Abstract
The magnetic properties and electronic structures of pure, doped and defective cerium oxide (CeO2) have been studied theoretically by means of ab initio calculations based on the density function theory (DFT) with the hybrid HF/DFT technique named PBE0. Carbon (C), nitrogen (N), phosphorus (P), sulphur (S), lanthanum (La) and praseodymium (Pr) doped in CeO2 and CeO2 containing oxygen vacancies (Ov) were considered. Our spin-polarized calculations show that C, N, Pr dopants and Ov defects magnetize the non-magnetic CeO2 in different degree. The optical band gap related to photocatalysis for pure CeO2, corresponding to the ultraviolet region, is reduced obviously by C, N, S, Pr impurities and oxygen vacancies, shifting to the visible region and even further to the infrared range. Especially, N-, S- and Pr-doped CeO2 could be used to photocatalytic water splitting for hydrogen production. As the concentration of Ov increasing up to 5%, the CeO2 exhibits a half-metallic properties.
Similar content being viewed by others
Introduction
CeO2, as one of the most important rare-earth metal oxides, has been in focus of intensive research during recent years due to its wide range of industrial applications. CeO2 special feature of high reactivity mediated by redox couple of Ce4+/Ce3+ could be used in catalysis1,2. Its unique oxygen storage capacity3 could make CeO2 material for solid oxide fuel cells4. Another potential application of CeO2 -based materials is in spintronics devices with advanced silicon based microelectronic devices5, due to an excellent match of crystal structure and high dielectric constant (ε = 26) of CeO2 with those of silicon6.
CeO2 is also a potential material for ultraviolet (UV) filtration7. CeO2 with a band gap of 3.2 eV, good transparency in the visible range, and no known toxicity, seems to be a promising inorganic material for use as a UV filter in sunscreen cosmetic products. Some previous studies regarding Zn-, Mg- and Ca-doped CeO2 demonstrated that the impurities shift the material’s band gap because of their effects on electronic transitions8. Furthermore, CeO2, as a wide band-gap semiconducting material which absorbs light in the near UV and slightly in the visible region, is a prospective material used in photocatalytic reactions, such as decomposing water to produce hydrogen and oxygen9. One of the most effective methods is doping. Zr- and La-doped CeO2 have found their potential application in solar cell devices10, Mao et al. demonstrated that N-doped CeO2 shows a visible-light absorbance shift11.
As mentioned above, the oxygen storage capacity of CeO2 makes it an important material. By releasing and storing oxygen during fuel-rich and lean conditions, an optimal oxygen pressure for the catalytic removal of harmful exhaust gases can be maintained. This is achieved by partial reduction/oxidation of the CeO2 and is related to the chemistry of oxygen vacancies in the material12. However, the description of oxygen vacancies in insulating transition and rare earth metal oxides is a challenge for modern electronic structure calculations13. In this paper, we used first-principles method to investigate the magnetic properties and electronic structures for doped and defective CeO2. Carbon (C), nitrogen (N), phosphorus (P), sulphur (S), lanthanum (La) and praseodymium (Pr) doped in CeO2 and CeO2 containing oxygen vacancies (Ov) were considered.
Results
Magnetic properties
Cerium oxide (CeO2), or ceria, is a lanthanide oxide with a cubic fluorite structure (Fm m) and a cell parameter of 5.41 Å at room temperature, in agreement with our PBE0 result of 5.49 Å, as shown in Fig. 1(a).
m) and a cell parameter of 5.41 Å at room temperature, in agreement with our PBE0 result of 5.49 Å, as shown in Fig. 1(a).
First, we invested the magnetic properties of pure, doped and defective CeO2. Table 1 lists the magnetic moment of doped and defective CeO2, and shows that C, N and Pr dopants and O vacancies (Ov) magnetize the pure CeO2 in different degree. C impurity introduces a global magnetic moment of 2.0 μB per supercell. The C atom does the most contribution (0.733 μB) to the total magnetic moment, and the four nearest Ce and six next-nearest neighboring O atoms provide 0.090 μB per Ce and 0.035 μB per O, respectively. Spin-polarized charge density calculation indicates that C 2p electrons diffuse to the second-nearest neighboring O atoms and lead to a polarization of surrounding O and Ce atoms, implying FM coupling between the doped C atom and its neighboring atoms (see Fig. 1(b)). N impurity magnetizes the pure CeO2 with a magnetic moment of 1.0 μB per surpercell, being smaller than that of C dopant. The N atom and six second-nearest O atoms contribute 0.563 μB and 0.048 μB per atom to the total magnetic moment respectively, whereas the spin-polarized contribution from the nearest Ce could be neglected. In a defective CeO2 system containing oxygen vacancies, two unpaired electrons localize in one Ov, leaving cation dangling bonds. When the Ov site is occupied by the C or N dopant, the two unpaired localized electrons fulfill 2p orbitals of the dopant, and according to Hund’s rules, the substitutional C or N has four or five 2p electrons with high-spin configuration of 2p4 (↑↓↑↑) for C or 2p5 (↑↓↑↓↑) for N, which may create magnetic moment of 2.0 μB or 1.0 μB per impurity atom. Additionally, we found that P and S dopants do not produce spin-polarization in pure CeO2. For the P-doped case, according to effective charge calculation, 3p elections of the P impurity are much more delocalized than in the N-doped case, which leads to a vanishing of spin polarization.
We have also calculated La- and Pr-doped CeO2, to investigate the magnetic properties affected by lanthanide elements. From our calculations, La does not introduce spin polarization, whereas Pr magnetizes the doped CeO2 with a magnetic moment of 1.0 μB per supercell. For the Pr-doped CeO2, the localized 4f electrons do the most contribution (1.211 μB) to the magnetization and the 2p electrons of the eight nearest O atoms occur opposite spin polarization with a non-negligible magnetic moment of −0.032 μB per O (see Fig. 1(c)). On the other hand, the La-doped CeO2 has no magnetizing phenomenon, and it is mainly due to the unpaired electron delocalization, being responsible for spin pairing.
Furthermore, we investigated the defective CeO2 system containing oxygen vacancies (Ov). For the non magnetic CeO2, the formation of an Ov is known to result in the donation of two electrons which results in magnetism. Thus, upon Ov formation in CeO2, two electrons are left behind, and they localize on the f-level traps of the neighbouring Ce atoms, introducing two unpaired electrons which magnetize the defective CeO2. According to our calculations, the defective CeO2 containing 1, 2, 3 and 4 Ovs introduces 2.0, 4.0, 6.0 and 8.0 μB magnetic moment per supercell, respectively. Our spin charge calculations demonstrate that the f electrons localizing on the neighbouring Ce atoms result in the spin polarization (see Fig. 1(d)).
Electronic structures and photocatalysis
Next, we mainly focus our discussion on band gaps and electronic structures of pure, doped and defective CeO2 systems. Our hybrid-DFT calculation for the band gap of pure CeO2 gives an overestimating value of 4.24 eV, compared to the experimental value of 3.2 eV10. Density of state (DOS) calculations demonstrate that the top of valance bands (VB) mainly consist of O 2p orbitals and the bottom of conduction bands (CB) primarily is formed by Ce 4f orbitals. Table 2 collects all the band gaps between the top of occupied states and the bottom of the unoccupied states for doped and defective CeO2. For the C-doped CeO2 system, four separated bands are introduced between the VB-CB gap. Because the C impurities magnetize CeO2, the band structure of C-doped system is polarized magnetically. Two bands are α-spin states and others β-spin states, as we can see in Fig. 2. Fermi level is located at the top of β1 band and the unoccupied β2 band is below the CB bottom, which leads to a reduced gap of 1.85 eV corresponding to an optical absorption. The two α-spin bands are all above the VB top and below the Fermi level, and the first possible optical absorption corresponds to an electron transition from the α2 band to CB bottom (2.68 eV) in α-spin state. So, the C impurities considerably reduce the forbidden gap of pure CeO2 by 37% for α-spin and 56% for β-spin states. When the band gap is reduced, electrons from the VB top can migrate easily to the CB bottom by absorbing light, forming electron-hole pairs. The electrons and holes that accumulate on the surface of material are then scavenged by oxygen molecules (O2) and hydroxides (OH−) dissolved in water to yield highly oxidative species, such as superoxide radical anions ( ) and hydroxyl radicals (•OH), which are responsible for decomposing and degenerating pollutants. Therefore, photocatalysis is closely related to the optical band gap of materials. C dopant shifts the optical response of CeO2 from the UV to the visible region and change the photocatalytic properties of CeO2.
) and hydroxyl radicals (•OH), which are responsible for decomposing and degenerating pollutants. Therefore, photocatalysis is closely related to the optical band gap of materials. C dopant shifts the optical response of CeO2 from the UV to the visible region and change the photocatalytic properties of CeO2.
CeO2 is not only a potential photocatalyst for water/air purification, but also a candidate used in photocatalytic water-splitting for hydrogen production. Both applications require essential photogeneration of electron-hole pairs, which is closely connected with the band gap. According to our calculations on C-doped CeO2, the bottom of unoccupied states of α- and β-spin are both higher (negative) than hydrogen production level ( ) by 2.5 eV for α-spin and 2.2 eV for β-spin, meeting the requirement to initiate hydrogen production. On the other hand, the top of the occupied states located much higher than water oxidation level (
) by 2.5 eV for α-spin and 2.2 eV for β-spin, meeting the requirement to initiate hydrogen production. On the other hand, the top of the occupied states located much higher than water oxidation level ( ) for both spin states, which does not satisfy the requirement for effective water oxidation. However, the α1 occupied band is close to the
) for both spin states, which does not satisfy the requirement for effective water oxidation. However, the α1 occupied band is close to the  , being slightly higher by 0.3 eV. Though it also does not meet the water oxidation requirement, quite small difference and the ineluctable errors from theoretical calculations make C-doped CeO2 a probable photocatalyst for hydrogen production.
, being slightly higher by 0.3 eV. Though it also does not meet the water oxidation requirement, quite small difference and the ineluctable errors from theoretical calculations make C-doped CeO2 a probable photocatalyst for hydrogen production.
DOS analysis shows that C and neighbour O 2p orbitals form the α1 band, and the unoccupied β2 band consists of C 2p and the neighbour Ce 4f orbitals. The C 2p states lead to the neighbour O 2p electrons and Ce 4f orbitals separate from the VB top and CB bottom respectively, forming hybridization orbitals, as we can see Fig. 2. Spin-polarized DOS calculations also can reveal the magnetization mechanism. The number of spin-up electrons should be more than that of spin-down states for a system with net magnetic moment. For C-doped CeO2, we found that there are obviously more α-spin electrons near the Fermi level, where the C 2p, neighbour O 2p and Ce 4f α-spin states are dominant. The integrated DOS calculations also demonstrated that the magnetism mainly results from the spin-exchange splitting between the α- and β-spin states near the Fermi level. Similar scenario is also observed in other C- or N-doped systems14.
Figure 3 shows that there are three separated β-spin bands introduced between the VB-CB gap and one α-spin band connected with the VB top for N-doped CeO2. Fermi level is at the top of β2 band. The gap related to the α-spin electron transition equals to 3.96 eV, being much larger than that of C-doped case and closing to the pure CeO2, whereas the corresponding gap for β-spin state is only 0.47 eV (see Table 2). Similar to the C-doped case, N 2p and the neighbour O 2p orbitals do the major contributions to the impurity bands. The N impurities pull the neighbour O 2p electrons from VB top towards Fermi level, however the affection on the neighbour Ce 4f orbitals could be neglected (see Fig. 3). Therefore, a degree of hybridization between the N 2p and neighbour O 2p states can be seen near the Fermi level. The α-spin gap related to photocatalysis meets the requirements for water spitting, whereas the slight reduction of the gap with respect to the pure CeO2 implies that photocatalytic water splitting occurs near the UV region. We found that occupied-β1 band approximately lies at the same level as  , and unoccupied-β3 bands locates 0.46 eV above the
, and unoccupied-β3 bands locates 0.46 eV above the  . Therefore, the optical absorption corresponding to an electron transition from occupied-β1 to unoccupied-β3 bands (1.6 eV), which satisfies the conditions of photocatalytic water splitting, suggests that N-doped CeO2 could be applied in visible-region photocatalysis for hydrogen production.
. Therefore, the optical absorption corresponding to an electron transition from occupied-β1 to unoccupied-β3 bands (1.6 eV), which satisfies the conditions of photocatalytic water splitting, suggests that N-doped CeO2 could be applied in visible-region photocatalysis for hydrogen production.
P- and S-doped CeO2 do not exhibit the magnetization like C- and N-doped cases, thus the band structures are not spin-polarized. P impurities introduce three separated bands between the VB-CB gap. Two of them are narrow and below the Fermi level, and the third band is expanded and crosses the Fermi level with a tail, which shows a degree of metallic properties, as we can see in Fig. 4. For the S-doped CeO2 system, there are also three approximately separated bands introduced between the VB-CB gap. The two lower bands are narrow and the third impurity band is expanded but does not cross the Fermi level, unlike the P-doped case, still showing insulating properties (see Fig. 4). The gap between the top of occupied states and the bottom of unoccupied states is 2.85 eV, reducing the VB-CB gap of pure CeO2 considerably by around 33%. On the other hand, the first impurity band for the P-doped case locates above the VB top 1.55 eV, being much higher than that of the S-doped case (0.49 eV), which implies that P 3p orbitals are more delocalized. Similar to the C- and N-doped cases, for P- and S-doped CeO2, 3p orbitals of dopants, neighbour O 2p and Ce 4f orbitals hybridize to the impurity bands located between the VB-CB gap. For the S-doped case, our calculations show that the occupied impurity bands lie closely to the  and the CB bottom is much higher than the
and the CB bottom is much higher than the  level, indicating a probability for water spitting.
level, indicating a probability for water spitting.
Lanthanide impurities, such as La and Pr, change the band and electronic structures of pure CeO2. We found that unlike the above-mentioned dopants, La impurities do not introduce separated bands between the VB-CB gap. There are a few states crossing the Fermi level, exhibiting a little metallicity for the La-doped CeO2 system, as we can see Fig. 5. O 2p states forming the VB top in pure CeO2 are pulled towards the Fermi level by La 4f and 5p orbitals, leading to a degree of p-f hybridization straddling the Fermi level. The electronic structure of Pr-doped CeO2 is quite different from that of La-doped case. Pr dopants magnetize the doped CeO2 system and polarize the band structure. Four separated vacant bands are introduced between the VB-CB gap, being far from the Fermi level and closing to the CB bottom (see Fig. 5). Three of them are α-spin states and the other is β-spin. The three α-spin bands lie below the β-spin band. The gap between the top of occupied and the bottom of unoccupied states, corresponding to an optical absorption, is 3.12 eV for α-spin and 4.17 eV for β-spin states, reducing the VB-CB gap of pure CeO2 by around 26% and 2% respectively. Because of the obvious reduction of the optical gap in α-spin state, photocatalysis in the visible region is possible for the Pr-doped CeO2 case. Further, the top of occupied states locate 0.3 eV below the  and 1.6 eV above the
and 1.6 eV above the  level, meeting the water-spitting requirements. Therefore, Pr-doped CeO2 also could be used in hydrogen production. DOS calculations demonstrate that these separated vacant bands mainly consist of Pr 4f orbitals and the contributions from other orbitals could be neglect, therefore the f-p hybridization is not remarkable for these bands. We also found that there are a few α-spin states moving out of the VB top towards the Fermi level (see Fig. 5). A slight f-p hybridization between Pr and neighbor O exists near the Fermi level.
level, meeting the water-spitting requirements. Therefore, Pr-doped CeO2 also could be used in hydrogen production. DOS calculations demonstrate that these separated vacant bands mainly consist of Pr 4f orbitals and the contributions from other orbitals could be neglect, therefore the f-p hybridization is not remarkable for these bands. We also found that there are a few α-spin states moving out of the VB top towards the Fermi level (see Fig. 5). A slight f-p hybridization between Pr and neighbor O exists near the Fermi level.
Further, we performed calculations on the supercell containing 1, 2, 3 and 4 oxygen vacancies (labeled with 1Ov, 2Ov, …) to investigate the electronic structure of defective CeO2. For the 1Ov case, two connected bands with α-spin state lie 3.08 eV above the VB top, governing the Fermi level, and the gaps between the top of occupied and the bottom of unoccupied bands are 0.79 eV and 4.60 eV for α- and β-spin states respectively (see Fig. 6). So, the Ov reduces considerably the optical gap (4.24 eV) by around 81% in one spin state, whereas slightly increases it in the other spin state. Therefore, the photocatalysis of CeO2 containing oxygen vacancies may even shift up to the infrared region. The formation of an Ov is known to result in the donation of two electrons which form the defect bands locating between the VB-CB gap. According to our DOS calculations, the defect bands show mostly neighbour Ce 4f character. Then we can conclude that the two electron left behind localize on the f-level traps of the neighbour Ce atoms.
For the 2Ov and 3Ov cases, there are several α-spin bands lying between the VB-CB gaps, as we can see Fig. 7. The gaps with α-spin state between the top of occupied and the bottom of unoccupied bands are 0.57 eV for the 2Ov case and 0.64 eV for the 3Ov case, even reducing the optical gap further. Both cases have separated vacant α-spin bands below the CB bottom. Finally, for the 4Ov case, corresponding to the defect concentration of around 5%, the defective CeO2 system shows an obvious half-metallic character (see Fig. 7). Therefore, the charge carriers within the defect bands are sufficiently mobile with an ideal 100% polarization, which meets the need for spin injection where a highly polarized spin current is desired.
Conclusions
We have investigated magnetic properties, band and electronic structures of pure, doped and defective CeO2, such as C, N, P, S, La and Pr dopants, as well as Ov defects, in the framework of DFT using the hybrid functional technique PBE0. C, N, Pr impurities and Ov defects introduce magnetic moments of 2.0 μB, 1.0 μB, 1.0 μB and 2.0 μB per supercell with one dopant or defect, respectively. The calculations on the gap between top of occupied and bottom of unoccupied band, which corresponds to an optical absorption, demonstrated that C, N, S, Pr dopants and Ov defects reduces the band gaps evidently, showing a visible-light and even further infrared absorbance shift. It implies that the photocatalysis related closely to the optical absorption maybe occur in the visible region. Especially, N-, S- and Pr-doped CeO2 are promising photocatalysts for water splitting. As the concentration of Ov increasing up to around 5%, the CeO2 exhibits a half-metallic properties.
Methods
We have studied the magnetic properties and electronic structures of doped and defective CeO2 by performing first-principles calculations based on the DFT. Ab initio simulations were performed using the projector augmented wave (PAW) method15 as implemented in the Vienna ab initio simulation package (VASP) code16,17. We have chosen the exchange-correlation functional proposed by Perdew et al. using the hybrid HF/DFT calculation denoted hereby PBE018. For Ce atoms, we have used PAW potentials with following orbitals treated as valence states: 5s25p66s24f15d1 configuration. The calculations were performed using a cutoff energy of 500 eV and sampling the Brillouin zone with fixed Monkhorst-Pack k points (3 × 3 × 3) for conventional cell and (5 × 3 × 1) for a 72-atom (1 × 2 × 3) supercell.
Additional Information
How to cite this article: Shi, H. et al. Role of vacancies, light elements and rare-earth metals doping in CeO2. Sci. Rep. 6, 31345; doi: 10.1038/srep31345 (2016).
References
Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catalysis Reviews 38, 439–520 (1996).
Campbell, C. T. & Peden, C. H. F. Oxygen vacancies and catalysis on ceria surfaces. Science 309, 713–714 (2005).
Bao, H., Chen, X., Fang, J., Jiang, Z. & Huang, W. Structure-activity relation of Fe2O3-CeO2 composite catalysts in CO oxidation. Catalysis Letters 125, 160–167 (2008).
Bishop, S. R., Tuller, H. L., Kuru, Y. & Yildiz, B. Chemical expansion of nonstoichiometric Pr0.1Ce0.9O2−d : Correlation with defect equilibrium model. J. Euro. Ceramic Soc. 31, 2351–2356 (2011).
Fernandes, V. et al. Dilute-defect magnetism: Origin of magnetism in nanocrystalline CeO2 . Phys. Rev. B 80, 035202 (2009).
Li, M., Zhang, R., Zhang, H., Feng, W. & Liu, X. Synthesis, structural and magnetic properties of CeO2 nanoparticles. IET Micro Nano Letters 5, 95–99 (2010).
Tsunekawa, S., Wang, J.-T., Kawazoe, Y. & Kasuya, A. Blueshifts in the ultraviolet absorption spectra of cerium oxide nanocrystallites. J. Appl. Phys. 94, 3654–3656 (2003).
Truffault, L. et al. Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater. Res. Bull. 45, 527–535 (2010).
Bamwenda, G. R. & Arakawa, H. Cerium dioxide as a photocatalyst for water decomposition to O2 in the presence of Ce aq4+ and Fe aq3+ species. J. Mol. Catal. A: Chem. 161, 105–113 (2000).
Corma, A., Atienzar, P., Garcia, H. & Chane-Ching, J.-Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 3, 394 (2004).
Mao, C., Zhao, Y., Qiu, X., Zhu, J. & Burda, C. Synthesis, characterization and computational study of nitrogen-doped CeO2 nanoparticles with visible-light activity. Phys. Chem. Chem. Phys. 10, 5633–5638 (2008).
Trovarelli, A. Catalysis by Ceria and Related Materials. London (2002).
Pacchioni, G. Modeling doped and defective oxides in catalysis with density functional theory methods: Room for improvements. J. Chem. Phys. 128, 182505 (2008).
Elfimov, I. S. et al. Magnetizing oxides by substituting nitrogen for oxygen. Phys. Rev. Lett. 98, 137202 (2007).
Bl:ochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthm:u ller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Acknowledgements
Thanks to SSF-Sweden and NRF-Korea for joint collaborative research funding. This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIP) (No. 2015-066177). H. Shi was supported by NSFC Grant No. 11004008. SNIC, HPC2N and UPPMAX are acknowledged for providing computing time.
Author information
Authors and Affiliations
Contributions
R.A. and W.L. designed and planned the research. T.H. performed the calculations. H.S. analyzed data and wrote the manuscript. R.A., W.L., T.H., H.S. and T.W.K. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shi, H., Hussain, T., Ahuja, R. et al. Role of vacancies, light elements and rare-earth metals doping in CeO2. Sci Rep 6, 31345 (2016). https://doi.org/10.1038/srep31345
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31345
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.


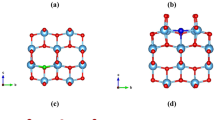

 m structure (big green and small red spheres represent Ce and O atoms respectively). Spin-charge density (nα−nβ) maps for C- (from the (001) side view) (b), Pr-doped (from the (110) side view) (c), and 1Ov (from (110) side view) (d) defective CeO2.
m structure (big green and small red spheres represent Ce and O atoms respectively). Spin-charge density (nα−nβ) maps for C- (from the (001) side view) (b), Pr-doped (from the (110) side view) (c), and 1Ov (from (110) side view) (d) defective CeO2.




