Abstract
The value of neoadjuvant chemotherapy (NAC) has not yet been fully defined. We aimed to systematically evaluate the influence of neoadjuvant chemotherapy (NAC) on survival and complete cytoreduction after debulking surgery in advanced epithelial ovarian cancer (AEOC) patients. We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for the randomized controlled trials (RCTs) comparing NAC and primary debulking surgery (PDS) in AEOC patients. The last search date is February 25, 2016. Cochrane systematic evaluation was used to evaluate bias risk of included studies. RevMan 5.3 software was used for statistical analysis. A total of 4 RCTs involving 1922 patients were included. Compared with PDS, NAC may contribute to the completeness of debulking removal [no residual disease (RR: 2.37; 95%CI: 1.94–2.91; P<0.00001), residual disease ≤1 cm (RR: 1.28; 95%CI: 1.04–1.57; P = 0.02), optimal cytoreduction rate (RR: 1.76; 95%CI: 1.57–1.98; P<0.00001)], but there were no significant differences in both groups with regard to overall survival (HR: 0.94; 95%Cl: 0.81–1.08; P = 0.38) and progression-free survival (HR: 0.89; 95%Cl: 0.77–1.03; P = 0.12). This meta-analysis indicates that the higher rate of optimal debulking made NAC more favorable as a treatment option for AEOC patients with non-inferior survival compared with PDS.
Similar content being viewed by others
Introduction
The standard of care for advanced epithelial ovarian cancer (AEOC) has been primary cytoreductive surgery (PDS) followed by systemic chemotherapy. Neoadjuvant chemotherapy (NAC) followed by interval debulking surgery (IDS) has recently been put forth, as an acceptable standard of care for AEOC stimulated by the results of numerous studies1,2. Despite being recommended by 2015 NCCN guideline for patients with bulky stage III/IV disease, who are diagnosed by fine-needle aspiration (FNA), biopsy, paracentesis, or poor surgical candidates due to high-risk comorbidity conditions or disease factors3, the utility of NAC remains in dispute, especially whether NAC improves the prognosis of AEOC and which patients benefit most from NAC1,2,4,5,6,7.
To our knowledge, 4 large randomized controlled trials have been conducted1,2,4,5, assessing the benefits of NAC for AEOC, however, their results are conflicting. Kehoe et al.1 published a randomized phase III trial comparing NAC versus PDS in patients with AEOC, which showed equivalent survival in these patients, though NAC was associated with fewer complications and lower treatment-related mortality after IDS. These findings correspond with the data from Vergote et al.2, but not of Van Der Burg et al.5, in which patients with AEOC achieved longer survival with NAC followed by IDS compared to PDS.
Several meta-analysises, based on retrospective studies, have been performed8,9. The present meta-analysis was restricted to RCTs in order to eliminate selection bias, and overcome the limitation of retrospective studies.
Methods
Data sources and search strategy
PubMed, Embase, and the Cochrane Central Register of Controlled Trials databases were comprehensively and systematically searched from inception to February 25, 2016 without language restrictions. The search was limited to RCTs that compared NAC versus PDS for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIB-IV epithelial ovarian cancer. The search terms included “ovarian cancer”, “ovarian carcinoma”, “ovarian tumor”, “neoadjuvant chemotherapy”, “preoperative chemotherapy”, and “cytoreductive surgery”. References of relevant literature were also manually screened.
Study selection and eligibility criteria
Articles were retained if they fulfilled the following predefined criteria: (1) subjects were patients whose pathological diagnosis was AEOC, with FIGO stage IIB-IV; (2) interventions were platinum-based NAC followed by IDS and chemotherapy OR PDS followed by NAC then IDS followed by chemotherapy, compared with PDS followed by platinum-based chemotherapy; (3) type of study was RCT. Articles were excluded if they were review articles or ongoing studies, conference abstracts, or without survival data. Two authors (LJ Zeng and CL Xiang) evaluated all articles to verify the inclusion and exclusion criteria independently. Differences of opinion were resolved by consensus after consultation between the two reviewers.
Data extraction and quality assessment
Two independent reviewers (LJ Zeng and CL Xiang) performed data extraction (first author, year of publication, sample size, age, FIGO stage, pathology, histological grade, intervention, and outcome data) and evaluated the quality of included studies according to Cochrane Collaboration’s risk of bias tool in Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. Consensus was used to resolve discrepancies. The end points of interest were: overall survival (OS), progression-free survival (PFS), no residual disease, residual disease ≤1 cm, and optimal cytoreduction rate. The definition of optimal cytoreductive surgery is residual tumor diameter ≤1 cm or no residual disease after debulking surgery.
Statistical analysis
Pooled hazard ratios (HRs) for OS or PFS and relative risks (RRs) for extent of surgical debulking with corresponding 95% confidence interval (CI) were calculated in Review Manager(RevMan)10. We using the I2 statistic to assess statistical heterogeneity between studies11. An I2 ≤ 25%, 26% to 50%, and >50% indicates low, moderate, and high heterogeneity, respectively12. When I2 ≤ 25%, fixed effects model was presented12,13,14. On the contrary, random effects model was applied and sensitivity analysis was also carried out to investigating the influence of a single study on the overall pooled estimate by omitting one study in each turn. All statistical analysis were carried out by using RevMan 5.3.
Results
Literature search and study characteristics
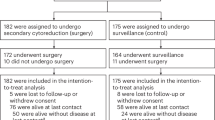
The search process of the study is shown in Fig. 1. The initial screening yielded 1341 references. After the exclusion of duplicate publications, 1250 references were withheld for screening of the title and/or abstract, then 1224 references were excluded because of other reasons (non-RCT, reviews, editorials, no use of NAC, no PDS control, ongoing trials, and conference abstract). Finally after screening of the full article, only 4 RCTs containing 1922 patients were included in the meta-analysis process1,2,4,5. The major characteristics of the 4 RCTs included in the meta-analysis are presented in Table 1. The sample size of these trials ranged from 278 to 670 (total 1922 patients). The average age of the patients was similar between trials. All studies reported the OS and PFS1,2,4,5, and 2 studies compared optimal cytoreduction rate between both groups1,2.
The assessment of the quality of the selected RCTs is presented in Fig. 2. As less than 10 studies were included, we did not evaluate the publication bias in our study15.
Overall survival
All studies evaluated the OS between NAC group and PDS group1,2,4,5. The outcomes of OS were pooled and compared with a random-effects model (Fig. 3). There was no significant difference in OS between NAC group and PDS group (HR: 0.94; 95%Cl: 0.81–1.08; P = 0.38), with high heterogeneity among the studies (I2 = 56%). From the sensitivity analysis, we found that Van der Burg et al.5 probably contributed to the heterogeneity. After excluding this study, the result suggested that OS of AEOC patients was still similar between NAC and PDS (HR 0.99, 95%CI: 0.90–1.09, P = 0.90), with low heterogeneity (I2 = 0%).
Progression-free survival
All studies evaluated the PFS between NAC group and PDS group1,2,4,5. Pooling data from the 4 RCTs by a random-effects model included no significant difference in PFS was found between the NAC group and PDS group (HR: 0.89; 95% Cl: 0.77–1.03; P = 0.12), with moderate heterogeneity among the studies (I2 = 46%) (Fig. 4).
Extent of surgical debulking
Two of the 4 studies reported data on the extent of surgical debulking, and thus were eligible to be included in the meta-analyses1,2. Compared with PDS, NAC may contribute to the completeness of lesions removal [no residual disease (RR: 2.37; 95%CI: 1.94–2.91; P < 0.00001; I2 = 0%), residual disease ≤1 cm (RR: 1.28; 95%CI: 1.04–1.57; P = 0.02; I2 = 0%), optimal cytoreduction rate (RR: 1.76; 95%CI: 1.57–1.98; P < 0.00001; I2 = 0%)] (Fig. 5).
Forest plot for extent of surgical debulking.
The definition of optimal cytoreductive surgery is residual tumor diameter ≤1 cm or no residual disease after debulking surgery; NAC, neoadjuvant chemotherapy followed by interval debulking surgery; PDS, primary cytoreductive surgery followed by systemic chemotherapy.
Discussion
We performed a meta-analysis of 4 RCTs to systematically evaluate the influence of NAC on survival and complete cytoreduction after debulking surgery in AEOC patients. In our meta-analysis, we found that NAC may contribute to the completeness of tumor removal [no residual disease (RR: 2.37; 95%CI: 1.94–2.91); residual disease ≤1 cm (RR: 1.28; 95%CI: 1.04–1.57); optimal cytoreduction rate (RR: 1.76; 95%CI: 1.57–1.98)], but OS and PFS of AEOC patients was similar between NAC and PDS [OS (HR: 0.94; 95%Cl: 0.81–1.08; P = 0.38); PFS (HR: 0.89; 95%Cl: 0.77–1.03; P = 0.12)]. Our findings are consistent with several previous meta-analysis which were based on retrospective cohort studies8,9.
According to the results of our analysis, NAC increased rate of optimal cytoreduction. It’s worth noting that Rose and colleagues (2004) state on that of 112 patients whose tumor exceeded 1 cm in diameter before they underwent secondary surgery, 79 (approximately 70%) had a residual mass of less than 1 cm and 33 (approximately 30%) had a residual mass of at least 1 cm after secondary surgery. The death rates in these two groups did not differ significantly (HR 1.25, 95% CI 0.785–2, p = 0.34). However, the studies by Rose et al.4 and van der Burg5 are fundamentally different in that all patients initially underwent a maximal cytoreductive effort complicated by inability to optimally debulk the cancer (interval debulking); this is in contrast to the more modern studies by Vergote and Kehoe in which patients underwent NO attempt at cytoreduction, only FNA/core biopsy often without laparotomy (or if laparotomy was performed, only biopsies were allowed) prior to randomization (pure neoadjuvant chemotherapy paradigm).
On the other hand, heterogeneity was noticed in OS between the included trials. From the sensitivity analysis, we found that the study conducted by Van der Burg et al. probably contributed to the heterogeneity5. Significantly different from other 3 studies, OS of NAC group was prolonged compared with PDS group in Van der Burg et al. 5 . A higher proportion of patients with large residual tumors in PDS group than the other 3 studies might have contributed to worse survival compared with the NAC group, and may thus be regarded as a potential source of heterogeneity16. Notably further sensitivity analysis excluding Van der Burg et al.5 did not appreciably alter our findings.
NAC has been controversial since its inception. Whether NAC improves the prognosis of AEOC has been the focus of much controversy. Optimal cytoreduction improves survival17, but the increase in optimal cytoreduction does not translate into a significant improvement of overall survival and progression-free survival in patients who receive NAC. In 2009, a meta-analysis8 of 21 non-randomized trials concluded that increased rate of optimal cytoreduction was found in NAC group, but survival was similar in both NAC and PDS groups5,18,19,20,21,22,23. In the retrospective setting, this may be due to better performance status and/or lesser extent of tumor in PDS group. In order to eliminate selection bias, the recent studies included RCTs only. Interestingly, we arrived at similar conclusions: NAC increased the rate of optimal cytoreduction, but did not provide equal survival compared with PDS. We hypothesize that chemotherapy before surgery might induce fibrosis, and the postoperative residual fibrosis tissue may contain cancer stem cells which promote chemotherapy resistance in AEOC patients who have received NAC2,8,24. More studies are needed to explore this mechanism. At a minimum, we can conclude that NAC was non-inferior to PDS.
With improvements in survival afforded by better therapies for original carcinoma, future studies should focus on quality of life (QoL). Recently, QoL outcome from final analysis of peri-operative outcome of Fagotti et al.25, using the EORTC quality of life questionnaire core-36 (QLQC-30) and the ovarian cancer-specific quality of life questionnaire (QLQ-Ov28), indicates QoL scores were shown to be more favorable in NAC group than PDS group in AEOC patients26,27. A similar trend towards better QoL was reported in Kehoe et al.1. More patients in NAC group reported improvement in QoL at least 5 points than PDS group at 6 and 12 months in Kehoe et al.1.
Which patients benefit most from NAC remains unknown. Due to poor performance status or older age of study population, unsatisfactory surgical outcomes were observed in PDS group of Vergote et al. and Kehoe et al.1,2. Though NAC is recommended for patients with bulky stage III/IV disease who are poor surgical candidates due to high-risk comorbidity conditions or disease factors by 2015 NCCN guideline, maximum surgical efforts and competent surgical skills are necessary, regardless of whether NAC is performed28.
Although we chose to include only RCTs in this study to minimize bias, there remain potential limitations of our meta-analysis. Due to different study designs, surgical techniques, chemotherapeutics regimens and surgical procedures, there was heterogeneity among the included trials. In order to mitigate the potential effect of heterogeneity on the validity of the results, we used a random-effects model and explored possible causes of heterogeneity by sensitivity analysis.
In summary, NAC is a reasonable treatment option for FIGO stage III and IV epithelial ovarian cancer patients with non-inferior survival compared with PDS. Furthermore emerging evidences suggests that NAC may be associated with improved QoL compared to PDS. Maximum surgical efforts and improvement of competent surgical skills are necessary, regardless of whether NAC is performed.
Additional Information
How to cite this article: Zeng, L.-J. et al. Neoadjuvant chemotherapy for Patients with advanced epithelial ovarian cancer: A Meta-Analysis. Sci. Rep. 6, 35914; doi: 10.1038/srep35914 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kehoe, S. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 386, 249–257 (2015).
Vergote, I. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363, 943–953 (2010).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: original cancer including fallopian tube cancer and primary peritoneal cancer. v. 2. Avaible at www.nccn.org (2015).
Rose, P. G. et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N. Engl. J. Med. 351, 2489–2497 (2004).
van der Burg, M. E. et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 332, 629–634 (1995).
Chi, D. S., Bristow, R. E., Armstrong, D. K. & Karlan, B. Y. Is the easier way ever the better way? J. Clin. Oncol. 29, 4073–4075 (2011).
Vergote, I. et al. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J. Clin. Oncol. 29, 4076–4078 (2011).
Kang, S. & Nam, B. H. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann. Surg. Oncol. 16, 2315–2320 (2009).
Bristow, R. E. & Chi, D. S. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 103, 1070–1076 (2006).
The Nordic Cochrane Centre. RevMan: A software for preparing and maintaining Cochrane reviews. The Cochrane Collaboration, Copenhagen, Denmark. URL http://tech.cochrane.org/revman/ (2012).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Song, F., Eastwood, A. J., Gilbody, S., Duley, L. & Sutton, A. J. Publication and related biases. Health. Technol. Assess 4, 1–115 (2000).
van der Burg, M. E. & Vergote, I. The role of interval debulking surgery in ovarian cancer. Curr. Oncol. Rep. 5, 473–481 (2003).
Hoskins, W. J. et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 170, 974–979, discussion 979–980 (1994).
Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 20, 1248–1259 (2002).
du Bois, A. et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115, 1234–1244 (2009).
Griffiths, C. T. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl. Cancer Inst. Monogr. 42, 101–104 (1975).
Polterauer, S. et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int. J. Gynecol. Cancer 22, 380–385 (2012).
Winter, W. E. 3rd et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 25, 3621–3627 (2007).
Winter, W. E. 3rd et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 26, 83–89 (2008).
Lim, M. C. et al. Residual cancer stem cells after interval cytoreductive surgery following neoadjuvant chemotherapy could result in poor treatment outcomes for ovarian cancer. Onkologie 33, 324–330 (2010).
Fagotti, A. et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 59, 22–33 (2016).
Greimel, E. et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur. J. Cancer 39, 1402–1408 (2003).
Osoba, D., Rodrigues, G., Myles, J., Zee, B. & Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 16, 139–144 (1998).
Kang, S. Neoadjuvant chemotherapy for ovarian cancer: do we have enough evidence? Lancet 386, 223–224 (2015).
Acknowledgements
This study is supported by the National Natural Science Foundation of China. Grant No. 81360389.
Author information
Authors and Affiliations
Contributions
Y.K. and L.-J.Z. designed the study, interpreted the data and wrote the first draft. L.-J.Z. and C.-L.X. performed statistical analysis. L.-J.Z. and Y.-Z.G. performed additional data analysis. C.-L.X., F.-F.L., S.-Y.Y., Y.Z. and M.L. contributed to the interpretation of the data and the final version of the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zeng, LJ., Xiang, CL., Gong, YZ. et al. Neoadjuvant chemotherapy for Patients with advanced epithelial ovarian cancer: A Meta-Analysis. Sci Rep 6, 35914 (2016). https://doi.org/10.1038/srep35914
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35914
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.