Abstract
Acute lung injury (ALI) is a severe respiratory disease with high mortality rates worldwide. Recent reports suggest that human neutrophil elastase (HNE) plays a key role in the inflammatory response that is characteristic of ALI, which indicates that the development of HNE inhibitors could be an efficient treatment strategy. In the current study, an enzyme-based screening assay was used to identify effective HNE inhibitors from a number of traditional Chinese medicines (TCMs). Among them, a water extract of Ilex kaushue (IKWE) effectively inhibited HNE activity (IC50, 11.37 ± 1.59 μg/mL). Using bioactivity-guided fractionation, one new compound and 23 known compounds were identified. Compound 6 (identified as 3,5-dicaffeoylquinic acid; 3,5-DCQA) exerted the most potent and selective inhibitory effect on HNE activity (IC50, 1.86 ± 0.06 μM). In a cell-based assay, 3,5-DCQA not only directly reduced superoxide generation and elastase activity but also attenuated the Src family kinase (SRKs)/Vav signaling pathway in N-formyl-L-Met-L-Leu-L-Phe (fMLF)-stimulated human neutrophils. In an animal disease model, both 3,5-DCQA and standardized IKWE protected against lipopolysaccharide-induced ALI in mice, which provides support for their potential as candidates in the development of new therapeutic agents for neutrophilic inflammatory diseases.
Similar content being viewed by others
Introduction
ALI and its more severe form, acute respiratory distress syndrome (ARDS), are inflammatory diseases triggered by direct and indirect pathogenic factors, such as sepsis, pneumonia, inhalation injury and trauma1. Lipopolysaccharide (LPS) is an endotoxin that plays a pathological determinant role in sepsis-related ALI2. In the majority of cases, ALI and ARDS result in respiratory failure leading to high mortality. No effective therapeutic agents are available for ALI and technical ventilation and supportive care constitute the primary approaches to avoid underlying complications1,3,4, highlighting the urgent requirement for novel treatment strategies and medicines.
Neutrophils form the first line of defense against pathogens in innate immunity mainly through phagocytosis. Further invasion of pathogens is prevented with the release of reactive oxygen species (ROS), serine proteases and neutrophil extracellular traps5. In ALI pathology, circulating neutrophils are recruited and activated by chemokines and cytokines from alveolar macrophages and alveolar-type II epithelial cells, triggering neutrophil serine protease release, alveolar edema and impaired oxygenation. Neutrophil elastase (NE) is one of the serine proteases released from activated neutrophils that cause pulmonary damage through hydrolysis of elastin-rich connective tissue. Additionally, NE acts as an inflammatory mediator and contributes to the migration and activation of neutrophils through effects on alveolar macrophage and lung epithelial cells6. α1-Antitrypsin, an endogenous secretory elastase inhibitor abundant in the peripheral alveolar region, naturally protects lung tissue from proteolysis by elastase. Enhanced NE activity has been observed due to inactivation of α1-antitrypsin under conditions of increased oxidative stress resulted from neutrophils4,7. The development of NE inhibitors is therefore considered an effective therapeutic strategy for ALI3,4.
Botanical products and TCM are recognized as important sources of novel drugs8,9. In an attempt to identify NE inhibitors, 22 TCM extracts were prepared and their inhibitory effects on human neutrophil elastase (HNE) activity evaluated. Among these, the I. kaushue water extract (IKWE) inhibited HNE activity with an IC50 value of 11.37 ± 1.59 μg/mL. I. kaushue, syn I. kudingcha (also known as Kudingcha) is an evergreen tree found in China10. The leaves have been used as a daily beverage and herbal medicine in TCM for nearly two thousand years11. Triterpenoids, polyphenols, cyanoglucosides and essential oils are the major metabolites of I. kaushue12,13. Traditionally, the plant has beneficial physiological effects, including thirst quenching, elimination of phlegm for resuscitation and removal of mucus from the lung14. Recent studies have additionally revealed anticancer, antidiabetes, antiobesity and antioxidant bioactivities12,13,15,16,17. In the current investigation, we focused on evaluating the beneficial effects of I. kaushue and its bioactive component on acute lung injury (ALI), both in vitro and in vivo.
Results
Chemical components isolated from I. kaushue water extracts (IKWE) via bioactivity-guided fractionation
Using bioassay-directed fractionation, ninteen compounds, including polyphenols (1-8), triterpenoid saponins (9-15), cyanoglucosides (17-19) and phytosteroid (16) (Fig. 1A and Supplementary information Fig. S1) were isolated from IKWE. Among these, compound 19 was new, while compounds 1~8 were identified as caffeoylquinic acids (CQA) or dicaffeoylquinic acids (DCQA) based on the position and number of caffeoyl groups conjugated with quinic acid. Saccharides of 11, 14 and 15 were further hydrolyzed to generate aglycons (11-1, 11-2, 14-1, 15-1 and 15-2) for proposing structure and activity relationships (SAR). All known compounds were identified by comparing their physical and spectral data with the values provided in the literatures.
Structural elucidation of menisdaurin F (compound 19)
Compound 19, [α]23D: −63.6° (c = 0.05, MeOH), was obtained as a colorless powder. The molecular formula, C14H19NO7, assigned based on HRESIMS data (336.1048 m/z, [M + Na]+), implied six unsaturation degrees. The presence of a hydroxyl group was suggested based on the absorption band at 3392 cm−1 in the IR spectrum. The characteristic bands at 2220 and 1621 cm−1 in the IR spectrum, 13C NMR signals at δC 93.7 (d), 117.9 (s), 124.5 (d), 143.3 (d), 159.1 (s) and 1H NMR signals at δH 5.77 (s), 6.24 (d, 10.0), 6.57 (dd, 10.0, 2.0), together with ultraviolet absorption at λmax 256 nm, signifying the presence of α, β, γ, δ-unsaturated nitrile18,19,20. NMR signals at δH 3.66 (dd, 11.6, 8.0), 3.86 (d, 11.6), 4.48 (d, 8.0); δC 62.6 (t) and 102.6 (d) suggested the presence of a glucopyranosyl group. Comparison of 1D and 2D NMR spectra revealed that compound 19 is a structural isomer of menisdaurin (18) (Fig. 1B, Supplementary information Figs S2–S8 and Table S1)19,20,21. The strong NOE (nuclear overhauser effect) correlations between H-4 and H-5a, H-5a and H-6 and H-6 and H-1’ demonstrate that compound 19 shares the same orientation of H-4 and H-6 with menisdaurin. Additionally, NOE correlations between H-6 and H-7 implied an E-configuration of the C-1/C-7 double bond in compound 1919,22. The downfield shift of the H-2 signal (calculated as 0.25 ppm) additionally supported the presence of the E-configuration19. Based on the collective data, the structure of 19 was determined as an E-form isomer of menisdaurin (18), designated menisdaurin F.
3,5-DCQA (compound 6) showed potent and selective inhibition of HNE activity
To identify the bioactive components and their specificities, enzyme inhibition assays involving human and bovine serine proteases were performed (Table 1). Among the isolates, 3,5-DCQA (6) exerted the most potent and selective inhibitory effect on HNE activity with an IC50 value of 1.86 ± 0.06 μM. All triterpenoid saponins (10-15) were non-active, while aglycons (11-2 and 15-2) and ursolic acid (9) exerted non-selective inhibitory effects on serine proteases.
3,5-DCQA reduced superoxide anion (O2.−) production and NE activity in fMLF-activated human neutrophils
O2.− and NE from activated neutrophils cause alveolar damage in response to acute inflammatory conditions in ALI. Therefore, the effects of all isolates and semi-synthetics on O2.− generation and NE release were determined using fMLF as an inducer in human neutrophils (Table 2). Our results showed that 3,5-DCQA inhibited O2.− generation and NE activity with IC50 values of 1.92 ± 0.54 and 12.02 ± 0.60 μM, respectively (Supplementary information Table S2). Three triterpenoids, 9, 15-1 and 15-2, exhibited good inhibitory effects on O2.− generation and NE release as well. However, triterpenoid saponins (compounds 10-15) showed no or weak inhibitory effects.
3,5-DCQA attenuated the activation of SFKs and Vav in fMLF-induced neutrophils
To investigated whether 3,5-DCQA modulated neutrophil activity through intracellular signaling pathway, the activation of SFKs, Vav, Akt and MAPKs were evaluated by Western blot. The results indicated fMLF triggered the phosphorylation of signaling proteins and 3,5-DCQA was able to reduce phosphorylation of SFKs and Vav, but not Akt and MAPKs (Fig. 2 and Supplementary information Fig. S9).
3,5-DCQA inhibited the phosphorylation of Src family kinase (Src) and Vav in fMLF-activated human neutrohpils.
Human neutrophils were pre-incubated with dimethylsulfoxide (DMSO) or 3,5-DCQA (10 μM) for 5 min before stimulation with or without fMLF (0.1 μM) for another 0.5 min. All the Western blotting experiments were performed under the same condition. After transferring the blots onto nitrocellulose membranes, the targeted blots were cropped immediately according to referenced indicating markers. The targeted proteins were immunoblotted with its specific monoclonal antibody. (A) Src family kinases; (B) Vav. Targeted bands were analyzed using a densitometer and normalized to the corresponding total protein or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The densitometric data were presented as mean ± S.E.M. (n = 3–4). Compared with fMLF group: #p < 0.05 and ##p < 0.01.
Establishment of CMC (chemistry, manufacturing and controls)
CMC data are essential to maintain the quality of botanical products in manufacturing. Accordingly, SOPs (standard operating procedures) and quality control were performed for the IKWE preparation. In HPLC fingerprints, three distinct peaks were identified as 3,4-DCQA (4), 3,5-DCQA (6) and 4,5-DCQA (7), compared to the respective pure compounds (Fig. 3). We defined and quantified these DCQAs as chemical reference standards based on calibration curves (Supplementary information Fig. S10). Biological identification was additionally validated with the HNE activity assay. The yield for three batches of IKWE was generally over 35% (Supplementary information Fig. S11) and amounts of 4, 6 and 7 obtained were 3.76 ± 0.26%, 4.70 ± 0.13% and 8.85 ± 0.15%, respectively. These data indicated that 3,5-DCQA served as the main bioactive component in KSWE. Besides, batches of IKWE exerted an inhibitory effect on HNE activity with an IC50 value of 10.50 ± 0.48 μg/mL, signifying the stable quality of bioactivity and chemical composition.
HPLC fingerprint chromatography and standard references of IKWE.
(A) 3,4-DCQA, 100 μg/mL, rt 13.5 min; (B) 3,5-DCQA, 100 μg/mL, rt 15.9 min; (C) 4,5-DCQA, 200 μg/mL, rt 25.0 min; (D) Combination of 3,4-DCQA, 3,5-DCQA and 4,5-DCQA at 50, 50, 100 μg/mL respectively; (E) IKWE, 2 mg/mL. Injection volume, 20 μL; Detection wavelength, 326 nm; Flow rate, 0.8 mL/min; Mobile phase, 42.5% MeOH solution with 1% formic acid.
Toxicity Evaluation of IKWE
Safety of botanical drugs is another important concern. To evaluate the toxicity of IKWE, acute oral toxicity studies were performed at a high dose of 10.0 g/kg23. All mice receiving IKWE with LD50 values higher than 10.0 g/kg survived. Moreover, no significant differences were observed with regard to movement and body weight growth in mice, suggesting no adverse effects (Fig. 4A). Organ toxicity was further assessed via determination of liver and kidney function (Fig. 4B). The data collectively indicated high safety of IKWE.
Acute oral toxicity evaluation of IKWE.
IKWE were dissolved with ddH2O to give IKWE solution. Body weights of mice received vehicle or IKWE solution through gavage were observed for 14 days. Serum was obtained 24 hours after gavage for chemical parameter determination. (A) Curves of body weight growth in vehicle-received or IKWE-received mice (n = 10 for each group); (B) Examination of liver and kidney functions between vehicle and IKWE groups. ALT, alanine transaminase; AST, aspartate aminotransferase; CRTN, creatinine; BUN, blood urea nitrogen. Data were presented as mean ± S.E.M. (n = 10).
IKWE and 3,5-DCQA protected against LPS-induced ALI in mice
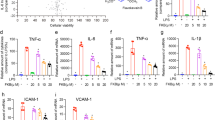
In view of the finding that IKWE and its bioactive component, 3,5-DCQA, exert anti-inflammatory effects against activated neutrophils, both were prepared and their protective effects evaluated in an LPS-induced ALI disease model. Infiltrating neutrophils, thickening of the alveolar wall, lung edema and alveolar hemorrhage are pathological features of ALI1,2,3,4. Dark red color and morphological swelling of the lung in LPS-induced ALI mice suggested alveolar hemorrhage and edema (Fig. 5A). In addition to hemorrhage, histological examination revealed infiltrating neutrophils and thickening of the alveolar wall in the LPS-treated group. These pathological features were significantly improved following pretreatment with IKWE (500 mg/kg) or 3,5-DCQA (50 mg/kg). Wet/dry (W/D) weight ratio, myeloperoxidase (MPO) activity and branchalveloar lavage fluid (BALF) were further assessed to confirm the protective effects and determine underlying molecular mechanisms. Our results showed that IKWE and 3,5-DCQA not only improve lung edema but also suppress accumulation of neutrophils in lung tissue (Figs 5B,C and 6A–C). Reduced levels of proinflammatory cytokines (TNF-α and IL-6) were additionally observed (Fig. 6D,E), clearly supporting the protective effects of both IKWE and 3,5-DCQA against LPS-induced ALI in mice. Besides, the post-treatment with 3,5-DCQA was performed to further evaluate its effects against LPS-induced ALI in mice24. The results showed that pathological features of ALI were also significantly improved following treatment with 3,5-DCQA (Supplementary information Fig. S12).
IKWE and 3,5-DCQA attenuated LPS-induced ALI in mice.
Mice pretreated with vehicle or drugs intraperitoneally received intratracheal instillation of LPS. After 6 hours, mice were anesthetized and their chests were opened. Whole lungs were obtained immediately and observed for morphological changes. Left lobe was then dissected for histology or MPO activity. Right lobes were applied for W/D weight ratio. (A) Morphological observation and histological examination (arrows indicated hemorrhage and infiltrated neutrophils), scale bar = 50 μm; (B). (C) Lung W/D weight ratio and MPO activity. Data were presented as mean ± S.E.M. (n = 6). Compared with vehicle group: ##p < 0.01; Compared with LPS group: *p < 0.05 and **p < 0.01.
Content analysis of BALF.
Mice were anesthetized and inserted with a plastic cannula into the trachea. After clamping the hilum of left lobe, PBS was injected and recovered to give BALF. Supernatant from centrifuged BALF were applied for total proteins, TNF-α and IL-6 measurement. Cell pellets were resuspended and cytospun for population analysis. (A) Examination of cell populations, N: neutrophils, M: macrophages, E: erythrocytes (scale bar = 50 μm in ×200 magnification or 10 μm in ×640 magnification); (B) Counts of BALF neutrohpils; (C) Levels of total proteins; (D) Levels of TNF-α; (E) Levels of IL-6. Data were presented as mean ± S.E.M. (n = 6). Compared with vehicle group: ##p < 0.01; compared with LPS group: **p < 0.01.
Discussion
ALI is a life-threatening disease for which no effective treatments are available. Botanical drugs are considered to be an important resource for drug development and they require stringent testing for efficacy, safety and quality9. Because of the ability of IKWE to inhibit HNE activity, the protective effects of I. kaushue against ALI were investigated, both in vitro and in vivo. In the in vivo studies, dexamethasone was used as a positive control. Although the applied dosage and possible side effects of dexamethasone in clinical settings are controversial, it is still a common positive control in ALI mouse models25,26,27.
Although serine proteases are responsible for several physiological functions in humans, abnormal and excessive levels can cause or promote disease28,29. Recent experimental and clinical studies have shown that enhanced HNE activity is associated with the degradation of elastin-rich proteins in the pathological progression of ALI. Consequently, HNE is considered to be a promising therapeutic target for treating ALI4,28,30. In our experiments, 3,5-DCQA exhibited high selectivity for HNE among the five serine proteases that were examined. Further exploration of the SAR of the caffeic acid analogues showed that the inhibitory effects on HNE activity of the compounds did not depend on the caffeic acid moiety, but were correlated with the regioselectivity of caffeic acids conjugated to quinic acid (Supplementary information Table S3).
In addition to serine proteases, enhanced oxidative stress also contributes to the pathogenesis of inflammatory diseases1,4,30. The production of superoxide anions from human neutrophils can be reduced through intracellular mechanisms or ROS scavenging agents4,30. 3,5-DCQA was previously reported to be a ROS-scavenger and to exert anti-oxidant effects31. The ortho-dihydroxyphenyl moiety significantly promotes ROS scavenging activity via a highly favorable electron acceptance and resonance system32,33. Our results showed that all caffeoylquinic acid derivatives, together with CA, RA and THBA, potently inhibit O2.− generation in neutrophils (Table 2). In contrast, compounds p-CA, 3-HCA and FA were non-active because the ortho-dihydroxyphenyl group was absent. Interestingly, the HNE inhibitory effects of 3,5-DCQA decreased in fMLF-induced neutrophils, which was demonstrated by comparing the results of an enzyme inhibition assay. To eliminate the influence of O2.− generation, leukotriene B4 (LTB4) was used as an inducer in human neutrophils (Supplementary information Table S2), which led to the recovery of 3,5-DCQA-induced HNE inhibition34. These data confirmed that 3,5-DCQA at a high dose (100 μM) was able to inhibit elastase release, myeloperoxidase activity and superoxide production in human neutrophils35.
SFKs belong to the family of tyrosine kinases and play important roles in neutrophil activation36,37. Previous reports have shown that SFKs are responsible for O2.− generation and cell migration in fMLF-stimulated neutrophils36. In addition, SFKs have been shown to cause elevated expression of TNF-α and chemokines in LPS-induced neutrophils37. These effects have been inhibited effectively by PP2, a highly selective SFK inhibitor36,37. Vav, a member of the guanine nucleotide exchange factors (GEFs) family, causes activation of NADPH oxidase by activating Rac38. A previous study revealed that SFKs were able to enhance Vav phosphorylation and activity39. Our immunoblotting assay showed that 3,5-DCQA inhibited SFKs and Vav phosphorylation in fMLF-induced neutrophils, which suggests that 3,5-DCQA might attenuate O2.− generation, cell migration and TNF-α and chemokine expression in stimulated neutrophils through the SFKs/Vav signaling pathway. Other signaling proteins such as ERK, p38 and Akt are also important in neutrophil activation36. However, 3,5-DCQA does not reduce the levels of phosphorylated ERK, p38 and Akt. Based on enzyme-based and cell-based data, we proposed that 3,5-DCQA modulates neutrophil function through intercellular and intracellular mechanisms simultaneously.
Ursolic acid (9) was previously reported to inhibit O2.− production and NE release from neutrophils through an intracellular mechanism40. The findings of the effects of ursolic acid on neutrophils were supported by our enzyme-based and cell-based data. In addition, randialic acid B (15-1) and sanguisorbigenin (15-2) exhibited 5-fold greater efficacy than ursolic acid. SARs analysis of triterpenoid saponins (10-15) and aglycons indicated that their efficacy in fMLF-induced neutrophils was affected by sugar moiety and structural modifications at the E-ring. For example, triterpenoid saponins showed weak or no inhibitory effects on O2.− generation and NE release, indicating that saccharides in saponins are unfavorable for bioactivity in human neutrophils. Further, when the carboxylic acid at C-15 was cyclized with a hydroxyl group at C-20 to form a δ-lactone ring (11-1 and 11-2), bioactivity vanished, suggesting that carboxylic acid is necessary for this activity. Conversely, the presence of a double bond at C-18/C-19 or C-19/C-20 (15-1 and 15-2) enhanced human neutrophil activity.
Increased oxidative stress has also been reported to enhance elastase activity by inactivating α1-antitrypsin4,7. Therefore, a combination of HNE inhibitors and free radical scavengers is considered to be an effective strategy for treating ALI. A previous study reported that better therapeutic effects could be achieved with a combined treatment of sivelestat sodium and edaravone in LPS-induced ALI in rats41. In our current experiments, enhanced protective effects were observed following treatment with IKWE (500 mg/kg) compared to treatment with 3,5-DCQA (25 mg/kg) alone. The amount of 3,5-DCQA in IKWE at a 500 mg/kg dose is ~25 mg/kg. Because caffeoylquinic acid derivatives may improve α1-antitrypsin activity via their ROS scavenging ability, our results suggest that there are synergistic ROS-scavenging effects and a protease-antiprotease balance of other caffeoylquinic acid derivatives in the extract.
Neutrophil recruitment into inflammatory tissue by chemokines is a key event in ALI. Neutrophil locomotion includes adhesion and migration through endothelial cells. L-selectin, β2-integrin and platelet-endothelial cell adhesion molecule-1 (PECAM-1) are important adhesion proteins and are quickly expressed in activated neutrophils. These adhesion proteins promote neutrophil attachment to endothelial cells and firm their migration30. Chlorogenic acid (3) is another major chemical component in I. Kaushue12 and it was able to attenuate the adhesive ability of neutrophils by inhibiting L-selectin cleavage and reducing β2 integrin levels, as well as suppressing the expression of PECAM-1 that is induced by LPS in neutrophils42. In the same study, chlorogenic acid also exhibited inhibitory effects on fMLF-induced neutrophil migration. In our experiments, both a BALF analysis and histological data showed that neutrophil infiltration in ALI mice was significantly reduced following treatment with IKWE or 3,5-DCQA. However, no difference was found between the IKWE (500 mg/kg) and 3,5-DCQA (25 mg/kg) groups. These findings suggest that the effects of chlorogenic acid on reducing neutrophil recruitment were not apparent in our in vivo experiments. Because the effects of 3,5-DCQA on neutrophil recruitment, adhesion and migration are unclear, further evaluation of the effects of 3,5-DCQA is necessary.
Macrophages are also important in inflammatory diseases. In ALI, there is an increased release of inflammatory cytokines from stimulated macrophages43. Neutrophil elastase has been shown to stimulate TNF-α and IL-6 expression in macrophages44. A previous study demonstrated that 3,5-DCQA was able to reduce TNF-α and IL-6 expression in LPS-stimulated RAW 264.7 cells45. In the current study, 3,5-DCQA inhibited neutrophil elastase and reduced BALF inflammatory cytokines in vitro and in vivo. These suggested that 3,5-DCQA might be able to modulate macrophage function by inhibiting elastase and intracellular mechanisms simultaneously44.
Pharmacokinetic properties are important in drug development. Previous studies have reported that although caffeoylquinic acids derivatives have similar chemical properties, their pharmacokinetic parameters are different46,47,48,49. Specifically, the terminal elimination half-life (T1/2z) and the mean residence time (MRT) of 3,5-DCQA were 227~292 min and 227~438 min, respectively, after oral administration and 3,5-DCQA was detectable in the plasma for 4 hours after intravenous administration46,47,49. In addition, chlorogenic acid was distributed in lung tissue more than 1.5 hours after oral administration48. These studies may be able to illustrate the efficacy of 3,5-DCQA and its possible metabolites in vivo; however, further investigation of the bio-distribution of 3,5-DCQA and its possible metabolites in lung tissue is necessary.
CMC data are critical to determine and maintain the quality of botanical products. Original identification, HPLC fingerprints, chemical and biological references and standardized manufacturing procedures are required for CMC. The internal transcribed spacer (ITS) sequence is the determining factor in genomic identification. However, no ITS information is available for I. kaushue, excepting a review article published in 201050. To identify the origin of I. kaushue from commercial, two gene sequences, trnS-trnG and trnH-psbA, were analyzed and compared using the genomic identification database. The results confirmed 100% and 99% sequence identity to I. kaushue, respectively. Subsequently, three batches of IKWE (IKWE-1–3) and water extracts from other two commercial Kudingcha materials (KUD-1 and NKUD-1) were prepared using a standardized manufacturing protocol, followed by determination of HPLC fingerprints, extraction yield, amounts of chemical references and biological activity of each extract. The results indicate good quality of each extract with high reproducibility and consistency (Supplementary information Fig. S11).
In addition to anti-oxidative capacity, 3,5-DCQAs and other DCQAs display diverse bioactivities, including hepatoprotection, anti-hyperlipidemia and anti-thrombosis51,52,53. Anti-viral activities against HIV, RSV, H1N1 and HBV have additionally been demonstrated54,55,56,57. Here, we identified three abundant DCQAs in IKWE, supporting the possibility of different biological applications. For example, Shuang-Huang-Lian, a TCM preparation composed of Flos lonicerae and other two herbs, is applied to treat various acute respiratory infections, such as influenza virus A-induced pneumonia in China46,47. Chlorogenic acid is the main bioactive component in F. lonicerae. A pharmacokinetic study reported that the amount of chlorogenic acid is 50-fold higher than that of 3,4-DCQA and 200-fold higher than that of 3,5-DCQA in rat blood plasma45. The CQA amount is comparable to that of DCQA in I. kaushue12. Considering the anti-inflammatory and broad anti-viral effects of DCQAs, further evaluation of the effects of IKWE on influenza virus A-induced pneumonia is warranted.
In conclusion, we have developed a standard protocol to prepare a water extract of I. kaushue with good reproducibility, consistency and safety. 3,5-DCQA, the bioactive component in I. kaushue, modulated neutrophil function not only by directly targeting HNE and ROS but also by inhibiting the SRKs/Vav signaling pathway. Both IKWE and 3,5-DCQA exhibited protective effects against LPS-induced ALI in mice. These data provide support for both IKWE and 3,5-DCQA as candidates in the development of new lead agents to treat ALI.
Methods
Genomic identification of I. kaushue
Three raw materials of Kudingcha were purchased from two retailers. Two (IK and KUD) were from Huang-De-An (New Taipei, Taiwan) on 2013/11/20. The third (NKUD) was from Da-Ye (Nantou, Taiwan) on 2015/06/01. Samples from IK were prepared for genomic analysis by Bioproduction Engineering Technology Department, Biomedical Technology and Device Research Laboratories, ITR (Hsinchu, Taiwan). Briefly, genomic DNA was obtained and two genes, trnS-trnG and trnH-psbA, subsequently sequenced. Sequence identities to I. Kaushue were determined as 100% and 99%, respectively, according to the NCBI genome database.
Preparation and quality control of water extracts
Each raw material sample (20 g) was refluxed twice with 200 mL ddH2O for 2 h. The solutions were filtered and concentrated under vacuum to generate crude extracts (IKWE-1~3, KUD-1 and NKUD-1). The specifications of quality control included extraction yield, HPLC fingerprint chromatography, as well as determination of chemical and biological references. To establish HPLC chromatographic fingerprints, each extract (2.0 mg/mL) was dissolved in mobile phase solution (1% formic acid in 42.5% MeOH aqueous solution), filtered through a 0.45 μm membrane filter and passed through an HPLC system. The Develosil™ C30-UG-5 column (4.6 × 250 mm, 5 μm) (Nomura, Japan) was eluted with a mobile phase consisting of 1% formic acid in 42.5% MeOH aqueous solution at a flow rate of 0.8 mL/min. The detector wavelength and injection volume were set at 326 nm and 20 μL, respectively. Chemical reference levels were determined from calibration curves generated at a concentration range of 40 to 120 μg/mL for 3,4-DCQA and 3,5-DCQA or 80 to 240 μg/mL for 4,5-DCQA. The HNE activity assay was further performed for biological validation.
Bioactivity-guided fractionation
The isolation procedures and 1D/2D NMR and physical properties of isolates and semi-synthetics are described in Supplementary information S1.
Menisdaurin F (Supplementary information Figs S2–S8).
Colorless plate; [α]23D: −63.6° (c = 0.05, MeOH); UV λmax (MeOH) nm (log ε): 256 (4.37); IR vmax (KBr) cm−1: 3392, 2924, 2220, 1621, 1018; Mp: 176–177°; ESIMS: m/z 336.2 [M + Na]+; HRESIMS: m/z 336.1048 [M + Na]+(calcd. for C14H9NO7Na, 336.1054); 1H NMR (CD3OD, 400 MHz) δ: 6.57 (1H, dd, J = 10.0, 2.0 Hz, H-2), 6.24 (1H, d, J = 10.0 Hz, H-3), 5.77 (1H, s, H-7), 4.62 (1H, m, H-6), 4.49 (1H, m, H-4), 4.48 (1H, d, J = 8.0 Hz, H-1’), 3.86 (1H, d, J = 11.6 Hz, H-6’a), 3.66 (1H, dd, J = 11.6, 5.2 Hz, H-6’b), 3.22~3.40 (4H, m, H-2’~H-5’), 2.56 (1H, m, H-5a), 1.66 (1H, m, H-5b); 13C NMR (CD3OD, 100 MHz) δ: 159.1 (s, C-1), 143.3 (d, C-3), 124.5 (d, C-2), 117.9 (s, C-8), 102.6 (d, C-1’), 93.7 (d, C-7), 78.2 (d, C-5’), 78.0 (d, C-3’), 74.8 (d, C-6), 74.7 (d, C-2’), 71.5 (d, C-4’), 67.6 (d, C-4), 62.6 (t, C-6’), 36.4 (t, C-5).
Serine protease inhibition
Enzyme inhibition assays are described in Supplementary information S2.
Determination of O2.− generation and elastase release from neutrophils
All assays were performed as described previously58,59. Neutrophils isolated from the blood of healthy volunteers (20–30 years old) were resuspended in a Ca2+-free HBSS buffer (pH 7.4) at 4 °C before use. O2.− generation was measured based on reduction of ferricytochrome c. In brief, after supplementation with 0.5 mg/ml ferricytochrome c and 1 mM Ca2+, neutrophils were equilibrated at 37 °C for 2 min and incubated with the specified drugs for 5 min. Neutrophils were activated using 30 nM fMLF or 100 nM LTB4 for 10 min after addition of cytochalasin B (CB, 1 μg/mL) for 3 min. Changes in absorbance concomitant with reduction of ferricytochrome c at 550 nm were continuously monitored. For elastase release, neutrophils (6 × 105 cells/mL) were mixed with methoxysuccinyl-Ala-Ala-Pro-Val-pNA (100 μM) substrate at 37 °C for 5 min. After incubation with DMSO or test agents, neutrophils were activated as described previously and changes in absorbance at 405 nm continuously monitored to assay elastase release. Inhibition of superoxide generation and elastase release were calculated in keeping with previous reports.
Immunoblotting assay
Neutrophils were pretreated with DMSO or 3,5-DCQA (10 μM) for 5 min before fMLF stimulation for 0.5 min at 37 °C. Cells were lysed with lysis buffer consisting of 50 mM HEPES (pH 7.4), 100 mM NaCl, 1 mM Ca2+, 2 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride, 5% b-mercaptoethanol, 10 mM p-nitrophenyl phosphate, 1% protease inhibitor cocktail (Sigma-Aldrich) and 1% Triton X-100. Cell lysates were collected by centrifugation at 14,000 rpm for 20 min at 4 °C. After gel electrophoresis and transferring to membranes, samples were blocked with 5% nonfat milk in a mixture of Tris-buffer saline and Tween 20. Target protein was identified by the corresponding primary antibody overnight at 4 °C. Membranes were incubated with horseradish peroxidase-conjugated, secondary anti-rabbit or anti-mouse antibodies at room temperature for 1 h. After washing, enhanced chemiluminescence solution was used and protein expression was analyzed by the BioSpectrum Imaging System (UVP, Upland, CA). The quantitative ratio of target protein was normalized to total protein or GAPDH.
Animals
All animal experiments were performed in accordance with the guidelines of the Animal Welfare Act and The Guide for Care and Use of Laboratory Animals from the National Institutes of Health. The animal protocols were approved by the Institutional Animal Care and Use Committee of Chang Gung University (Taoyuan, Taiwan, IACUC Approval no.: CGU12-011, period of protocol valid from June 01, 2012 to May 31, 2015). Male ICR mice (5–6 weeks; 25–30 g) were purchased from BioLasco (Ilan, Taiwan). Mice were housed under standard laboratory conditions and fed a standard laboratory diet and water ad libitum. Animals were allowed to adapt to the environment for at least one week before experiments.
Acute oral toxicity
IKWE powder was dissolved in 600 μL ddH2O to generate a solution at a dose equivalent to 10.0 g/kg. Mice were administered IKWE solution (300 μL) or ddH2O via gavage at intervals of 4 h. After 24 h, blood (ca. 1.0 mL) was collected through cardiac puncture under anesthesia with intraperitoneal injection of Zoletil 50 (50 mg/kg) and Xylazine (10 mg/kg). Blood samples were immediately mixed with 100 μL acid citrate dextrose solution (BD Vacutainer, 364606), centrifuged at 1,000 rpm for 15 min and stored at −20 °C. Supernatant fractions were further analyzed to determine the AST, ALT, CRTN and BUN levels using FUJI DRI-CHEM 3000 and four FUJI DRI-CHEM SLIDE products (n = 10). LD50 and body weight growth were additionally determined for 14 days (n = 10)23. Data were presented as mean ± S.E.M.
LPS-induced ALI in mice
Mice were randomly divided into nine groups (n = 6 per group): (1) Vehicle, (2) IKWE (500 mg/kg), (3) 3,5-DCQA (50 mg/kg), (4) LPS, (5) LPS + DEX (10 mg/kg), (6) LPS + IKWE (250 mg/kg), (7) LPS + IKWE (500 mg/kg), (8) LPS + 3,5-DCQA (25 mg/kg) and (9) LPS + 3,5-DCQA (50 mg/kg). Agents were dissolved in vehicle solution (PBS containing 10% Tween-80). All animals were pretreated intraperitoneally with 100 μL vehicle solution or agents, respectively, 1 h before PBS or LPS (Sigma, Escherichia coli. 055:B5) intratracheal injection (5 mg/kg in 50 μL of PBS)26,27. Mice were sacrificed at 6 h post-dosage and the left lung collected for histological examination or MPO activity measurement. Lung tissue was fixed with 10% formalin before embedding with paraffin wax and routine H&E staining. MPO activity was measured as described earlier59. The right lobes were prepared for W/D weight ratio determination or BALF analysis. To obtain the W/D weight ratio, wet tissues were weighed immediately after collection, dried in the oven at 80 °C for 48 h and re-weighed. For post-treatment experiments, mice treated with vehicle or 3,5-DCQA (50 mg/kg) intraperitoneally 1 h after LPS treatment24.
Bronchoalveolar lavage fluid preparation and analysis
Mice were sacrificed and hilum of the left lung sealed. BALF was collected following injection with 0.2, 0.2, 0.3, 0.3 or 0.5 mL PBS. BALF samples were combined and centrifuged at 1,000 rpm for 15 min at 4 °C. Total proteins in the supernatant fraction were determined using the protein assay dye (BIO-RAD, 500-0006) with BSA as the reference and TNF-α and IL-6 levels measured with ELISA kits (eBioscience, 88-7324-88 and 88-7064-88). Cell pellets from BALF were resuspended in 100 μL PBS and cytospun (200 g, 3 min) onto a glass microscope slide, followed by staining with Liu’s stain. Neutrophils were counted under a light microscope in five randomly selected fields (×200 magnification)60.
Statistical analysis
All data were expressed as mean ± S.E.M. and analyzed with two-tailed indirect Student tests or one-way ANOVA followed by Dunnet’s multiple comparison test. GraphPad Prism 5.01 was applied for statistical analysis (GraphPad Software, Inc., USA).
Additional Information
How to cite this article: Chen, Y.-L. et al. Ilex kaushue and Its Bioactive Component 3,5-Dicaffeoylquinic Acid Protected Mice from Lipopolysaccharide-Induced Acute Lung Injury. Sci. Rep. 6, 34243; doi: 10.1038/srep34243 (2016).
References
Modrykamien, A. M. & Gupta, P. The acute respiratory distress syndrome. Proc. (Bayl. Univ. Med. Cent.) 28, 163–171 (2015).
Rojas, M., Woods, C. R., Mora, A. L., Xu, J. & Brigham, K. L. Endotoxin-induced lung injury in mice: structural, functional and biochemical responses. Am. J. Physiol. Lung. Mol. Physiol. 288, L333–L341 (2005).
Standford, T. J. & Ward, P. A. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl. Res. 167, 183–191 (2016).
Aikawa, N. & Kawasaki, Y. Clinical untility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther. Clin. Risk Manag. 10, 621–629 (2014).
Mantovani, A., Cassatella, M. A., Costantini, C. & Jaillon, S. Neurophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Williams, A. E. & Chambers, R. C. The mercurial nature of neutrophils: still an enigma in ARDS? Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L217–L230 (2014).
Sandhaus, R. A. & Turino, G. Neutrophil elastase-mediated lung disease. COPD. 10, 60–63 (2013).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335 (2012).
Tang, T. Y., Li, F. Z. & Afseth, J. Review of the regulations for clinical research in herbal medicines in USA. Chin. J. Integr. Med. 20, 883–893 (2014).
Flora Reipublicae Popularis Sinicae, Vol. 45(2) (eds Wu, C. Y. et al.) 105–107 (Science, 1999).
Qin, L. F., Qu, X. C., Hu, G., Huang, Y. F. & Zhang, Q. W. Development of microsatellite markers in Ilex kaushue (Aquifoliaceae), a medicinal plant species. Appl. Plant Sci. 3, 1500040 (2015).
Li, L. et al. The large-leaved kudingcha (Ilex latifolia Thunb and Ilex kudingcha C. J. Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J. Nat. Med. 67, 425–437 (2013).
Hao, D. et al. Research progress in the phytochemistry and biology of Ilex pharmaceutical resources. Acta Pharm. Sin. B 3, 8–19 (2013).
Zhongyaodacidian 2nd edn, Vol. 1 (eds Nanjing University of Chinese Medicine) 1763–1765 (Shanghai Scientific & Technical Publisher, 2006).
Xu, d. et al. Inhibitory activities of caffeoylquinic acid derivatives from Ilex kudingcha C. J. Tseng on α-glucosidase form saccharomyces. J. Agric. Food Chem. 63, 3694–3703 (2015).
Zhu, K., Li, G., Sun, P., Wang, R., Qian, Y. & Zhao, X. In vitro and in vivo anti-cancer activities of Kuding tea (Ilex kudingcha C. J. Tseng) against oral cancer. Exp. Ther. Med. 7, 709–715 (2014).
Song, J. L., Qian, Y., Li, G. J. & Zhao, X. Anti-inflammatory effects of kudingcha methanol extract (Ilex kudingcha C. J. Tseng) in dextran sulfate sodium-induced ulcerative colitis. Mol. Med. Rep. 8, 1256–1262 (2013).
Takahashi, K., Matsuzawa, S. & Takani, M. The constituent of the vines of Menispermum dauricum DC. Chem. Pharm. Bull. 26, 1677–1681 (1978).
Yogo, M., Ishiguro, S., Murata, H. & Furukawa, H. Coclauril, a nonglucosidic 2-cyclohexen-1-ylideneacetonitrile, form Cocculus lauriforium DC. Chem. Pharm. Bull. 38, 225–226 (1990).
Ueda, K., Yasutomi, K. & Mori, I. Structure of a new cyanoglucoside from Ilex warburgii Loesn. Chem. Lett. 12, 149–150 (1983).
Seigler, D. S. et al. Cyanogenic glycosides and menisdaurin from Guazuma ulmifolia, Ostrya virginiana, Tiquilia plicata and Tiquilia canescens. Phytochemistry 66, 1567–1580 (2005).
Nahrstedt, A. & Wray, V. Structural revision of a putative cyanogenic glucoside from Ilex aquifolium. Phytochemistry 29, 3934–3936 (1990).
Lee, Y. J. et al. Toxicity of fermented soybean product (cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on liver and kidney of ICR mice. Lab. Anim. Res. 30, 54–63 (2014).
Zhang, J. L., Huang, W. M. & Zeng, Q. Y. Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 765, 94–99 (2015).
Horita, N. et al. Impact of corticosteroids on mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intern. Med. 54, 1473–1479 (2015).
Ma, C. et al. Anti- inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysacchride-induced inflammation in acute lung injury by suppressing PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 168, 349–355 (2015).
Huang, G. J. et al. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-κB and MAPK pathways in mice. J. Agric. Food Chem. 62, 5321–5329 (2014).
Korkmaz, B., Horwitz, M. S., Jenne, D. E. & Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G as therapeutic targets in human diseases. Pharacol. Rev. 62, 726–759 (2010).
Chen, D. et al. Design, synthesis and antithrombotic evaluation of novel dabigatran etexilate analogs, a new series of non-peptides thrombin inhibitors. Bioorg. Med. Chem. 23, 7405–7416 (2015).
Grommes, J. & Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 17, 293–307 (2011).
Iwai, K., Kishimoto, N., kakino, Y., Mochida, K. & Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food. Chem. 52, 4893–4898 (2004).
Greunke, C. et al. A systematic study on the influence of the main ingredients of an ivy leaves dry extract on the β2-adrenergic responsiveness of human airway smooth muscle cells. Pulm. Pharmacol. Ther. 31, 92–98 (2015).
Bors, W., Michel, C., Stettmaier, K., Lu, Y. & Foo, L. Y. Antioxidant mechanisms of polyphenolic caffeic acid oligomers, constituents of Salvia officinalis. Biol. Res. 37, 301–311 (2004).
Omann, G. M., Traynor, A. E., Harris, A. L. & Sklar, L. A. LTB4 induced activation signals and responses in neutrophils are short-lived compared to formylpeptide. J. Immunol. 138, 2626–2632 (1987).
Góngora, L. et al. Effects of caffeoyl conjugates of isorenyl-hydroquinone glucoside and quinic acid on leukocyte function. Life Sci. 71, 2995–3004 (2002).
Fumagalli, L., Zhang, H., Baruzzi, A., Lowell, C. A. & Berton, G. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formayl-methionyl-leucyl-phenylalanine. J. Immunol. 178, 3874–3885 (2007).
Mazzi, P., Caveggion, E., Lapinet-Vera, J. A., Lowell, C. A. & Berton, G. The src-family kinases Hck and Fgr regulate early lipopolysaccharide-induced myeloid cell recruitment into the lung and their ability to secrete chemokines. J. Immunol. 195, 2383–2395 (2015).
Roepstorff, K. et al. Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1 and PAK1 signaling axis. J. Biol. Chem. 283, 7983–7993 (2008).
Turner, M. & billadeau, D. D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486 (2002).
Hwang, T. L. et al. Ursolic acid inhibits superoxide production in activated neutrophils and attenuates trauma-hemorrhage shock-induced organ injury in rats. PLoS One 9, e111365 (2014).
Yang, T. et al. Combined effects of a neutrophil elastase inhibitor (sivelestat sodium) and a free radical scavenger (edaravone) on lipopolysaccharide-induced acute lung injury in rats. Inflamm. Res. 61, 563–569 (2012).
Hebeda, C. B. et al. Effects of chlorogenic acid on neutrophil locomotion functions in response to inflammatory stimulus. J. Ethnopharmacol. 135, 261–269 (2011).
Tsushima, K. et al. Acute lung injury review. Intern. Med. 48, 621–630 (2009).
Benabid, R. et al. Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa-induced pneumonia. J. Biol. Chem. 287, 34883–34893 (2012).
Chen, X. et al. The anti-inflammatory activities of Ainsliaea fragrans Champ. extract and its components in lipopolysaccharide-stimulated RAW264.7 macrophages through inhibition of NF-κB pathway. J. Ethnopharmacol. 170, 72–80 (2015).
Zhou, W. et al. Simultaneous determination of caffeic acid derivatives by UPLC-MS/MS in rat plasma and its application in pharmacokinetic study after oral administration of Flos lonicerae-Fructus forsythiae herb combination. J. Chromatogr. B 949–950, 7–15 (2014).
Zhou, W. et al. Study on the main components interaction from Flos lonicerae and Fructus forsythia and their dissolution in vitro and intestinal absorption in rats. PLoS One 9, e109619 (2014).
Zhou, Y., Zhou, T., Pei, Q., Liu, S. & Yuan, H. Pharmacokinetics and tissue distribution study of chlorogenic acid from Lonicerae japonicae flos following oral administrations in rats. Evid. Based Complement. Alternat. Med. 2014, 979414 (2014).
Wang, W. et al. Simultaneous quantitation of dicaffeoylqinic acids in rat plasma after an intravenous administration of mailuoning injection using liquid chromatography-mass spectrometry. J. Chromatogr. Sci. 47, 216–222 (2009).
Manen, J. F., Barriera, G., Loizeau, P. A. & Naciri, Y. The history of extant Ilex species (Aquifoliaceae): Evidence of hybridization within a Miocene radiation. Mol. Phylogenet. Evol. 57, 961–977 (2010).
Basnet, P., Matsushige, K., Hase, K., Kadota, S. & Namba, T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent Hepatoprotective activity in experimental liver injury models. Biol. Pharm. Bull. 19, 1479–1484 (1996).
Zhang, X. et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS One 8, e61922 (2013).
Satake, T., Kamiya, K., An, Y., Oishi, T. & Yamamoto, J. The anti-thrombotic active constituents from Centella asiatica. Biol. Pharm. Bull. 30, 935–940 (2007).
Hu, Z., Chen, D., Dong, L. & Southerland, W. M. Prediction of the interaction of HIV-1 intergrase and its dicaffeoylquinic acid inhibitor through molecular modeling approach. Ethn. Dis. 20, 45–49 (2010).
Ojwang, J. O. et al. A novel inhibitor of respiratory syncytial virus isolated from ethnobotanicals. Antiviral Res. 68, 163–172 (2005).
Takemura, T. et al. 3,4-Dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza A virus. Evid. Based Complement. Alternat. Med. doi: 10.1155/2012/946867 (2012).
Zhao, Y. et al. UFLC/MS-IT-TOF guided isolation of anti-HBV active chlorogenic acid analogues from Artemisia capillaris as a traditional Chinese herb for the treatment of hepatitis. J. Ethnopharmacol. 156, 147–154 (2014).
Hwang, T. L., Wang, W. H., Wang, T. Y., Yu, H. P. & Hsieh, P. W. Synthesis and pharmacological characterization of 2-aminobenzaldehyde oxime analogs as dual inhibitors of neutrophil elastase and proteinase 3. Bioorg. Med. Chem. 23, 1123–1134 (2015).
Tsai, Y. F. et al. Sirtinol inhibits neutrophil elastase activity and attenuates lipopolysaccharide-mediated acute lung injury in mice. Sci. Rep. 5, 8347 (2015).
McKenzie, C. G. et al. Peripheral blood monocyte-derived chemokine blockage prevents murine transfusion-related acute lung injury (TRALI). Blood 123, 3496–3503 (2014).
Acknowledgements
We appreciated Mr. Jui-Hung Yen (Bioproduction Engineering Technology Department, Biomedical Technology and Device Research Laboratories, ITR) for performing genomic analysis of plant material. This work was supported by a grant from the Ministry of Science and Technology (MOST102-2320-B-182-008-MY3) and Chang Gung Memorial Hospital and Chang Gung University (CMRPD1F0241~3 and BMRPB23 to P.-W.H. and CMRPD1B0332, CMRPF1F0061~3 and EMRPD1D1F0311 to T.-L.H.). The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.-L.C. and P.-W.H. participated in the study designs. Y.-L.C. performed the preparation of herbal constituents and extracts, enzyme inhibition assays and in vivo studies. T.-L.H., W.-Y.C. and H.-W.Y. carried out human neutrophil assays. W.-Y.C. performed Western blotting assays. Y.-L.C., H.-P.Y., J.-Y.F., K.-Y.C., Y.-W.C. and C.-Y.C. carried out BALF or MPO analysis in animal tests. Y.-L.C. wrote the manuscript. P.-W.H. was in charge of the whole experimental conduction and proofread manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, YL., Hwang, TL., Yu, HP. et al. Ilex kaushue and Its Bioactive Component 3,5-Dicaffeoylquinic Acid Protected Mice from Lipopolysaccharide-Induced Acute Lung Injury. Sci Rep 6, 34243 (2016). https://doi.org/10.1038/srep34243
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34243
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.