Abstract
The aim of the study was to evaluate the diagnostic accuracy of urinary neutrophil gelatinase- associated lipocalin (uNGAL) in patients with chronic kidney disease (CKD) as an early biomarker for contrast induced acute kidney injury (CI-AKI) and to investigate whether patients with an uNGAL increase might benefit from an additional intravenous volume expansion with regard to CI-AKI-incidence. We performed a prospective randomized controlled trial in 617 CKD-patients undergoing intra-arterial angiography. Urinary NGAL was measured the day before and 4–6hrs after angiography. In the event of a significant rise of uNGAL patients were randomized either into Group A, who received intravenous saline post procedure or Group B, who did not receive post-procedural i.v. fluids. Ten patients (1.62%) exhibited a significant rise of uNGAL after angiography and were randomized of whom one developed a CI-AKI. In the entire cohort the incidence of CI-AKI was 9.4% (58 patients) resulting in a specificity of 98.4% (95% CI: 97.0–99.3%) and a sensitivity of 1.72% (95% CI: 0.044–9.2%) of uNGAL for the diagnosis of CI-AKI. In this study uNGAL failed to predict CI-AKI and was an inadequate triage tool to guide an early intervention strategy to prevent CI-AKI. Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01292317.
Similar content being viewed by others
Introduction
Contrast induced acute kidney injury (CI-AKI) also called contrast induced nephropathy (CIN) represents a serious complication of radiocontrast procedures and has consistently been associated with adverse clinical outcomes1,2. Chronic kidney disease (CKD) alone or coupled to other risk factors such as diabetes or heart failure significantly increases the incidence of CI-AKI to 25–45%3,4. Although plasma creatinine only poorly reflects early changes in glomerular filtration it is still the mainstay of all current definitions of acute kidney injury (AKI) and CI-AKI5. The use of plasma creatinine for the detection of AKI fosters a therapeutic intervention not before 24 hours following the insult to the kidney. Hence, considerable effort has been put into the search for new biomarkers as early indicators of AKI6. One of the promising candidate biomarkers is neutrophil gelatinase - associated lipocalin (NGAL), a 25 kDa siderophore binding protein7. In response to a renal tubular injury the monomeric form of NGAL is highly expressed in the kidney followed by a rapid increase of NGAL-levels in plasma and urine8,9. Clinical trials in adults reported a convincing performance of NGAL to diagnose AKI as early as 4–8 hours after a renal injury, far earlier than by an increase in creatinine concentrations4,10,11. However, it has not been evaluated yet if a therapeutic intervention prompted by an early NGAL-rise can prevent further kidney damage and/or a subsequent deterioration of glomerular filtration. Given the reported high incidence of CI-AKI in patients with CKD, we hypothesized that administration of intravenous saline after an early post procedural rise of uNGAL would prevent further kidney damage reducing the incidence of CI-AKI12. We further aimed to quantify the diagnostic accuracy of uNGAL as an early biomarker of CI-AKI in patients with chronic kidney disease (CKD).
Materials and Methods
Study population
We conducted a single-center, open-label, prospective randomized trial on patients with CKD who either underwent a percutaneous coronary intervention (PCI) or a percutaneous transluminal angiography/angioplasty (PTA) of peripheral arteries, renal arteries or carotids (clinicaltrials.gov: NCT01292317, registered February 8, 2011). No patients undergoing emergency procedures were included. Briefly, inclusion criteria were age >18 years, stable CKD with an estimated glomerular filtration rate (eGFR) <70 mL/min per 1.73 m2 which was confirmed by checking the institutional medical record system and the laboratory values from out-of hospital testing provided by the patients, and the need for intra-arterial angiography/angioplasty. Criteria for exclusion were evidence of acute renal failure according to the Acute Kidney Injury Network (AKIN) criteria13, dependence on dialysis, rhabdomyolysis, intolerance of volume expansion, acute life threatening conditions, pregnancy and administration of iodinated contrast media within 7 days prior to intervention. The study was conducted in accordance with the Declaration of Helsinki at the Medical University of Graz and was approved by the Institutional Review Board of the Medical University of Graz (IRB00002556, Study Registration Number: 21–278 ex 09/10). All patients gave their written informed consent prior to enrollment in the study.
Study design and conduct
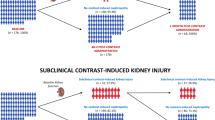
The study design has been previously published in detail12. As a nephroprotective measure all study participants received 3–5 ml/kgBW 0.9% saline per hour for 3 hours prior to angiography (=1000 ml over 3 hours in a 70 kg person). Monomeric, non-ionic, low osmolar iomeprol (Iomeron 300®, Bracco Vienna, Austria) was exclusively used as contrast medium for all study subjects in doses adjusted for body weight and determined by the numbers and locations of angiograms. Urinary NGAL (uNGAL) was measured the day before (day-1) and 4–6 hours after CM-application (day 0). Urine samples were taken from spontaneously voided urine and immediately processed thereafter. For practical reasons we chose a time period instead of an exact point in time for the uNGAL-measurement. Patients whose uNGAL was elevated above a pre-determined threshold after angiography (day 0) were randomized into two groups (A and B) using an online-randomization tool (http://www.randomizer.at). Thresholds for randomization were an increase of uNGAL from <75 ng/ml at baseline (day-1) to >150 ng/ml post angiography (day 0) or a doubling of uNGAL if baseline values were >75 ng/ml. These increments of uNGAL were considered significant accounting for a standard deviation of uNGAL that is approximately twice its mean concentration in healthy subjects14,15. Patients allocated to Group A received 3–4 ml/kg/BW 0.9% saline intravenously for 6 hours post procedure, Group B did not receive any i.v. fluids.
Assays
Plasma creatinine was measured by the standard Jaffe colorimetric method, Cystatin C by a nephelometric immunoassay and the estimated glomerular filtration rate (eGFR) was calculated via the abbreviated Modification of Diet in Renal Disease (MDRD) study equation16. Midstream urinary NGAL (uNGAL) was measured the day before and 4–6 hours after CM-application by the ARCHITECT® NGAL Test (Abbott Laboratories, Abbott Park, Illinois, US). Blood was drawn the day preceding angiography (day-1), the day after angiography (day 1) and the following day (day 2).
Outcomes
The primary outcome of the study was the development of CI-AKI by a ≥25% increase of plasma creatinine from baseline within 48 hours after angiography. This was the most commonly used definition and timing of CI-AKI at the time of creating the study protocol1,17. For a renal follow-up, plasma creatinine and eGFR were derived from the institutional medical record system one and three months after hospital discharge if available. In a secondary analysis we tested the sensitivity and specificity of uNGAL for CIN in the non-randomized cohort12.
Statistical analysis
Descriptive statistics were given as median 25% percentile (Q1) - 75% percentile (Q3) or mean ± standard deviation. Associations (correlations) between continuous variables were tested by the Spearman rank correlation coefficient, associations between dichotomous variables and continuous or polytomous variables were tested by the Wilcoxon rank sum test or Kruskal-Wallis test. Associations between dichotomous variables were tested by Fisher’s exact test. Continuous variables were dichotomized at the median in order calculate descriptive statistics of uNGAL for the high and the low group. Risk factors for CI-AKI were assessed by univariate logistic regression. Differences between treatment arms were assessed by the Fisher’s exact test. Because of its skewed distributions uNGAL was transformed by the natural logarithm.
Sample size
Based on the reported high positive predictive value of NGAL for an early diagnosis of CI-AKI18,19,20 we considered the reduction in CI-AKI incidence by 20% in the hydration group A as clinically relevant. Therefore 108 patients per treatment arm are required at an alpha of 0.05 and power of 0.8 (two-sided, chi-squared test). Assuming a 20% CI-AKI incidence rate21,22,23 and a 10% drop out rate, then 1200 patients had to be included. It was pre-specified that if after inclusion of 240 patients less than 48 patients had been randomized, the NGAL criteria would be adapted to improve the recruitment rate to intervention. All statistical tests were two-sided. R 3.2.0 was used for calculations.
Results
Patients
Between September 2010 and April 2012 1538 potential study participants were screened. After the screening process 921 patients had to be excluded: 789 did not meet the inclusion criteria, 27 declined to participate, 23 cases had to be excluded because patients underwent multiple angiographic procedures and 82 had miscellaneous reasons. (Fig. 1). This left 617 (287 female) patients (median age 74.0 years, range 37–91 years) who were recruited and finished the study in compliance with the study protocol. 39.9% of patients were diabetics, 85.9% had hypertension and 81.2% had an underlying heart disease which was defined as any history of ischemic heart disease, congestive heart failure, cardiac valvular defects or chronic cardiac arrhythmias. The mean plasma creatinine at study entry was 1.36 ± 0.41 mg/dl, and the mean eGFR was 48.7 ± 12.7 ml·min−1·1.73 m−2. 62.4% of participants underwent a PCI, 34.5% had a percutaneous transluminal angioplasty of peripheral arteries and 3.1% had a PTA of renal arteries or carotids, respectively (Table 1). The pre-procedural hydration according to our protocol was given to 97.2% of patients.
At the pre-planned interim assessment of the first 240 patients only two had been randomized. This unexpectedly low number meant that it was not possible to adjust the uNGAL criteria to sufficiently improve recruitment to meet the minimum needed number to test the primary hypothesis. This led to the decision not to change criteria for randomization and to continue to collect data to test the sensitivity and specificity of uNGAL for the diagnosis of CI-AKI in non-randomized patients. A sample size of 600 was considered sufficient for this.
Contrast induced acute kidney injury (Primary outcome)
From all 617 patients included into our study, 58 patients (9.4%) developed a ≥25% increase of serum creatinine from baseline either 24 or 48 hours after angiography with a peak creatinine of 2.6 mg/dl on day 1 and 3.6 mg/dl on day 2. The incidence was 9.6% with the KDIGO-criteria of AKI5. By the RIFLE-classification of AKI stages, no patients were classified as RIFLE R (1.5fold increase of creatinine), 43 patients were classified as RIFLE I (a two-fold increase in creatinine) and 3 patients RIFLE F (a threefold increase of creatinine)24. No patients had oliguria.
Ten patients (9 female) showed a significant increase of uNGAL after angiography and were randomized. Six patients were assigned to group B, of whom one developed a CI-AKI, whereas no CI-AKI was observed in group A (p = 1.0, Fig. 2). In group B two adverse events (AE) occurred on day 0 and one severe adverse event (SAE) on day 1. In group A there was one AE and one SAE on day 0 and two AE on day 1. Detailed information about types and numbers of adverse events and adverse reactions is provided in Supplemental Tables 1 and 2 of the Supplementary appendix.
Black: Patients without CI-AKI; Red: Patients with CI-AKI; Shaded area: Patients with significant increase of NGAL. Diagonal dotted line: Patients without significant change of uNGAL. Diagonal dashed line: Patients with doubling of uNGAL after angiography. Area below dotted line: Patients with decrease of uNGAL.
In the whole group patients with CI-AKI had lower creatinine and a higher eGFR at baseline. Apart from baseline creatinine and eGFR no other independent predictors of CI-AKI could be identified by logistic regression analysis (Table 2). Patients who developed a CI-AKI were significantly longer hospitalized (median two days, Q1–Q3 2–4 days) than patients without CI-AKI (median three days, Q1–Q3 2–7 days; p = 0.01). One month after angiography kidney function parameters of 363 patients could be derived from the institutional medical record system. Patients with CI-AKI had an eGFR of 56.50 ± 18.02 ml·min−1·1.73 m−2 and did not differ from patients without CI-AKI (eGFR 58.92 ± 24.92 ml·min−1·1.73 m−2; p = 0.76). Three months after hospital discharge kidney function parameters of 422 patients were available and remained stable in both groups (eGFR 55.25 ± 13.05 ml·min−1·1.73 m−2 versus 60.40 ± 22.72 ml·min−1·1.73 m−2; p = 0.22).
Characteristics of uNGAL and prediction of contrast induced acute kidney injury (Secondary analysis)
In the whole group median uNGAL (Q1–Q3) was 19 (9–49) ng/ml at day-1. uNGAL was ≤75 ng/ml in 521 patients (84.4%). Baseline uNGAL was associated with female sex, diabetes, age, lower eGFR, Cystatin C, urine osmolality and proteinuria (Table 3). These associations applied for both absolute uNGAL and uNGAL normalized to creatinine (Table 3). In the whole group uNGAL decreased from median 19 (9–49) ng/ml at day-1 to median 11 (6–28) ng/ml after contrast application (p < 0.0001, Fig. 2). uNGAL at day 0 did not differ between patients who subsequently developed CI-AKI and those who did not (p = 0.76) (Table 2). A pro forma receiver operating characteristic (ROC) analysis gave an area under curve (AUC) of 0.51 (95% CI: 0.42–0.60), (Fig. 3). The threshold of uNGAL at day 0 that would have identified 20% patients as eligible for randomization as had been expected would have needed to be only 39 ng/ml. This corresponded to a sensitivity of only 28% (95% CI: 17–41%), a specificity of 80% (95% CI: 77–83%), and a positive predictive value of 10% (95% CI: 0.253–44.5%).
The relative changes of absolute uNGAL defined by the quotient of uNGAL at day 0 divided by day-1 were significantly correlated with the relative changes of the urine osmolality (Spearman r = 0.46, p < 0.0001), but this association was attenuated using uNGAL normalized to creatinine (Spearman r = 0.09, p = 0.03).
Discussion
The main findings of this prospective randomized study of 617 patients with CKD undergoing intra-arterial angiography was that only 10 patients exhibited a significant rise of uNGAL exceeding the threshold for randomization and only one single patient out of these developed CI-AKI. Therefore, insufficient patients could be recruited to test the hypothesis that post-procedural volume expansion ameliorates CI-AKI. This was the first study of contrast induced nephropathy to attempt to triage patients to an intervention following the elevation of a kidney injury biomarker. Our findings contrast CI-AKI-studies in CKD-patients showing a good predictive power of uNGAL in the diagnosis of CI-AKI with a marked increase between six to twelve hours post angiography4,25,26. On the other hand, there are also observational studies demonstrating that in patients with an eGFR <60 ml/min uNGAL-levels did not differ between those with and without AKI until 24 hours after cardiac surgery10 and intra-arterial angiography27,28, respectively. A very recently published study of patients with an eGFR <30 ml/min revealed that NGAL was a reliable marker for ruling out CI-AKI but with a poor positive predictive power at 6 hours after CM-exposure29. A key conclusion from these studies as well as ours is that in CKD-patients uNGAL does not provide an early intervention strategy following contrast media application to prevent further kidney damage and a decline of kidney function. Two early intervention clinical trial paradigms have been proposed: (1) An early intervention after a renal insult or (2) an intervention after an early detection of a kidney injury prior to an observed change in kidney function6. Our results suggest that uNGAL is an inadequate tool for the latter strategy after contrast application. In general there is a great ambiguity of results inherent to studies on urinary and serum biomarkers for the diagnosis of AKI30 and this also seems to hold true for NGAL31. The diagnostic performance of NGAL is critically determined by baseline renal function and after a renal insult NGAL-levels in CKD-patients increase with a considerable delay10,32. Following cardiopulmonary bypass surgery, McIlroy and colleagues noted poor performance of NGAL in CKD patients at all time points following surgery10. In critically ill patients in the ICU Endre and colleagues noted that it was not until some 12 to 36 hours following the putative time of insult that the diagnostic performance of NGAL was optimized in this group32. If the findings in surgical and critically ill patients were to translate to the current setting, then this may explain the poor performance of NGAL as a tool to triage to early treatment. This clearly hampers a clinical decisive time advantage for an early diagnosis of AKI and the possibility of an early therapeutic intervention in CKD-patients. From ten randomized patients according to a relevant NGAL increase, only one patient developed a CI-AKI, whereas nine patients with a significant rise of uNGAL did not experience any subsequent deterioration of glomerular filtration rate. Due to the small number of randomized patients no meaningful conclusions about the benefits of additional intravenous volume expansion can be drawn.
In the whole cohort, baseline uNGAL-levels were significantly associated with diabetes, lower eGFR, Cystatin C, proteinuria, age and female sex. These associations are in line with previous studies and thus support the validity of our measurements33,34. On the other hand, these findings also underline that NGAL concentrations are associated with various parameters with relevance for CIN and kidney function thus questioning or limiting the additional prognostic and diagnostic value of uNGAL35. Baseline urine osmolality and its longitudinal changes were positively correlated with baseline absolute uNGAL concentrations and its relative changes. This finding favors the preferential use of the ratio to creatinine, thus minimizing distortions by fluctuating urine osmolality36,14,37. Normalization of urinary biomarkers to creatinine, however, is also under debate as creatinine secretion shows a high variability during changes in glomerular filtration rate38. In patients with normal kidney function a cut off NGAL-value >150 ng/ml seems to be suitable for the diagnosis of AKI7. CKD-patients exhibit higher NGAL concentrations at baseline and higher cut-off levels for the identification of evolving AKI are therefore presumed39. Taking the high biological variability of NGAL into account, the cut off levels in our study seem to be thoroughly appropriate for a CKD-population14,15,37. Surprisingly, in 70% of our patients the concentrations of both absolute and uNGAL normalized to creatinine dropped after contrast application with a more pronounced decrease of absolute uNGAL concentrations. In parallel urine osmolality also decreased, albeit to a much lesser extent suggesting that a diluting effect is probably involved. Consistent with our results an observational study showed that half of the patients exhibited a decrease of both absolute and uNGAL normalized to creatinine concentrations after coronary angiography40. The incidence of CI-AKI in our study was 9.4% and at the lower end of the range reported in the literature that is characterized by a great variability of results1. In more recent studies, frequencies of CI-AKI in CKD patients varied from 3.2% to 12.3%4,25,26,27,29 and our findings are in good agreement with that. As a limitation of these studies as well as ours it cannot be fully excluded that CI-AKI cases occurring beyond 48 hours after contrast application might have been missed. In contrast to previous studies, established and recognized risk factors for CI-AKI such as diabetes, age, proteinuria, contrast volume and renal impairment could not be identified as independent predictors of CI-AKI by logistic regression analysis in our cohort. Quite contrary, baseline renal function determined by plasma creatinine and eGFR but not by Cystatin C was better in patients developing a CI-AKI. A possible explanation for this surprising finding might be the regression to the mean phenomenon, especially as Cystatin C did not show to have any predictive significance. The occurrence of CI-AKI necessitated a longer hospital stay, but after one as well as after three months after hospital discharge, there was no significant difference in renal function between CI-AKI and non-CI-AKI patients. These results suggest that in our study, contrast media caused only minor kidney damage with only a transient deterioration of glomerular filtration rate.
Our study has some limitations. As it is a single-center study our data need confirmation in a multi-center study setting. In order to test our hypothesis, we did not provide intravenous fluid post contrast application to all our patients as current CI-AKI prevention guidelines recommend5. Indeed, the comparably low incidence of CI-AKI in our high risk cohort demonstrated that we did not harm our patients and pre-hydration alone might be sufficient enough to prevent CI-AKI. However, this finding has to be confirmed by appropriate studies. The aim of the study was to evaluate uNGAL as a triage tool and for that reason serial uNGAL-measurements were not performed. We therefore cannot provide sufficient information on uNGAL-kinetics in CKD-patients. On the other hand, to the best of our knowledge the ANTI-CI-AKI-study is the largest prospective randomized trial so far evaluating the diagnostic performance of uNGAL in identifying evolving CIN and an early intervention strategy in CKD-patients. The results of our study, along with the aforementioned negative biomarker studies, demonstrate that in order to be a successful triage tool the correct biomarker for a specific kidney injury measured at the correct time point has to be selected. We know of no study that has measured multiple biomarkers at multiple time points following contrast administration to provide guidance as to what may be the optimal biomarker in this setting.
In conclusion, in this study uNGAL failed to guide an early intervention strategy post contrast application and failed to identify evolving CI-AKI in CKD-patients after intra-arterial angiography. No conclusions can be drawn about the benefits of additional intravenous volume expansion post angiography in this cohort.
Additional Information
How to cite this article: Ribitsch, W. et al. Neutrophil gelatinase-associated lipocalin (NGAL) fails as an early predictor of contrast induced nephropathy in chronic kidney disease (ANTI-CI-AKI study). Sci. Rep. 7, 41300; doi: 10.1038/srep41300 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
McCullough, P. A. et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am. J. Cardiol. 98, 5K–13K (2006).
Weisbord, S. D. et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J. Am. Soc. Nephrol. 17, 2871–2877 (2006).
Rudnick, M. R., Goldfarb, S. & Tumlin, J. C ontrast-induced nephropathy: is the picture any clearer? Clin. J. Am. Soc. Nephrol. 3, 261–262 (2008).
Lacquaniti, A. et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology 267, 86–93 (2013).
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. Suppl. 2, 1–138 (2012).
Pickering, J., Ralib, A., Nejat, M. & Endre, Z. New considerations in the design of clinical trials of acute kidney injury. Clin. Invest. 5, 637–650 (2011).
Haase-Fielitz, A., Haase, M. & Devarajan, P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann. Clin. Biochem. 51, 335–351 (2014).
Cai, L., Rubin, J., Han, W., Venge, P. & Xu, S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 5, 2229–2235 (2010).
Paragas, N. et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 17, 216–222 (2011).
McIlroy, D. R., Wagener, G. & Lee, H. T. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin. J. Am. Soc. Nephrol. 5, 211–219 (2010).
Bachorzewska-Gajewska, H. et al. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int. J. Cardiol. 127, 290–291 (2008).
Schilcher, G. et al. Early detection and intervention using neutrophil gelatinase-associated lipocalin (NGAL) may improve renal outcome of acute contrast media induced nephropathy: a randomized controlled trial in patients undergoing intra-arterial angiography (ANTI-CIN Study). BMC Nephrol. 12, 39-2369-12–39 (2011).
Mehta, R. L. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31 (2007).
Delanaye, P., Rozet, E., Krzesinski, J. M. & Cavalier, E. Urinary NGAL measurement: biological variation and ratio to creatinine. Clin. Chim. Acta 412, 390 (2011).
Grenier, F. C. et al. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin. Biochem. 43, 615–620 (2010).
Levey, A. S. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 145, 247–254 (2006).
Endre, Z. H. & Pickering, J. W. Outcome definitions in non-dialysis intervention and prevention trials in acute kidney injury (AKI). Nephrol. Dial. Transplant. 25, 107–118 (2010).
Hirsch, R. et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr. Nephrol. 22, 2089–2095 (2007).
Bachorzewska-Gajewska, H. et al. Could neutrophil-gelatinase-associated lipocalin and cystatin C predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press. Res. 30, 408–415 (2007).
Shaker, O. G., El-Shehaby, A. & El-Khatib, M. Early diagnostic markers for contrast nephropathy in patients undergoing coronary angiography. Angiology 61, 731–736 (2010).
Mehran, R. & Nikolsky, E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int. Suppl. 100, S11–5 (2006).
Weisbord, S. D. et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch. Intern. Med. 168, 1325–1332 (2008).
Stone, G. W. et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA 290, 2284–2291 (2003).
Bellomo, R. et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 8, R204–12 (2004).
McCullough, P. A. et al. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am. J. Nephrol. 35, 509–514 (2012).
Tasanarong, A., Hutayanon, P. & Piyayotai, D. Urinary Neutrophil Gelatinase-Associated Lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol. 14, 270-2369-14–270 (2013).
Alharazy, S. M. et al. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after coronary angiography. Angiology 65, 216–223 (2014).
Liebetrau, C. et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand. J. Clin. Lab. Invest. 74, 81–88 (2014).
Quintavalle, C. et al. Neutrophil Gelatinase-Associated Lipocalin and Contrast-Induced Acute Kidney Injury. Circ. Cardiovasc. Interv. 8, 10.1161/CIRCINTERVENTIONS.115.002673 (2015).
Vanmassenhove, J., Vanholder, R., Nagler, E. & Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol. Dial. Transplant. 28, 254–273 (2013).
Singer, E. et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol. (Oxf) 207, 663–672 (2013).
Endre, Z. H. et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 79, 1119–1130 (2011).
Tomonaga, Y. et al. Insights on urinary NGAL obtained in a primary care setting. Clin. Chim. Acta 413, 733–739 (2012).
Thrailkill, K. M. et al. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine 37, 336–343 (2010).
Nickolas, T. L. et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 148, 810–819 (2008).
Ralib, A. M. et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J. Am. Soc. Nephrol. 23, 322–333 (2012).
Helmersson-Karlqvist, J., Arnlov, J. & Larsson, A. Day-to-day variation of urinary NGAL and rational for creatinine correction. Clin. Biochem. 46, 70–72 (2013).
Waikar, S. S., Sabbisetti, V. S. & Bonventre, J. V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 78, 486–494 (2010).
Di Somma, S. et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit. Care 17, R29 (2013).
Weber, C. L., Bennett, M., Er, L., Bennett, M. T. & Levin, A. Urinary NGAL levels before and after coronary angiography: a complex story. Nephrol. Dial. Transplant. 26, 3207–3211 (2011).
Acknowledgements
We are indebted to our study nurse, Ms. Maria Berger for her great assistance with the data management. We also thank Abbott Company for donating the Architect kits used for testing and partly funding our study nurse’s appointment.
Author information
Authors and Affiliations
Contributions
W.R., G.S. and J.H.H. conceived and designed the study, W.R. wrote the paper, M.B., R.Z., P.S. and R.H.P. helped collecting the data and conducting the study, M.T.W. did the lab assays, F.Q. and S.P. analyzed the data, F.Q. prepared Figures 2, 3, J.W.P. and A.R.R. made specific comments on the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ribitsch, W., Schilcher, G., Quehenberger, F. et al. Neutrophil gelatinase-associated lipocalin (NGAL) fails as an early predictor of contrast induced nephropathy in chronic kidney disease (ANTI-CI-AKI study). Sci Rep 7, 41300 (2017). https://doi.org/10.1038/srep41300
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41300
This article is cited by
-
Evaluating biomarkers for contrast-induced nephropathy following coronary interventions: an umbrella review on meta-analyses
European Journal of Medical Research (2024)
-
Contrast Induced Acute Kidney Injury and its Impact on Mid-Term Kidney Function, Cardiovascular Events and Mortality
Scientific Reports (2019)
-
Lack of impact of iodinated contrast media on kidney cell-cycle arrest biomarkers in critically ill patients
BMC Nephrology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.