Abstract
A series of CuBi co-doped mesoporous zeolite Beta (CuxBiy-mBeta) were prepared by a facile one-pot hydrothermal treatment approach and were characterized by XRD, N2 adsorption-desorption, TEM/SEM, XPS, H2-TPR, NH3-TPD and in situ DRIFTS. The catalysts CuxBiy-mBeta were applied to the removal of NOx by selective catalytic reduction with ammonia (NH3-SCR), especially the optimized Cu1Bi1-mBeta achieved the high efficiency for the removal of NOx and N2 selectivity, superior water and sulfur resistance as well as good durability. The excellent catalytic performance could be attributed to the acid sites of the support and the synergistic effect between copper and bismuth species. Moreover, in situ DRIFTS results showed that amides NH2 and NH4+ generated from NH3 adsorption could be responsible for the high selective catalytic reduction of NOx to N2. In addition, a possible catalytic reaction mechanism on Cu1Bi1-mBeta for the removal of NOx by NH3-SCR was proposed for explaining this catalytic process.
Similar content being viewed by others
Introduction
Nowadays, it is still of great challenges for the effectively catalytic purification of diesel exhausts, especially for the NOx from diesel engine, since the conventional three-way catalysts are no longer effective in selective reducing NOx1. The commercial selective catalytic reduction with ammonia (NH3-SCR) catalyst for the removal of NOx, i.e.,V2O5-WO3/TiO2, only shows high catalytic efficiency in a narrow temperature window of 300–400 °C2, besides, the poor water and sulfur resistance as well as the toxicity of V2O5 also greatly prohibit the popularity of vanadium-based composite oxides. Therefore, researchers have devoted to develop a new kind of non-vanadia catalysts to overcome the disadvantages of vanadium-based composite oxides3. It was reported that compared to the traditional V2O5-WO3/TiO2, non-vanadia catalyst not only presents the wider NH3-SCR temperature windows with high N2 selectivity, but also owns good durability and strong resistance against H2O and SO2.
Very recently, zeolite-based catalysts with high surface areas and pore volume, abundant acidity sites, outstanding thermal and hydrothermal stability, as good catalyst supports, have attracted much research attention in selective catalytic reduction NOx by ammonia4,5. However, small microporous channels of zeolite greatly prevented the diffusion and transport of some large molecules, resulting in the low catalytic performance in a great majority of traditional catalytic reactions6. Therefore, a novel zeolite with mesoporous structure has been developed, which combines the advantages of conventional crystalline zeolite and mesoporus material, to enable the quick access for the diffusion and transport attributed to the hierarchically porous structure. It is generally believed that mesoporous zeolites possess remarkably higher catalytic activity and longer catalytic lifetime than conventional zeolites owing to the crystalline framework and the hierarchically porous structure7. Thereinto, the mesoporous Beta zeolite (mBeta) with unique three-dimensional network of large pores (12MR) exhibits much high surface area and excellent hydrothermal stability, which is widely applied in fine chemistry8. In the past decades, a number of synthetic approaches of mBeta have been explored and some encouraging results have been obtained8,9,10. Such as, Xiao et al., synthesized highly mesoporous single-crystalline zeolite beta by using a commercial polymer, polydiallyldimethylammonium chloride (PDADMA) as both structure-directing agent and porogen, which showed better hydrothermal stability and higher catalytic activity than conventional zeolite Beta in large molecules involved acid-catalyzed reactions8. In addition, many metal oxides were reported to have good performance in the removal of NOx by NH3-SCR, e.g., Cu-loaded zeolite beta exhibited a good activity and hydrothermal stability in the NH3-SCR of NOx11,12. It is also reported that the introduction of Bi2O3 can improve the SO2 resistance13.
On the basis of our previous work14, a novel CuBi co-doped mesoporous zeolite Beta (CuxBiy-mBeta) has been synthesized by one-pot hydrothermal treatment approach, by which the copper and bismuth species can be well dispersed into the framework of mesoporous zeolite Beta. Therein, the optimized prepared catalyst Cu1Bi1-mBeta exhibits very high catalytic activity for the selective catalytic reduction of NOx with NH3. In addition, the N2 selectivity, water vapor and sulfur resistance and durability of the Cu1Bi1-mBeta catalyst have been detailedly investigated. Finally, a possible catalytic mechanism of SCR of NOx with ammonia on this prepared catalyst CuBi-mBeta is proposed to clarify the catalytic process.
Results
Structure Characteristics
The powder XRD patterns of mBeta, Cu-mBeta, Bi-mBeta and CuxBiy-mBeta are shown in Fig. 1. It is found that all prepared samples keep the diffraction peak of typical zeolite beta structure, and no diffraction peaks corresponding to copper and bismuth species can be detected. Therefore, it is believed that the copper and bismuth species could be well-incorporated into the framework of zeolite as ions or highly dispersed into the mesoporous channels as metal oxides. It is noted that compared with the mBeta, the doping of Bi species can cause the inevitable destruction of zeolite framework to a certain extent, and the intensity of XRD peak of CuxBiy-mBeta decreases with the increase of Bi-loading content, as shown in Fig. 1.
The N2 adsorption isotherms and pore size distribution curves for samples mBeta, Cu-mBeta, Bi-mBeta and CuxBiy-mBeta are shown in Fig. 2 and the corresponding pore structure parameters of all the samples are summarized in Table 1. All the samples exhibit typical type IV isotherms, confirming the presence of mesoporous structure. The reference sample mBeta shows well-defined mesopore of 3.8 nm, and the BET surface area and total pore volume are calculated to be 556 m2/g and 0.34 cm3/g, respectively, including the mesoporous surface area (166 m2/g) and mesoporous volume (0.16 cm3/g), respectively. Compared with the mBeta, the surface areas of Cu-mBeta, Bi-mBeta and CuxBiy-mBeta show obvious decrease after loading amount of copper and bismuth species, which is resulted from the generation of non-framework Cu or/and Bi ions (i.e., CuO and Bi2O3) with the increase of Cu or Bi content, and inducing the collapse of pore structure of zeolite to some extent. Even so, the prepared Cu1Bi1-mBeta still keep high BET surface area and pore volume after doping with Cu and Bi species (Table 1), indicative of the unblocked mesoporous channels due to the high dispersity of Cu and Bi species. It is noted that the optimized Cu1Bi1-mBeta with the mesopore size of 3.6 nm shows high BET surface area (539 m2/g) and total pore volume (0.46 cm3/g).
The SEM image of as-prepared Cu1Bi1-mBeta, as shown in Fig. 3a, presents a rough surface morphology, demonstrating that the mesoporous structure has penetrated into the zeolite crystals by one-pot hydrothermal synthesis process. Additionally, no oxide aggregations can be found, as shown in Fig. 3a,b, indicating that the doped metal oxides are highly dispersed into the carrier mBeta. The clearly crystal lattices can be found in the high-magnification TEM image (Fig. 3c), confirming the zeolite crystallized structure. The element mapping in Fig. 3d–h further confirms that Cu and Bi species are highly dispersed into mesoporous zeolite Beta, which is consistent with the above results of XRD patterns.
Spectroscopy Characteristics
The X-ray transmission spectroscopy (XPS) result of the Cu1Bi1-mBeta is show in Fig. 4a. The binding energy levels of Cu 2p3/2 and Cu 2p1/2 at around 934.5 eV and 954.2 eV, respectively (denoted with ★), accompanied by a shoulder peak of about 10 eV higher binding energy, are attributable to Cu(II). Additionally, the distinctive peaks at 936.6 eV and 956.4 eV, (denoted with ▼) can be ascribed to Cu(I), indicating that Cu species have variable valencies in the obtained sample Cu1Bi1-mBeta. In addition, the prepared Cu1Bi1-mBeta presents higher binding energy peak intensities of Cu(II) (934.5 eV) than that of Cu(I) (936.6 eV), as shown in Fig. 4a, suggesting that Cu1Bi1-mBeta contains much more amounts of Cu(II) than Cu(I)15,16. Figure 4b shows the Bi 4f XPS spectrum of Cu1Bi1-mBeta catalyst. Compared to pure Bi2O3 at 164.2 eV and 158.9 eV, the Cu1Bi1-mBeta shows the Bi 4f5/2 and Bi 4f7/2 a little higher binding energies at 164.6 eV and 159.5 eV, respectively, which is attributed to the interaction between Bi and Cu or the support mBeta17,18,19.
The H2-TPR profiles of Cu-mBeta, Bi-mBeta and a series of CuxBiy-mbeta samples are shown in Fig. 5. It is found that the reference single loaded sample Cu-mBeta shows two weak reduction peaks at around 220 °C and 290 °C, indicating a two-step reduction of Cu2+ ions, i.e., firstly to Cu+ and then to Cu20,21. In addition, the reduction peak at 310 °C of reference Bi-mBeta can be ascribed to the reduction of Bi2O3. The H2-TPR profiles of co-loaded samples CuxBiy-mBeta show a distinctively different redox behavior from either Cu-mBeta or Bi-mBeta, and present enhanced reduction peak at 250–350 °C, attributed to the strong interaction between the active species CuO and Bi2O3. In addition, the reduction peak gradually shifts toward lower temperature range (from 350 to 310 °C) with the increase of Bi content (Fig. 5 and Table 1), confirming that the addition of Bi2O3 is beneficial to promote the catalytic redox reaction22.
NH3-TPD experiments were carried out to obtain the acidity information of the prepared catalysts, as shown in Fig. 6. It is clear that a low-temperature peak at 150 °C and a high-temperature peak at 300 °C can be observed for the sample mBeta, which is assigned to weakly weak Lewis acid sites and strong Brønsted acid sites, respectively23,24. It is noted that all the samples Cu-mBeta, Bi-mBeta and CuxBiy-mBeta show weaker low-temperature peaks than that of the reference mBeta, owing to the part destruction of zeolite framework structure after introducing Cu and Bi species. However, it is interesting that only the Cu1Bi1-mBeta shows a similar desorption peak at high-temperature range (300–400 °C) to the mBeta, indicating that the Cu1Bi1-mBeta sample still keeps the strong acidity site of mBeta. The presence of rich acidic sites (Brønsted acid and Lewis acid) produced from the framework Al atoms and copper/bismuth species are helpful to the adsorption and activation of NH3, and thus producing many ammonia species, including NH2, coordinated NH3 and ionic NH4+, which can greatly promote the selective catalytic reduction of NOx, as shown in Fig. 7.
The reaction of adsorbed NH3 species towards NO + O2 was evaluated by the in situ IR spectra at 250 °C and the results are shown in Fig. 7. When the catalyst are exposed to NH3 for the 60 min and purged with N2, the peaks related to coordinated NH3 on Lewis acid sites (3125, 3002, 1611, 1245 and 1115 cm−1) and ionic NH4+ bound to Brønsted acid sites (3601 and 1440 cm−1) are clearly observed15,25,26,27. Afterwards, the coordinated NH3 on acidic sites could undergo the oxidative dehydrogenation to form NH2 species (1560 cm−1), then produce intermediate specie NH2NO when NO and O2 were added into reaction gas. It is noted that all the ammonia species, including NH2, coordinated NH3 and ionic NH4+ bound, disappeared after NO + O2 purge, indicating that those ammonia species could participate in the reduction of NOx. Meanwhile, when NO and O2 were added into reaction gas, the bands at 1235, 1367, 1542 and 1601 cm−1 could be detected in IR spectra. Thereinto, the bands at 1235 and 1367 cm−1 were assigned to monodentate nitrate, while the bands at 1542 and 1601 cm−1 are associated with bidentate nitrate and adsorbed NO2, respectively15,25. More interestingly, two peculiar peaks at 3335 and 3265 cm−1 related to the adsorbed NH3 on acid sites became stronger with the increase of exposing time in the NO + O2, indicating that some acidic sites on the surface of the catalyst were released and then preferably adsorbed the NH3 after NO and O2 pass over the catalyst.
Catalytic performance
Figure 8 shows the NH3-SCR results of mBeta, Cu-mBeta, Bi-mBeta and a series of CuxBiy-mBeta catalysts. The NOx conversions over the prepared catalysts under high hourly space velocity of 64000 h−1 are shown in Fig. 8a. Compared with the references mBeta, Bi-mBeta and Cu-mBeta, the sample CuxBiy-mBeta show higher catalytic activity for the SCR of NOx. Especially, the optimized sample Cu1Bi1-mBeta with the 4.38 wt% Cu and 4.83 wt% Bi exhibits the highest catalytic performance, i.e., the NOx conversion efficient is above 90% within the wide operation temperature window of 170 °C to 400 °C. While, the excess doping of Cu and Bi species could induce the formation of oxides aggregates and thus decrease the active surface area (Table 1), e.g., the excess Bi would cover part of active center and results a lower catalytic activity. Therefore, the optimized sample Cu1Bi1-mBeta with the 1:1 ratio of Cu and Bi shows the highest catalytic performance. The results of N2 selectivity of these catalysts were shown in Fig. 8b. For comparison, the reference Bi-mBeta shows a low N2 selectivity, particular in 200 °C, owing to the part destruction of zeolite framwork with the addition of Bi species, as demonstrated by XRD results, which can affect the strong acidic sites (Fig. 6), thus decrease the adsoption ability of NH3 on the reference Bi-mBeta. Furthermore, the Cu1Bi1-mBeta catalyst also presents as high as up to 100% N2 selectivity in the whole temperature range investigated. It is believed that the high dispersity of active species Cu and Bi on the support mesoporous zeolite, the richer acidic sites and the strong interaction between the active copper and bismuth species on the Cu1Bi1-mBeta could be main contributions to the catalytic activity.
NOx conversion (a), N2 selectivity (b), H2O and SO2 resistance (c) and durability test at 250°C (d) for the NH3-SCR over mBeta, Cu-mBeta, Bi-mBeta and a series of CuxBiy-mBeta catalysts with different Cu/Bi ratios (500 ppm NO, 500 ppm NH3, 0 or 3% H2O, 0 or 50 ppm SO2, 5% O2, balance Ar, GHSV = 64 000 h−1).
It is well known that the real diesel exhaust presents large number of water vapor and trace S compounds, therefore, the effects of H2O and SO2 on the SCR catalytic activity are also investigated on the samples Cu1Bi1-mBeta. It is evident that compared with the reference mBeta, Cu1Bi1-mBeta catalyst shows an excellent SO2 resistance in the NH3-SCR above 200 °C (Fig. 8c). Even in the co-presence of H2O and SO2, the Cu1Bi1-mBeta catalyst still exhibits high activity of over 85% NOx conversion from 200 °C to 430 °C. The results of durability tested at 250 °C, as shown in Fig. 8d, indicate that the Cu1Bi1-mBeta is very stable in the presence of SO2 (or H2O and SO2). It is believed that the highly crystalline zeolite framework enables the catalyst to keep good stability against H2O, and the highly dispersity active speicies Bi can improve the SO2 resistance to some extent13, which is very important for the NH3-SCR of NOx in the co-existence of H2O and SO2.
Discussion
Based on the above results and discussions, a possible catalytic reaction mechanism for the SCR of NOx was proposed, as illustrated in Fig. 9. Firstly, there are a large amount of oxygen vacancies ( ) presented in the sample support mBeta due to the doping of hetero atoms Cun+, Bi3+ and Al3+ in the [SiO4], leading to the generation of numerous surface activated oxygen (O*) by adsorbing the O2, as shown in Step 1 (Fig. 9). Meanwhile, the existence of Bi2O3 was reported22,28 to be beneficial for the reduction of Cu2+ to Cu+, and NO could be easily adsorbed and activated by the Cun+ and generated the
) presented in the sample support mBeta due to the doping of hetero atoms Cun+, Bi3+ and Al3+ in the [SiO4], leading to the generation of numerous surface activated oxygen (O*) by adsorbing the O2, as shown in Step 1 (Fig. 9). Meanwhile, the existence of Bi2O3 was reported22,28 to be beneficial for the reduction of Cu2+ to Cu+, and NO could be easily adsorbed and activated by the Cun+ and generated the  and
and  at higher temperatures or lower temperatures, respectively29,30. Afterwards, these activated
at higher temperatures or lower temperatures, respectively29,30. Afterwards, these activated  and
and  could react with the surface activated oxygen (O*) and thus produce large numbers of NO2, as shown in Step 1 (Fig. 9). When the concentration ratio of NO and NO2 in reaction gas reaches to 1:1, the quick SCR reaction (NO + NO2 + 2NH3 = 2N2 + 3H2O) occurs, during which the NOx conversion efficiency at low temperature can be greatly improved. Secondly, the presence of rich acidic sites (Brønsted acid and Lewis acid) produced from the framework Al atoms and copper/bismuth species is helpful to the adsorption and activation of NH3, i.e., the NH3 adsorbed on strong Brønsted acid sites could be activated and generate NH4+, which was discovered from the in situ DRIFTs (Fig. 7), as shown in Step 2 (Fig. 9). Meanwhile, the NH3 molecules could also be adsorbed on the weak Lewis acid sites to generate NH3(ads) and react with the activated oxygen (O*) to produce the amines NH/NH2, as confirmed by the in situ DRIFTs (Fig. 7). Finally, the produced amide NH/NH2 and NH4+ could directly react with NOx on the highly dispersed active sites and generate N2, as shown in Step 3 (Fig. 9). It is believed that the existence of highly dispersed varied valence Cu species and acidic sites can accelerate the adsorption and activation of NO and NH3 in this reaction system, which greatly increases the NH3-SCR in the removal of NOx.
could react with the surface activated oxygen (O*) and thus produce large numbers of NO2, as shown in Step 1 (Fig. 9). When the concentration ratio of NO and NO2 in reaction gas reaches to 1:1, the quick SCR reaction (NO + NO2 + 2NH3 = 2N2 + 3H2O) occurs, during which the NOx conversion efficiency at low temperature can be greatly improved. Secondly, the presence of rich acidic sites (Brønsted acid and Lewis acid) produced from the framework Al atoms and copper/bismuth species is helpful to the adsorption and activation of NH3, i.e., the NH3 adsorbed on strong Brønsted acid sites could be activated and generate NH4+, which was discovered from the in situ DRIFTs (Fig. 7), as shown in Step 2 (Fig. 9). Meanwhile, the NH3 molecules could also be adsorbed on the weak Lewis acid sites to generate NH3(ads) and react with the activated oxygen (O*) to produce the amines NH/NH2, as confirmed by the in situ DRIFTs (Fig. 7). Finally, the produced amide NH/NH2 and NH4+ could directly react with NOx on the highly dispersed active sites and generate N2, as shown in Step 3 (Fig. 9). It is believed that the existence of highly dispersed varied valence Cu species and acidic sites can accelerate the adsorption and activation of NO and NH3 in this reaction system, which greatly increases the NH3-SCR in the removal of NOx.
In conclusion, a series of CuxBiy-mBeta catalysts have been prepared by a facile one-pot hydrothermal treatment approach. The optimized Cu1Bi1-mBeta shows an excellent NH3-SCR activity and high N2 selectivity (closely to 100%) toward NOx in a broad operation temperature window (170–400 °C). On the one hand, the mesopores structure is helpful for the homogeneous dispersion of active species, which can improve the diffusion and transport of reactants and products. Also, the highly crystalline zeolite framework enables the catalyst to keep good resistence toward water vapor. On the other hand, the highly dispersity of copper and bismuth active species, as well as the synergetic catalytic effect between copper and bismuth species and the richer acidic sites of the zeolite promote the reduction of CuO and generation of intermediate NO2. Meanwhile, the large number of amide NH/NH2 and NH4+ generated from NH3 adsorption, as the key intermediates, greatly accelerate the selective catalytic reduction of NOx. More interesting, the prepared catalyst shows good durability and high resistance against H2O and SO2, which could also be ascribed to the crystalline zeolite framework and the highly dispersity active sites. The CuBi-mBeta catalyst with distinctive mirco-mesoporous structure demonstrates excellent NH3-SCR activity and high stability, which, as we believe, will present promising prospect in the practical application of catalytic purification of diesel exhausts.
Methods
Preparation of the mesoporous zeolite Beta (mBeta)
Typically, 0.05 g NaCl and 0.15 g KCl were added into 2 mL distilled water and 14.4 g TEAOH solution. Afterwards, 3.9 g H2SiO3 was dissolved into the above mentioned solution and stirred at 313 K for 6 h. Next, the solution containing 0.033 g NaOH, 0.17 g NaAlO2 and 2 mL distilled water was slowly added into the resultant solution and further stirred at 313 K for 6 h. Finally, 0.5 g CTAB solution was added into the obtained solution and further stirred at 353 K for 8 h. The obtained mixed solution was hydrothermally treated for 48 h at 423 K. Subsequently, the products were washed with distilled water and dried at 383 K for 12 h. The final product mBeta was obtained after calcinations at 823 K for 6 h to remove any organics.
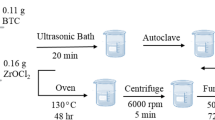
Preparation of Cu1Bi1-mBeta
Typically, 0.05 g NaCl and 0.15 g KCl were added into 2 mL distilled water and 14.4 g TEAOH solution. Afterwards, 3.9 g H2SiO3 was dissolved into the above mentioned solution and stirred at 313 K for 6 h. Then, the solution containing 0.033 g NaOH, 0.17 g NaAlO2 and 2 mL distilled water was slowly added into the resultant solution and stirring. Next, 1 mmol Cu(NO3)2•3H2O, 1 mmol Bi(NO3)3•5H2O and 2 mL distilled water was slowly added into the above precursor solution and further stirred at 313 K for 6 h. Then, 0.5 g CTAB solution was added into the obtained solution and further stirred at 353 K for 8 h. Next, the obtained mixed solution was hydrothermally treated for 48 h at 423 K. Subsequently, the products were washed with distilled water and dried at 383 K for 12 h. The final product, Cu1Bi1-mBeta, was obtained after calcinations at 823 K for 6 h to remove any organics.
For comparison, the Cu-mBeta, Bi-mBeta and CuxBiy-mBeta were synthesized by a facile one-pot hydrothermal treatment approach, similarly to the above process of Cu1Bi1-mBeta. Herein, the x and y represent the millimole amount of Cu and Bi in the initial precursor solution, respectively. In addition, the final pH value of the synthetic gel including copper and bismuth species is about 11.
Sample characterization
The XRD patterns were recorded on a Rigaku D/Max-2200PC X-ray diffractometer using Cu target at 40 kV and 40 mA. The N2 adsorption and desorption measurements were performed using Micromeritics Tristar 3000 at 77 K. The total surface area and pore volume were calculated using the BET and BJH method. Field emission scanning electron microscopy (SEM) analysis was performed on a JEOL JSM6700F electron microscope. Field emission transmission electron microscopy (TEM) analysis was conducted with a JEOL 200CX electron microscope operated at 200 keV. X-ray photoelectron spectroscopy (XPS) signals were collected on a Thermo Scientific ESCALAB 250 instrument using monochromated Al X-ray resource at 1486.6 eV operated at 15 kW. The temperature-programed reduction with hydrogen (H2-TPR) and temperature programmed desorption of ammonia (NH3-TPD) were performed on Micromeritics Chemisorb 2750 instrument attached with ChemiSoft TPx software. TPR was carried out from room temperature to 900 °C under 5% H2 in Ar at a flow rate of 25 mL/min. The H2 signal was detected by a thermal conductivity detector (TCD). The NH3-TPD of the samples was carried out from room temperature to 800 °C at a flow rate of 25 mL/min. The amount of NH3 desorbed was measured using a thermal conductivity detector (TCD). In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was performd on a Bruker spectrometer equipped with an MCT detector. Prior to each experiment, the sample was pretreated at 400 °C for 1 h in a flow of N2 and then cooled down to 200 °C. The background spectrum was collected in flowing N2 and automatically subtracted from the sample spectrum. The reaction conditions were controlled as follows: 100 mL min−1 total flow rate, 500 ppm NH3, 500 ppm NO, 5% O2 and N2 as the balance. All spectra were recorded by accumulating 100 scans with a resolution of 4 cm−1. The contents of Cu and Bi were measured by using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) analyzer on a Vista AX.
Catalytic activity test
The catalysis measurements were carried out in a fixed-bed quartz reactor using 0.2 g catalyst of 40–60 meshes. Before catalytic test, the catalysts were dried at 423 K for 16 h. The feed gas mixture contained 500 ppm NO, 500 ppm NH3, 0 or 3% H2O, 0 or 50 ppm SO2, 5% O2 and Ar as the balance gas. The total flow rate of the feed gas was 300 mL/min, corresponding to a GHSV of 64,000 h−1. The composition of the product gas was analyzed by a chemiluminescence NO/NO2 analyzer (Thermal Scientific, model 42i-HL) and gas chromatograph (Shimadzu GC 2014 equipped with Porapak Q and Molecular sieve 5A columns). The activity data were collected when the catalytic reaction practically reached steady-state condition at each temperature.
The NOx (XNOx) and NH3 (XNH3) conversions and N2 selectivity (SN2) were calculated as



where NOx includes NO and NO2, Ci presents the concentration of the “i” species, and the “in” and “out” present the gas concentration of inlet and the exit of the reactor, respectively.
Additional Information
How to cite this article: Xie, Z. et al. One-pot hydrothermal synthesis of CuBi co-doped mesoporous zeolite Beta for the removal of NOx by selective catalytic reduction with ammonia. Sci. Rep. 6, 30132; doi: 10.1038/srep30132 (2016).
References
Su, W. K., Chang, H. Z., Peng, Y., Zhang, C. Z. & Li, J. H. Reaction pathway investigation on the selective catalytic reduction of NO with NH3 over Cu/SSZ-13 at low temperatures. Environ. Sic. Technol. 49, 467–473 (2015).
Zhang, Q. L. et al. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2-ZrO2-Al2O3 . Catal. Today 175, 171–176 (2011).
Peng, Y., Qu, R. Y., Zhang, X. Y. & Li, J. H. The Relationship between Structure and Activity of MoO3-CeO2 Catalysts for NO Removal: Influences of Acidity and Reducibility. Chem. Commun. 49, 6215–6217 (2013).
Jin, R. B. et al. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B 148–149, 582–588 (2014).
Chang, X. F., Lu, G. Z., Guo, Y., Wang, Y. Q. & Guo, Y. L. A high effective adsorbent of NOx: Preparation, characterization and performance of Ca-beta zeolites. Microporous Mesoporous Mater. 165, 113–120 (2013).
Lethbridge, Z. A. D., Williams, J. J., Walton, R. I., Evans, K. E. & Smith, C. W. Methods for the synthesis of large crystals of silicate zeolites. Microporous Mesoporous Mater. 79, 339–352 (2005).
Xu, L. et al. Enhancement of low-temperature activity over Cu-exchanged zeolite beta from organotemplate-free synthesis for the selective catalytic reduction of NOx with NH3 in exhaust gas streams. Microporous Mesoporous Mater. 200, 304–310 (2014).
Zhu, J. et al. Highly Mesoporous Single-Crystalline Zeolite Beta Synthesized Using a Nonsurfactant Cationic Polymer as a Dual-Function Template. J. Am. Chem. Soc. 136, 2503–2510 (2014).
Chen, C. Y. et al. Enhanced performance in catalytic combustion of toluene over mesoporous Beta zeolite-supported platinum catalyst. Appl. Catal. B 140–141, 199–205 (2013).
Yin, C. Y. et al. One-step synthesis of hierarchical mesoporous zeolite Beta microspheres from assembly of nanocrystals. J. Colloid. Interface Sci. 397, 108–113 (2013).
Baerdemaeker, T. D. et al. Catalytic applications of OSDA-free Beta zeolite. J. Catal. 308, 73–81 (2013).
Deka, U., Lezcano-Gonzalez, I., Weckhuysen, B. M. & Beale, A. M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx . ACS Catal. 3, 413–427 (2013).
Karlsson, H. T. & Rosenberg, H. S. Flue gas denitrification. Selective catalytic oxidation of NO to NO2 . Ind. Eng. Chem. Proc. Des. Dev. 23, 808–814 (1984).
Zhou, X. X. et al. A facile one-pot synthesis of hierarchically porous Cu(I)-ZSM-5 for radicals-involved oxidation of cyclohexane. Appl. Catal. A 451, 112–119 (2013).
Zhang, R. R., Li, Y. H. & Zhen, T. L. Ammonia selective catalytic reduction of NO over Fe/Cu-SSZ-13. RSC Adv. 4, 52130–52139 (2014).
Chadwick, D. & Hashemi, T. Adsorbed corrosion inhibitors studied by electron spectroscopy: Benzotriazole on copper and copper alloys. Corros. Sci 18, 39–51 (1978).
Bian, Z. F. et al. Self-Assembly of Active Bi2O3/TiO2 Visible Photocatalyst with Ordered Mesoporous Structure and Highly Crystallized Anatase. J. Phys. Chem. C 112, 6258–6262 (2008).
Shan, W. J., Hu, Y., Zheng, M. M. & Wei, C. H. The enhanced photocatalytic activity and self-cleaning properties of mesoporous SiO2 coated Cu-Bi2O3 thin films. Dalton Trans. 44, 7428–7436 (2015).
Jiang, H.-Y. et al. Efficient organic degradation under visible light by α-Bi2O3 with a CuOx-assistant electron transfer process. Appl. Catal. B 163, 267–276 (2015).
Richter, M. et al. Gas-phase carbonylation of methanol to dimethyl carbonate on chloride-free Cu-precipitated zeolite Y at normal pressure. J. Catal. 245, 11–24 (2007).
Xue, J. J. et al. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 297, 56–64 (2013).
Yang, G. H. et al. MCM-41 supported CuO/Bi2O3 nanoparticles as potential catalyst for 1,4-butynediol synthesis. Ceram. Int. 40, 3969–3973 (2014).
Zhou, X. X. et al. Dual-mesoporous ZSM-5 zeolite with highly b-axis-oriented large mesopore channels for the production of benzoin ethyl ether. Chem.-Eur. J. 19, 10017–10023 (2013).
Wang, D. et al. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B 165, 438–445 (2015).
Liu, Z. M. et al. A superior catalyst with dual redox cycles for the selective reduction of NOx by ammonia. Chem. Commun. 49, 7726–7728 (2013).
Meng, D. M. et al. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 5, 5973–5983 (2015).
Yu, T. et al. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C 118, 6565–6575 (2014).
Lin, G. et al. Universal Preparation of Novel Metal and Semiconductor Nanoparticle-Glass Composites with Excellent Nonlinear Optical Properties. J. Phys. Chem. C 115, 24598–24604 (2011).
Chen, B. H., Xu, R. N., Zhang, R. D. & Liu, N. Economical Way to Synthesize SSZ-13 with Abundant Ion-Exchanged Cu+ for an Extraordinary Performance in Selective Catalytic Reduction (SCR) of NOx by Ammonia. Environ. Sci. Technol. 48, 13909–13916 (2014).
Zhang, R. Q. et al. NO Chemisorption on Cu/SSZ-13: A Comparative Study from Infrared Spectroscopy and DFT Calculations. ACS Catal. 4, 4093–4105 (2014).
Acknowledgements
This research was sponsored by National Key Basic Research Program of China (2013CB933202), China National Funds for Distinguished Young Scientists (51225202) and National Natural Science Foundation of China (51502330).
Author information
Authors and Affiliations
Contributions
Z.X. conceived the preparation method, synthesized and characterization of the CuBi co-doped mesoporous zeolite Beta. Z.X., X.Z., H.W. and H.C. analyzed the experimental results and preparation of the manuscript draft. L.C., H.Z., Y.L. and L.P. supervised and finalized the project. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, Z., Zhou, X., Wu, H. et al. One-pot hydrothermal synthesis of CuBi co-doped mesoporous zeolite Beta for the removal of NOx by selective catalytic reduction with ammonia. Sci Rep 6, 30132 (2016). https://doi.org/10.1038/srep30132
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30132
This article is cited by
-
Synthesis of CuCe co-modified mesoporous ZSM-5 zeolite for the selective catalytic reduction of NO by NH3
Environmental Science and Pollution Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.