘inflammasome’ whose activation results in conversion of pro-caspase-1 to caspase-1 which results in conversion of several substrates like pro IL-1β, IL-18 to their active forms and mediate activation of NF-κB and MAPK signaling pathways2,3. One of the most intensively studied inflammasomes is the NLRP3 inflammasome that contains NLRP3 sensor, ASC adaptor and caspase-1 protease. Upon stimulation, NLRP3 inflammasome components assemble into large cytoplasmic complexes and activation of caspase-1 eventually leads to the maturation and secretion of IL-1β4. The NLRP3 inflammasome is an important contributor to various inflammatory diseases5. The rapidly growing evidence for excessive inflammasome activation in a variety of common diseases highlights the need for discovery of potentially therapeutic inhibitors of NLRP3 inflammasome.
The conventional anti-inflammatory drugs used to relieve inflammatory processes are either too expensive or come with several side effects. In this sense, pharmaceutical companies have supported the search and development of drugs with anti-inflammatory activity from plants. Natural products have long been used in medicine and drugs based on these products have been also used in the prevention and treatment of many inflammatory disorders. They interfere with inflammatory mechanisms preventing prolonged inflammatory processes that are problematic for human health. Several natural products were known to have anti-inflammatory effects but very few products like Aloe Vera, Curcumin, Ginseng and Resveratrol have demonstrated their anti-inflammatory effects via inactivation of NLRP3 inflammosome6,7,8,9,10,11,12,13.
Mangiferin is a natural polyphenol with a C-glycosylxanthone structure. The primary source of Mangiferin is the mango tree (Mangifera indica); however, it is also present in other medicinal herbs like Iris unguicularis and in the honey bush, a popular herbal tea from South Africa14. In addition, Magiferin is also found in Anemarrhena asphodeloides Bunge, which has been widely used in Chinese traditional medicine system for the treatment of diabetes15. Mangiferin is known for its analgesic, antidiabetic, antisclerotic, antibacterial and antiviral activities15,16,17. Few studies have demonstrated the anti-inflammatory property of Mangiferin, however, the molecular mechanism of its effect on inflammaosomes has not been completely elucidated18,19,20.
In this study we isolated Mangiferin from the tubers of Pueraria tuberosa (PT-Mangiferin) and determined its anti-inflammatory mechanism in most reliable mouse air pouch model of inflammation.
Results
PT-Mangiferin is non-toxic to primary cells but exerts toxic and anti-proliferative effect on cancer cell lines
PT-Mangiferin didn’t show any significant level of toxicity to normal cell lines or primary cells (mouse fibroblast NIH-3T3, RAW264.7, HEK293 and mouse lymphocytes) even at concentration of 100 μM as determined by MTT assay (Fig. 1A). However, it showed significant level of cytotoxicity to various cancer cell lines like K562 (IC50 60 μM), MCF7 (IC50 56 μM), HEPG2 (IC50 69 μM), Jurkat cells (IC50 48 μM) and A549 (IC50 40 μM) at different inhibitory concentrations (Fig. 1B). PT-Mangiferin induced maximum anti-proliferative effect in A549 cells and also induced apoptosis in these cells in dose dependent manner (Fig. 1C). This data correlated with expression of active caspase-3 in A549 cells as there was significant increase (p < 0.05) in its level with increasing concentration of PT-Mangiferin (Fig. 1D).
(A) Various primary cells and cell lines (murine fibroblast NIH-3T3, RAW264.7, HEK293 and mouse lymphocytes) were incubated with or without PT-Mangiferin (0.01–100 μM) for 48 h and then the cell viability was measured by MTT assay as described in materials and methods. (B) Various cancer cell lines (K562, MCF7, HEPG2, Jurkat E6.1 cells and A549) were incubated with or without PT-Mangiferin (0.01–100 μM) for 48 h and then the cell viability as measured by MTT assay as described in materials and methods. The data represents concentration of PT-Mangiferin inducing toxicity in 50% cells (IC50). (C) Apoptosis induced by PT-Mangiferin (20, 40 μM) in A549 cells at 72 h was determined by Annexin V binding using flow cytometry and represented as percent apoptotic cells. (C) Caspase-3 levels in A549 cells were detected by ELISA using specific antibody. Data are mean ± SEM and representative of three independent experiments. *P < 0.05. PBS- Phosphate buffer saline, LPS-Lipopolysaccharide (1 μg/ml), CXB-Celecoxib (20 μM), MF-20- PT-Mangiferin (20 μM), MF-40- PT-Mangiferin (40 μM).
PT-Mangiferin significantly reduced the generation of ROS and inhibited the LPS induced NF-κB translocation in mouse macrophages
The anti-inflammatory effect of PT-Mangiferin was evaluated by examining its effect on generation of Reactive Oxygen Species (ROS). PT-Mangiferin significantly reduced the LPS induced generation of ROS (p < 0.05) in dose dependent manner (Fig. 2A). PT-Mangiferin at 40 μM dose was more effective in reducing the level of ROS than standard ROS inhibitor, N-acetyl cysteine (NAC). NF-κB translocation is responsible for expression of inflammatory proteins like COX-2, iNOS, TNF-α. However, pre-incubation of cells with PT-Mangiferin significantly inhibited LPS induced NF-κB translocation in dose dependent manner as significantly reduced levels of p50 and p65 (p < 0.05) were observed in nuclei of cells (Fig. 2B).
(A) RAW 264.7 cells were treated with PBS, LPS (1 μg/ml) followed by treatment with Celecoxib (20 μM) or different doses of PT-Mangiferin (20, 40 μM) and cells were kept for 16 h at 37 °C/5% CO2. ROS levels were measured by Flow cytometry. N-Acetyl Cysteine, a ROS inhibitor was used as a positive control. (B) Nuclear extract of the cells with above treatments were prepared and analyzed for translocation of p50 and p65 sub units using specific antibodies as described in materials and methods. Histone 3(H3) was used as internal control. The gels were run under same experimental conditions. PBS- Phosphate buffer saline, LPS-Lipopolysaccharide (1 μg/ml), CXB- Celecoxib (20 μM), MF-20- PT-Mangiferin (20 μM), MF-40- PT-Mangiferin (40 μM).
PT-Mangiferin reduced the production of LPS induced pro-inflammatory mediators by mouse macrophages
PT-Mangiferin significantly reduced the expression of inflammatory mediators (COX-2, iNOS, and TNF-α) induced by LPS in RAW 264.7 cells (p < 0.05). The reduced expression was observed both at gene expression level (Fig. 3A) which correlated with protein levels (Fig. 3B). PT-Mangiferin has shown to be more effective than standard non-steroidal anti-inflammatory drug (NSAID), Celecoxib (CXB) at similar dose (20 μM) in reducing the levels of COX-2 and iNOS (Fig. 3). PT-Mangiferin inhibited both COX-1 and COX-2 as revealed by their reduced enzymatic activities with IC50 value of 85 μM and 40 μM respectively (Fig. 3C). PT-Mangiferin also induced production of significant levels of anti-inflammatory cytokine (IL-10) in RAW 264.7 cells (Fig. 3D). These results indicate that PT-Mangiferin is potent anti-inflammatory compound.
(A) RAW 264.7 cells were treated with PBS, LPS (1 μg/ml) and LPS followed by treatment with Celecoxib (20 μM) or different doses of PT-Mangiferin (20, 40 μM). The cells were kept for 16 h at 37 °C/5% CO2. Semi-quantitative PCR was performed for analyzing expression of cox-2, iNOS and TNF-α at transcription level. GAPDH was used as internal control. (B) Cytoplasmic extract of the cells with above treatment were run on SDS-PAGE and then transferred to nitrocellulose membrane followed by probing with specific antibodies for analyzing expression of COX-2, iNOS and TNF-α at protein level. β-actin was used as internal positive control. (C) RAW 264.7 cells were treated with different concentration of PT-Mangiferin (0.01–100 μM) and enzymatic activity of COX-1 and COX-2 was determined as described in materials and methods. The data is represented as percent enzyme activity (D) Analysis of IL-10 by ELISA in culture supernatants of RAW264.7 cells. Data are mean ± SEM and representative of three independent experiments. *P < 0.05. PBS- Phosphate buffer saline, LPS-Lipopolysaccharide (1 μg/ml), CXB- Celecoxib (20 μM), MF-20- PT-Mangiferin (20 μM), MF-40- PT-Mangiferin (40 μM).
PT-Mangiferin significantly reduced the carrageenan induced inflammation in a mouse air pouch model
The air pouch exudates of the mice treated with carrageenan and PT-Mangiferin were analyzed after 24 h for total number of cells, phenotypes and cytokines. PT-Mangiferin treatment significantly reduced the volume and infiltrating cells in the air pouch cavity (Fig. 4A,B). Carrageenan induced infiltration of large number of polymorphonuclear cells (PMNs) predominantly monocytes and neutrophils which were significantly reduced after treatment with PT-Mangiferin. Cells within the inflammatory exudate identified by flow cytometry were B cells, T cells, Monocytes, Dendritic Cells and Neutrophils. B and T cells were present in low numbers (Fig. 4C). There was significant reduction in the level of neutrophils (p < 0.05) after PT-Mangiferin treatment in dose dependent manner as determined by FACS which correlated to reduced MPO activity in the exudate (Fig. 4D,E). The low number of cells in the PT-Mangiferin treated animals also correlated to significantly reduced production of pro-inflammatory cytokines (p < 0.05) like IL-6, TNF-α and IL-1β in the exudate (Fig. 4F). PT-Mangiferin also significantly reduced the expression of inflammatory mediators (COX-2, iNOS, and TNF-α) as analyzed in air pouch tissue (p < 0.05). Reduced expression was observed at the gene expression level (Fig. 5A) which correlated with protein levels (Fig. 5B). The classical symptoms of acute inflammation - redness, swelling and infiltration of cells were clearly observed in the air pouch lining of carrageenan treated animal (Fig. 6A). The inflammatory reaction gradually progressed with time and reached a peak at 24 h after carrageenan treatment. The carrageenan treated pouch tissue showed increase in the size of blood vessels which were significantly reduced in mice treated with PT-Mangiferin (10 mg/kg body weight) and were almost completely abrogated in mice treated with higher dose (Fig. 6A). Histopathological analysis clearly showed that in PT-Mangiferin treated animals the inflammatory reaction and cell infiltration was less when compared to animals treated with carrageenan alone (Fig. 6B). PT-Mangiferin at a dose of 20 mg/kg body weight was more effective as tissue histology results were close to normal or PBS control.
Animals from different treatment groups were killed, air pouch were exposed and exudates were recovered with 500 μl PBS as described in materials and methods. (A) Volume of exudate recovered from each group. (B) Total cells in the exudates from each group as determined by counting. (C) Percent of each cell type in the exudates as analyzed by Flow cytometry. (D) FACS plots showing neutrophil population in each group. (E) MPO analysis. (F) Analysis of cytokines (IL-6, TNF-α, IL-1β) in the inflammatory exudates by ELISA. PBS- Phosphate buffer saline, CGN-Carrageenan (1.5%, 0.5 ml), CXB- Celecoxib (10 mg/kg body weight), MF-10- PT-Mangiferin at 10 mg/kg body weight, MF-20- PT-Mangiferin at 20 mg/kg body weight.
(A) cDNA obtained from air pouch tissues with different treatments as described in material and methods were analyzed for expression of cox-2, iNOS and TNF-α at transcription level. GAPDH was used as positive control. (B) Proteins obtained from air pouch tissue was subjected to Immunoblot for analyzing expression of COX-2, iNOS and TNF-α. β-actin was used as internal positive control. Data are mean ± SEM and representative of thee independent experiments. *P < 0.05. PBS- Phosphate buffer saline, CGN- Carrageenan (1.5%, 0.5 ml), CXB-Celecoxib (10 mg/kg body weight), MF-10- PT-Mangiferin at 10 mg/kg body weight, MF-20- PT-Mangiferin at 20 mg/kg body weight.
(A) Pictures of mouse air pouch tissue from various groups taken 24 h after treatment. (B) Photomicrographs showing the histological sections of pouch tissue 24 h after administration. The data is representative of two independent experiments. PBS- Phosphate buffer saline, CGN- Carrageenan (1.5%, 0.5 ml), CXB- Celecoxib (10 mg/kg body weight), MF-10- PT-Mangiferin at 10 mg/kg body weight, MF-20- PT-Mangiferin at 20 mg/kg body weight.
PT-Mangiferin reduces inflammation by inactivating NLRP3 inflammasome
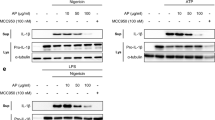
PT-Mangiferin significantly inhibited various components involved in inflammosome activation in LPS induced macrophages (RAW 264.7) both at gene expression and protein level (p < 0.05). There was significant reduction in transcript and protein levels of NLRP3, Caspase-1 and IL-1β in cells treated with PT-Mangiferin (Fig. 7A,B). The significant reduced levels (p < 0.05) of IL-1β and IL-18 cytokines further supported this observation (Fig. 7C). PT-Mangiferin also significantly reduced the expression of NLRP3, Caspase-1 and IL-1β in carrageenan treated air pouch tissue both at gene expression and protein level. These results indicate that PT-Mangiferin reduces inflammation by inactivating or inhibiting the NLRP3 inflammasome.
(A) cDNA obtained from RAW 264.7 cells with different treatments as described in material and methods were analyzed for expression of NLRP3, Caspase-1 and IL-1β at transcription level. GAPDH was used as positive control. (B) Analysis of expression of NLRP3, Caspase-1 and IL-1β in RAW 264.7 cells at protein level. β-actin was used as internal positive control. (C) Analysis of IL-1β and IL-18 by ELISA in the culture supernatants of RAW264.7 cells. (D) cDNA obtained from air pouch tissues with different treatments were analyzed for expression of NLRP3, Caspase-1 and IL-1β α at transcription level as described in material and methods. GAPDH was used as positive control. (E) Analysis of expression of NLRP3, Caspase-1 and IL-1β from air pouch tissues at protein level. β-actin was used as internal positive control. Data are mean ± SEM and representative of three independent experiments. *P < 0.05 PBS- Phosphate buffer saline, LPS-Lipopolysaccharide (1 μg/ml), CXB- Celecoxib (20 μM), MF-20- PT-Mangiferin (20 μM), MF-40- PT-Mangiferin (40 μM), CGN- Carrageenan (1.5%, 0.5 ml), CXB-Celecoxib (10 mg/kg body weight), MF-10- PT-Mangiferin at 10 mg/kg body weight, MF-20- PT-Mangiferin at 20 mg/kg body weight.
Discussion
The currently available anti-inflammatory agents mainly consisting of NSAIDs, glucocorticoids, immunosuppressant drugs and biologicals are either not effective enough or associated with intolerable side effects21. Thus, the discovery of new anti-inflammatory compounds is currently in great demand. The NLRP3 inflammasome is an important contributor to diverse inflammatory diseases such as Alzheimer, atherosclerosis, diabetes etc. Few recent studies have discovered some promising candidates, however, there are currently no clinically available inhibitors of NLRP322,23. The extracts of many plants are known to act as anti-inflammatory agents however mechanism of action is largely unknown24,25 Mangiferin isolated from Mangifera indica has shown to mediate anti-oxidant, antidiabetic and anti-inflammatory property mainly by targeting MAP kinases and NF-κB signalling pathways26,27,28,29,30,31. Recent studies have shown that Mangiferin inhibits NLRP3 inflammasome in liver and endothelial cells to protect against liver injury and endothelial dysfunction32,33. In this study we have exploited the mouse air pouch model which is most reliable model for inflammation and confirmed that anti-inflammatory activity of Mangiferin isolated from P. tuberosa (PT-Mangiferin) is indeed associated with its ability to inhibit NLRP3 inflammosomes.
In the current study, PT-Mangiferin effectively reduced inflammation in dose dependent manner and provided protection against the acute inflammatory response induced by carrageenan in mice. Although we used three doses of PT-Mangiferin both in vitro and in vivo, the lowest dose (10 μM or 5 mg/kg body weight) had no significant effect in reducing the inflammation (data not shown). On testing the effect of PT-Mangiferin on various normal cell lines and primary cells (mouse fibroblast, macrophages and spleenocytes) our results showed that it did not cause any significant level of toxicity upto 100 μM concentration but caused anti-proliferative effect in various cancer cells with IC50 value ranging from 40 μM to 70 μM. It showed maximum toxicity to A549 lung cancer cell line by inducing significant level of apoptosis in these cells (Fig. 1C,D). Our data is in accordance to recent report of Mangiferin showing anti-proliferative effect on various cancer cell lines with maximum effect on A549 cells34. PT-Mangiferin was found to be more effective in reducing the generation of ROS in RAW 264.7 cells than standard inhibitor NAC (Fig. 2A). The xanthonoid structure with C-glycosyl linkage and polyhydroxy component of PT-Mangiferin is believed to be crucial for its ability to scavenge ROS. The reduced generation of ROS correlated to inhibition of NF-κB signaling as there was reduced levels of p50 and p65 in nuclei (Fig. 2B). NF-κB p50/p65 heterodimers in most cells are held in the cytoplasm by an inhibitor molecule, IκBα. The specific stimulatory signals such as LPS, PMA or TNF-α can cause degradation of IκBα and release of p50/p65 causing transport of transcriptionally active heterodimers to the nucleus leading to induction of transcription of target genes35. ROS interacts with NF-κB signaling pathways in many ways. The transcription of NF-κB dependent genes influences the levels of ROS in the cell, and in turn, the levels of NF-κB activity are also regulated by the levels of ROS. Depending on the context, ROS can both activate and inhibit NF-κB signaling. For instance ROS often stimulates the NF-κB pathway in the cytoplasm, but inhibits NF-κB activity in the nucleus35. Since our data shows that PT-Mangiferin inhibited production of COX-2 enzyme it is likely that NF-κB is regulating the production of ROS though COX-2 (Fig. 3A,B).
Further, PT-Mangiferin inhibited the LPS induced production of proinflammatory mediators like COX-2, iNOS, TNF-α by RAW 264.7 cells (Fig. 3). Cyclooxygenase exist in two isoforms, COX-1 and COX-2 where latter is rapidly induced during inflammation and contributes to acute inflammation though production of proinflammatory signaling molecules36,37. Although COX-2 appears to be the dominant source of prostaglandin formation in inflammation, the role of COX-1 in contributing to the acute inflammatory response cannot be ruled out. COX-1 is constitutively expressed in resident inflammatory cells and there is evidence for induction of COX-1 during LPS-mediated inflammatory response and cellular differentiation38. Consistent to our previous study, our data shows that PT-Mangiferin is inhibiting both COX-1 and COX-2 (Fig. 3C)39. However we have used much lower dose where it is predominantly targeting COX-2. In contrast to standard NSAIDs, it seems that anti-inflammatory mechanism of PT-Mangiferin is via targeting both COX-1 and COX-2. Nitric oxide (NO) is another important pro-inflammatory mediator released during the inflammatory process. It is produced by the inducible nitric oxide synthase (iNOS) during inflammation. This mediator can induce oxidative stress in macrophages and also modulate T cell development and cytokine production40. In our study, PT-Mangiferin treatment caused a significant reduction in levels of pro-inflammatory mediators including iNOS and TNF-α. The reduction in these mediators may be due to inhibition of NF-κB signaling as iNOS (NOS2) is heavily upregulated by NF-κB35.
Several studies have described the use of the mouse air pouch model as a very consistent and straightforward model to investigate inflammatory response, creating an ideal environment for collection and phenotypic analysis of cells migrating into the pouch space41,42. In this study, we exploited this model to evaluate the effect of PT-Mangiferin on carrageenan induced infiltration of inflammatory cells, mediators and cytokines. PT-Mangiferin significantly reduced the exudate volume, infiltration of monocytes and neutrophils, pro-inflammatory cytokines (IL-6, TNF-α and IL-1β) and MPO in the air pouch of carrageenan treated animals (Fig. 4). This effect was dose dependent and level of neutrophils correlated to MPO activity which is an enzyme present in primary granules and is considered to be an important marker for inflammatory response in a variety of acute and chronic inflammatory conditions43. Since NO acts on the endothelium increasing the vascular permeability and consequently increasing exudation, it seems that the reduced level of pro-inflammatory mediator like iNOS in PT-Mangiferin treated animals may have contributed to reduced generation of NO leading to reduction in exudate volume44. The reduction in cell number achieved by treatment of PT-Mangiferin seems mainly due to the inhibition of neutrophil migration and reduction in inflammatory mediators. These results indicate that PT-Mangiferin showed an anti-inflammatory property by inhibiting migration of leukocytes (mainly monocytes and neutrophils) and reducing pro-inflammatory enzymes and cytokines in the exudates (Figs 4 and 5). The type of infiltrating cells and their numbers present in inflammatory responses generally varies with time from induction with phagocytes being predominant during the early phases of inflammation, whereas lymphocytes become more evident in chronic inflammation45. Since we have not analyzed the lavage post 24 h it was not possible to determine if PT-Mangiferin induced the migration of lymphocytes in the pouch cavity. Consistent with the flow cytometry analysis of exudate cells, at 24 h histology data also showed that PT-Mangiferin treated pouches appear less inflamed, with decreased cellularity (lower number of infiltrating cells) similar to PBS-treated pouches (Fig. 6).
To investigate whether the anti-inflammatory mechanism of PT-Mangiferin occurs via inactivation of NLRP3 inflammasome, we analyzed the expression of NLRP3, caspase-1 and IL-1β in LPS stimulated macrophages and carrageenan induced inflamed air pouch tissue. Our results showed that PT-Mangiferin significantly inhibited expression of NLRP3, caspase-1 and IL-1β in both macrophages and air pouch tissue. The reduced production of these mediators correlated to levels of IL-18 and IL-1β in mouse macrophages (Fig. 7). It is likely that PT-Mangiferin is inhibiting the expression of Caspase-1 and IL-1β by reducing the generation of ROS as it has been shown that mitochondrial ROS plays an important role in NLRP3 inflammasome activation46,47,48.
In conclusion, we have uncovered the fact that anti-inflammatory mechanism of PT-Mangiferin is via inhibiting NF-κB signaling, COX-1, COX-2 and inactivation of NLRP3 inflammaosomes highlighting its ability to modulate several points in the inflammatory pathway. The major advantage of using PT-Mangiferin as anti-inflammatory compound is that it may confer greater protective effects than compounds that have single targets and can also be effective at lower doses. Therapeutically, our data indicates that PT-Mangiferin has potent anti-inflammatory property and has potential to be repurposed as drug against various inflammatory diseases.
Materials and Methods
Chemicals and Reagents
MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), Tris, Ethylene diamine tetra acetic acid (EDTA), Trypsin-EDTA, Di-ethyl dithio-carbamate (DDC), Tween-20, Hematin, Glycerol, Phenol, Ammonium sulphate, Carrageenan, COX-1 and COX-2 enzymes were purchased from Sigma-Aldrich (St. Louis, MO, USA). Celecoxib was a generous gift from Unichem Laboratories (Mumbai, India). The stock solutions of PT-Mangiferin (100 mg/ml) was prepared in dimethyl sulphoxide and further dilutions were made in PBS for treatment in vitro and in vivo. Polyclonal antibodies to, COX-2, IL-1β and TNF-α were purchased from Santa Cruz Biotechnology (California, U.S.A.). iNOS antibody was from Thermo scientific. All other chemicals and solvents were of analytical grade and purchased from authorized standard companies.
Cell culture
Human Leukemic cell line (K562), Hepatocellular carcinoma cells (HepG2), Breast carcinoma cells (MCF-7), Human T cell leukemic cells (Jurkat E6.1), Human lung carcinoma cells (A549), Mouse fibroblast (NIH-3T3), Human Embryonic Kidney cells (HEK293) and mouse macrophage cell line (RAW 264.7) were obtained from NCCS (Pune, India). The cells were grown in their recommended mediums either in Dulbecco’s Modified Eagle Medium (DMEM) or RPMI1640 (GIBCO, USA) supplemented with 2 mM glutamine, 10% heat-inactivated FBS, 100IU/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
Isolation and purification of Mangiferin from Pueraria tuberosa
Mangiferin was isolated from the methanolic extract of tubers of P. tuberosa (here referred to as PT-Mangiferin) as described previously39. Briefly, the tubers of P. tuberosa were collected from Tirumala hills, Andhra Pradesh, South India. A voucher specimen (DG-11) has been deposited in the herbarium of the Department of Botany, Sri Venkateswara University, Tirupati. Air-dried and powdered (3 kg) tubers of P. tuberosa was successively extracted with n-hexane (3 × 5 L), Ethyl acetate (EtOAc, 3 × 5 L) and Methyl alcohol (MeOH, 3 × 5 L). The MeOH extract was concentrated under reduced pressure to yield a brown colored viscous residue. The MeOH extract on purification over a silica gel column using n-hexane and n-hexane-EtOAc step gradient afforded 3 compounds and one among them was PT-Mangiferin. The structures of compounds 1–3 were elucidated by various NMR techniques including 1H, 1H COSY, HSQC, HMBC, NOESY experiments and ESI-TOF Mass Spectrometry. PT-Mangiferin was dissolved in DMSO and prepared as stock of 100 mM.
Cell proliferation assay
Various cell lines or primary cell (mouse fibroblast NIH-3T3, RAW264.7, HEK293 and mouse lymphocytes) and various cancer cell lines (K562, MCF7, HEPG2, Jurkat E6.1 cells and A549) were seeded in 96 well plates in recommended medium at a density of 5 × 104 cells/well. The cells were treated with different concentrations of PT-Mangiferin (0.01 μM to 100 μM) for 48 h and cell proliferation was assessed colorimetrically by standard MTT assay described by Mossman49. The effect of PT-Mangiferin on growth inhibition was assessed as percent cell viability; control cells treated with the PBS were considered 100% viable. Supernatant from 0.1% Triton X-100 treated cells (100% lysis) was used as positive control.
Apoptosis assay
Apoptosis induced by PT-Mangiferin on A549 cells was measured using flow cytometry (to quantify the levels of detectable phosphatidylserine on the outer membranes of apoptotic cells) and caspase-3 activity. A549 cells were seeded in 6 well cultures plate. At 60–70% confluency, cells were incubated with different concentrations of PT-Mangiferin (10, 20 and 40 μM) and Celecoxib (20 μM) for 72 h. Cells were stained with FITC labelled Annexin V using Apoptosis Detection Kit (Cat#556547, BD, USA) according to manufacturer’s protocol and then analyzed by FACS. For determining the activated caspase-3 protein levels, the cells were lysed and total protein was quantified with the BCA Protein Assay Reagent Kit. The active caspase-3 in the lysate was detected by ELISA by using mouse cleaved caspase-3 antibody (Cat#DYC835, R&D Systems).
Measurement of ROS
RAW 264.7 cells were seeded in 6 well plates at a density of 2 × 105 cells/well and then pre incubated with different concentrations of PT-Mangiferin (10, 20 and 40 μM). The cells were stimulated with LPS (1 μg/ml) for 16 h and then treated with 10 μM DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) and further incubated for 15 min at 37 °C. The cells were collected, washed with PBS and total of 10000 events were analyzed in FL1 channel by Flow cytometry (FACS caliber, BD, USA).
Animals
Adult Balb/c male mice weighing 20–25 g were used in the present study. They were fed with a standard chow pellet diet, had free access to water, and were maintained on a 12:12-h light-dark cycles. All procedures in this study were approved by the Animal Ethical Committee of National Institute of Animal Biotechnology, Hyderabad and Sri Krishnadevaraya University, Anantapur. All the methods were carried out in accordance with the approved protocols and relevant guidelines.
Air pouch model of inflammation
Carrageenan treated mice air pouch model of inflammation was developed as described previously10. Air cavities were produced by sub-cutaneous injections of 5 ml of sterile air into the intra-capsular area on the dorsal side of the animal. An additional 3 ml of air was injected into the cavity every three days. Seven days after the initial air injection, 0.5 ml of 1.5% (w/v) solution of carrageenan dissolved in saline was injected directly into the pouch to produce an inflammatory response. Control animals received 0.5 ml of saline only. PT-Mangiferin and Celecoxib were injected thee hours prior to injection of carrageenan into the pouch cavity. Animals were divided into 5 different groups as follows: PBS treated; carrageenan (0.5 ml of 1.5% (w/v) carrageenan in saline) treated; carrageenan + Celecoxib (10 mg/Kg body weight); carrageenan + PT-Mangiferin (5 mg/Kg body weight) treated; carrageenan + PT-Mangiferin (10 mg/Kg body weight) treated; carrageenan + PT-Mangiferin (20 mg/Kg body weight) treated. For the time course studies, animals were sacrificed by cervical dislocation at various time points after the injection. Pouch tissue was carefully dissected and inflammatory exudates were recovered by lavaging the pouches with 500 μl of sterile PBS. The pouch lining was separated from the muscle and dissected out, and rinsed in saline before processing further. The tissue was used to study the expression of inflammatory genes at RNA and protein level and lavage/exudate was used to analyze infiltrating cells and cytokines. For checking toxicity of PT-Mangiferin, splenocytes were isolated from normal untreated animals using standard procedure.
Preparation of cytoplasmic and nuclear extracts
RAW 264.7 cells were cultured in 6-well plates (4 × 106cells/well) with or without LPS (1 μg/ml), Celecoxib (20 μM) and in the presence or absence of different concentration of PT-Mangiferin (10, 20, 40 μM). After culture the cells were collected and washed twice with cold PBS, lysed in 400 μL of cold buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 1 mM EDTA, 1 mM phenylmethanesulphonylfluoride (PMSF), 1 mM EGTA, 1 mM dithiotheitol (DTT1mg/ml Aprotinin, 1 mg/ml Leupeptin and 1 mg/ml Pepstatin A). After 15-min incubation on ice, 0.1% NP-40 was added to the homogenates and the tubes were vigorously rocked for 1 min. Then the homogenates were centrifuged (20,800 g × g, 5 min) at 4 °C. The supernatant fluid (cytoplasmic extracts) was collected and stored in aliquots at −70 °C. The nuclear pellets were washed once with cold buffer A, then suspended in 50 μL of cold buffer B (20 mM HEPES, pH 7.9, 420 mM NaCl, 0.1 mM EDTA, 1 mM PMSF, 1 mM DTT, 1 mg/ml Aprotinin, 1 mg/ml Leupeptin and 1 mg/ml Pepstatin A) and vigorously rocked at maximum speed at 4 °C for 30 min. The solution was clarified by centrifugation at 20,800× g for 5 min, and the supernatant fluid (nuclear extract) was stored in aliquots at −70 °C. The protein concentration was determined according to the Bradford method. Air pouch tissue homogenate was prepared by homogenizing in 100 mM Tris-HCl (pH 8.0) buffer containing 0.3 M Mannitol, 1 mM EGTA, 1 mM EDTA, 4 mM K2HPO4, 1 mM DTT, 1 mM Sodium orthovanadate, 0.1% SDS, 2 mM PMSF and 40 μl/ml complete protease inhibitor. The homogenate was centrifuged for 30 min at 10,000 rpm at 4 °C and the protein content in the supernatant was measured by Bradford method9.
Western Blotting
Equal amounts of protein from RAW 264.7 cells and tissue homogenates were separated by SDS-PAGE and then transferred electrophoretically to nitrocellulose membranes (Millipore, Bedford, MA). After blocking with 5% nonfat milk powder in PBS (pH 7.4, 0.05% (v/v) Tween-20), the membranes were incubated overnight at 4 °C with corresponding primary Abs, for the detection of COX-2, iNOS, Caspase-1, NLRP3, TNF-α, IL-1β and β-actin according to the manufacturer’s instructions. After washing with PBS, the membranes were incubated for 1–2 h at 37 °C with the appropriate HP-conjugated anti-rabbit IgG secondary Ab (1:5000, CST, USA). The peroxidase-positive bands were detected using an ECL detection kit (Bio-Rad Cat#1705060).
Enzymatic assay
Enzymatic activities of both COX-1 and COX-2 were measured according to the method of Copeland et al. with slight modifications, using a chomogenic assay based on the oxidation of N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) during the reduction of PGG2 to PGH250,51,52.
Cytokine analysis
Cytokine sandwich ELISA kits (R&D systems) were used to measure cytokine levels, following the manufacturer’s instructions. IL-10, IL-18 and IL-1β were measured from culture supernatant of RAW264.7 cells. IL-6, TNF-α and IL-1β were measured from exudates obtained from various groups.
Flow cytometric analysis of inflammatory exudate
The cells in the exudate were counted on an automatic cell counter (Bio-Rad) using trypan blue to assess viability. The cells (1 × 106) were suspended in FACS buffer (PBS, 2% (v/v) fetal calf serum, and 0.05% (w/v) NaN3), blocked with Fc block (anti-CD16/32) and then stained with lineage specific and fluorochrome labelled monoclonal antibodies against T cells (CD3-FITC), B cells (B220-PE), Monocytes (CD11b-APC), Dendritic cells (CD11c-PerCP), Macrophages (F4/80-PE-Cy7) and Neutrophils (Ly6G-APC-Cy7). The cells were washed with FACS buffer and then 50,000 cells were acquired in Flow cytometer (FACS caliber, BD, USA). The data was analyzed by FLOW JO software (Tree star Inc.).
Myeloperoxidase assay
The Myeloperoxidase (MPO) activity was evaluated in the exudates obtained from various groups by using MPO colorimetric assay kit (Cat# MAK068-1KT Sigma, USA) following manufacturer’s instructions. Briefly, 100 μL of lavage fluid was mixed with 400 μL (4 volume) of MPO Assay Buffer and homogenized with sonication for 10 seconds and three subsequent freeze and thaw cycles. The sample was then centrifuged at 13,000 g for 15 min at 4 °C. The supernatant was then used for the measurement of MPO activity using kit according to manufacturer’s protocol. The results were expressed in milli units per milliliter.
Histopathology of air pouch tissue
Air pouch tissues from control and experimental animals were rinsed in PBS and fixed in Bouin’s fixative (70% saturated picric acid, 25% formaldehyde and 5% glacial acetic acid) overnight followed by thorough washing with distilled water. Tissues were then dehydrated sequentially in 70%, 80%, 90% alcohol and finally in absolute alcohol for 10 min each. After dehydration, the tissue was processed in alcohol and benzene (3:1 for 10 min, 1:1 for 10 min, benzene and paraffin (1:1) for 10 min) to embed in paraffin wax. The tissue was placed in molten paraffin for 3 h to allow infiltration of paraffin into the tissue and then allowed to harden. Thin sections (10 μm) were taken on Leitz microtome and mounted on polylysine-coated slides. Sections were deparaffinised by incubating in xylene for 10 min, rehydrated by sequential incubations in 90%, 80% and 70% alcohol for 10 min each. The tissue sections were observed under light microscope at 400x magnification and photographs were taken.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
RAW 264.7 cells (5 × 106 cells) were seeded in 90 mm Petri plates. Cells were pre incubated with different concentrations of PT-Mangiferin (10, 20 and 40 μM) or celecoxib (20 μM) for 3 h and then stimulated with LPS (1 μg/ml) for 24 h. Cells were recovered and suspended in Trizol. Similarly pouch tissues obtained from different groups were homogenized in Trizol reagent. Total cellular RNA was extracted using RNAeasy kit (Qiagen) according to the manufacturer’s instructions. RNA quality was checked by running on a Formaldehyde gel for 16 S and 18 S RNA bands and also on Bio-analyzer. The RNA quantity was assessed by UV spectroscopy and purity by 260/280 ratio. cDNA of each sample was prepared by using 2 μg of RNA, 1 μl M-MLV reverse transcriptase, 1 mM dNTP and 1 μl oligo dT according to manufacturer’s standardized protocol (Promega Corporation, WI, USA). RT-PCR was performed in 20 μl reaction containing 50 ng cDNA, 2 μl 10X reaction buffer, 1U Taq DNA polymerase, 0.2 mM dNTP, 100 pmol of forward and reverse primer (Table 1- Supp. info) to detect cox-2, iNOS, TNF-α, NLRP3, Caspase-1, IL-1β, and GAPDH. After amplification, PCR products were electrophoresed on 1% agarose gels and visualized by ethidium bromide staining on UV irradiation (BIO-RAD, Universal hood II).
Statistical analysis
Statistical analysis was performed by multiple software’s like Excel, Analysis of Variance (ANOVA) and Bonferroni Post hoc test for multiple comparisons wherever applicable. P-value was determined by the Student’s T-test. P-value of less than 0.05 was considered as a significant difference.
Additional Information
How to cite this article: Bulugonda, R. K. et al. Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Sci. Rep. 7, 42683; doi: 10.1038/srep42683 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ozaki, E., Campbell, M. & Doyle, S. L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 8, 15–27, doi: 10.2147/JIR.S51250 (2015).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29, 707–735, doi: 10.1146/annurev-immunol-031210-101405 (2011).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology 10, 241–247, doi: 10.1038/ni.1703 (2009).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13, 397–411, doi: 10.1038/nri3452 (2013).
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21, 677–687, doi: 10.1038/nm.3893 (2015).
Devi, K. P. et al. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res 99, 1–10, doi: 10.1016/j.phrs.2015.05.002 (2015).
McFarlin, B. K. et al. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin 5, 72–78, doi: 10.1016/j.bbacli.2016.02.003 (2016).
Zorman, J., Susjan, P. & Hafner-Bratkovic, I. Shikonin Suppresses NLRP3 and AIM2 Inflammasomes by Direct Inhibition of Caspase-1. PloS one 11, e0159826, doi: 10.1371/journal.pone.0159826 (2016).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 285, 9792–9802, doi: 10.1074/jbc.M109.082305 (2010).
Shi, J. Q. et al. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-kappaB and NLRP3 inflammasome activation. CNS Neurosci Ther 19, 262–268, doi: 10.1111/cns.12066 (2013).
Zhu, T. et al. Scropolioside B inhibits IL-1beta and cytokines expression through NF-kappaB and inflammasome NLRP3 pathways. Mediators Inflamm 2014, 819053, doi: 10.1155/2014/819053 (2014).
Gong, Z. et al. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol Nutr Food Res 59, 2132–2142, doi: 10.1002/mnfr.201500316 (2015).
Tozser, J. & Benko, S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1beta Production. Mediators Inflamm 2016, 5460302, doi: 10.1155/2016/5460302 (2016).
Mahomoodally, M. F. Traditional medicines in Africa: an appraisal of ten potent african medicinal plants. Evid Based Complement Alternat Med 2013, 617459, doi: 10.1155/2013/617459 (2013).
Miura, T. et al. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine 8, 85–87 (2001).
Carvalho, A. C. et al. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med 73, 1372–1376, doi: 10.1055/s-2007-990231 (2007).
Na, L. et al. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: a double-blind randomized controlled trial. Sci Rep 5, 10344, doi: 10.1038/srep10344 (2015).
Marquez, L., Garcia-Bueno, B., Madrigal, J. L. & Leza, J. C. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur J Nutr 51, 729–739, doi: 10.1007/s00394-011-0252-x (2012).
Garrido, G. et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res 50, 143–149, doi: 10.1016/j.phrs.2003.12.003 (2004).
Rivera, D. G. et al. Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J Pharm Pharmacol 63, 1336–1345, doi: 10.1111/j.2042-7158.2011.01328.x (2011).
Ong, C. K., Lirk, P., Tan, C. H. & Seymour, R. A. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res 5, 19–34, doi: 10.3121/cmr.2007.698 (2007).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21, 248–255, doi: 10.1038/nm.3806 (2015).
Daniels, M. J. et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat Commun 7, 12504, doi: 10.1038/ncomms12504 (2016).
Cragg, G. M. & Newman, D. J. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830, 3670–3695, doi: 10.1016/j.bbagen.2013.02.008 (2013).
Atanasov, A. G. et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 33, 1582–1614, doi: 10.1016/j.biotechadv.2015.08.001 (2015).
Dou, W. et al. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-kappaB and MAPK signaling inactivation. Int Immunopharmacol 23, 170–178, doi: 10.1016/j.intimp.2014.08.025 (2014).
Fu, Y. Y. et al. Mangiferin regulates interleukin-6 and cystathionine-b-synthase in lipopolysaccharide-induced brain injury. Cell Mol Neurobiol 34, 651–657, doi: 10.1007/s10571-014-0039-8 (2014).
Leiro, J. et al. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol 4, 763–778, doi: 10.1016/j.intimp.2004.03.002 (2004).
Gong, X. et al. Anti-inflammatory effects of mangiferin on sepsis-induced lung injury in mice via up-regulation of heme oxygenase-1. J Nutr Biochem 24, 1173–1181, doi: 10.1016/j.jnutbio.2012.09.003 (2013).
Wang, B. et al. Mangiferin attenuates renal ischemia-reperfusion injury by inhibiting inflammation and inducing adenosine production. Int Immunopharmacol 25, 148–154, doi: 10.1016/j.intimp.2014.11.011 (2015).
Mahmoud-Awny, M., Attia, A. S., Abd-Ellah, M. F. & El-Abhar, H. S. Mangiferin Mitigates Gastric Ulcer in Ischemia/Reperfused Rats: Involvement of PPAR-gamma, NF-kappaB and Nrf2/HO-1 Signaling Pathways. PloS one 10, e0132497, doi: 10.1371/journal.pone.0132497 (2015).
Song, J., Li, J., Hou, F., Wang, X. & Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism 64, 428–437, doi: 10.1016/j.metabol.2014.11.008 (2015).
Pan, C. W. et al. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 770, 85–91, doi: 10.1016/j.ejphar.2015.12.006 (2016).
Shi, W. et al. Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A549 human lung carcinoma cells. Mol Med Rep 13, 3423–3432, doi: 10.3892/mmr.2016.4947 (2016).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21, 103–115, doi: 10.1038/cr.2010.178 (2011).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31, 986–1000, doi: 10.1161/ATVBAHA.110.207449 (2011).
Rajakariar, R., Yaqoob, M. M. & Gilroy, D. W. COX-2 in inflammation and resolution. Mol Interv 6, 199–207, doi: 10.1124/mi.6.4.6 (2006).
McAdam, B. F. et al. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest 105, 1473–1482, doi: 10.1172/JCI9523 (2000).
Sumalatha, M. et al. Isoorientin, a Selective Inhibitor of Cyclooxygenase-2 (COX-2) from the Tubers of Pueraria tuberosa. Nat Prod Commun 10, 1703–1704 (2015).
Luiking, Y. C., Ten Have, G. A., Wolfe, R. R. & Deutz, N. E. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab 303, E1177–1189, doi: 10.1152/ajpendo.00284.2012 (2012).
Duarte, D. B., Vasko, M. R. & Fehrenbacher, J. C. Models of inflammation: carrageenan air pouch. Curr Protoc Pharmacol Chapter 5, Unit5 6, doi: 10.1002/0471141755.ph0506s56 (2012).
Matte, C. & Olivier, M. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. The Journal of infectious diseases 185, 673–681, doi: 10.1086/339260 (2002).
Nussbaum, C., Klinke, A., Adam, M., Baldus, S. & Sperandio, M. Myeloperoxidase: a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid Redox Signal 18, 692–713, doi: 10.1089/ars.2012.4783 (2013).
Dos Reis, G. O. et al. Croton antisyphiliticus Mart. attenuates the inflammatory response to carrageenan-induced pleurisy in mice. Inflammopharmacology 22, 115–126, doi: 10.1007/s10787-013-0184-6 (2014).
Akbar, M., Fraser, A. R., Graham, G. J., Brewer, J. M. & Grant, M. H. Acute inflammatory response to cobalt chromium orthopaedic wear debris in a rodent air-pouch model. J R Soc Interface 9, 2109–2119, doi: 10.1098/rsif.2012.0006 (2012).
Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282, 2871–2879, doi: 10.1074/jbc.M608083200 (2007).
Kepp, O., Galluzzi, L. & Kroemer, G. Mitochondrial control of the NLRP3 inflammasome. Nature immunology 12, 199–200, doi: 10.1038/ni0311-199 (2011).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225, doi: 10.1038/nature09663 (2011).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65, 55–63 (1983).
Copeland, R. A. et al. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proceedings of the National Academy of Sciences of the United States of America 91, 11202–11206 (1994).
Egan, R. W., Paxton, J. & Kuehl, F. A. Jr. Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem 251, 7329–7335 (1976).
Pagels, W. R. et al. Immunochemical evidence for the involvement of prostaglandin H synthase in hydroperoxide-dependent oxidations by ram seminal vesicle microsomes. J Biol Chem 258, 6517–6523 (1983).
Acknowledgements
This work was supported partly from internal funding to Dr. Muralidhara Rao at SKU and partly from Ramalingaswami Fellowship Grant No- BT/RLF/2012 (SP003-NIAB) funded to Dr. Faisal from Department of Biotechnology, Ministry of Science and Technology, Government of India. Financial support from NIAB core fund to Dr. Faisal is duly acknowledged. The authors would like to thank Director, NIAB for providing necessary infrastructural facility. We are grateful to Dr. Aparna Rachamallu, NIAB for critically reviewing and editing the manuscript. Thanks to Mr. Vivek Varma (Student of Dr. Faisal), NIAB for help Flow cytometry and Ms. Rama Devi, NIAB for help in animal experiments. R.K. Bulugonda at SKU would like to thank ICMR for providing fellowship. Gangappa wants to acknowledge DST-SERB for providing National Post-Doctoral fellowship.
Author information
Authors and Affiliations
Contributions
S.M.F., K.A.K. and D.M.R. designed the experiments. R.K.B., K.A.K., G.D., H.B., G.H.P., D.M.R. and S.M.F. performed the experiments. S.M.F. and K.A.K. analyzed the data. S.M.F. and K.A.K. wrote the manuscript and all authors reviewed and approved the final version. R.K.B. and K.A.K. share first authorship whereas D.M.R. and S.M.F. share senior authorship.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bulugonda, R., kumar, K., Gangappa, D. et al. Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Sci Rep 7, 42683 (2017). https://doi.org/10.1038/srep42683
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42683
This article is cited by
-
Nano-delivery Systems and Therapeutic Applications of Phytodrug Mangiferin
Applied Biochemistry and Biotechnology (2024)
-
Pharmacological properties of mangiferin: bioavailability, mechanisms of action and clinical perspectives
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
The multifaceted role of mangiferin in health and diseases: a review
Advances in Traditional Medicine (2021)
-
Anti-inflammatory Effect of Curcuma longa and Allium hookeri Co-treatment via NF-κB and COX-2 Pathways
Scientific Reports (2020)
-
Pharmacological and biochemical studies on protective effects of mangiferin and its interaction with nitric oxide (NO) modulators in adjuvant-induced changes in arthritic parameters, inflammatory, and oxidative biomarkers in rats
Inflammopharmacology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.