Abstract
Fe2O3 nanorods exposing (001) and (010) plane as well as Fe2O3 nanosheets exposing (001) plane have been successfully synthesized. Fe2O3 nanosheets exhibit better cycle performance and rate capabilities than that of Fe2O3 nanorods. The discharge capacity of Fe2O3 nanosheets can stabilize at 865 mAh/g at the rate of 0.2 C (1C = 1000 mA/g) and 570 mAh/g at the rate of 1.2 C after 80 cycles, which increased by 90% and 79% compared with 456 mAh/g and 318 mAh/g of Fe2O3 nanorods. In comparison with (010) plane, the (001) plane of hematite possesses larger packing density of Fe3+ and O2−, which is responsible for the superior electrochemical performances of Fe2O3 nanosheets than that of Fe2O3 nanorods. In addition, potentiostatic intermittent titration (PITT) results show the diffusion coefficients of Li+ (DLi) of Fe2O3 nanosheets is higher than that of Fe2O3 nanorods. The higher diffusion coefficients of Li+ is favorable for the excellent lithium-storage capabilities and rate capability of Fe2O3 nanosheets. Inspired by our results, we can design and synthesize Fe2O3 or other electrodes with high performances according to their structure features in future.
Similar content being viewed by others
Introduction
3d transition-metal oxides, which can be used as anode materials, such as iron oxide, cobalt oxide, and nickel oxide have attracted a great deal of attentions for their much higher capacity than that of conventional graphite (372 mAhg−1)1,2,3,4,5,6,7,8,9,10,11,12. For instance, the theoretical capacity of Co3O4 is about 890 mAhg−1, which is almost two and a half times higher than that of graphite. However, high price and toxicity of Co limit the application of Co3O413,14. Interestingly, Fe2O3 also exhibits high capacity (1007 mAhg−1) like Co3O4. More importantly, due to its low cost, nontoxicity and high resistance to corrosion, Fe2O3 has attracted special attentions in recent years15,16. For example, various morphologies of Fe2O3 such as nanoparticles, nanotubes, hollow structure and thin films have been studied as electrodes for lithium-ion batteries17,18,19,20,21,22,23,24,25. Lou et al. prepared a series of hollow microspheres of iron oxides which showed significantly improved lithium-storage capabilities24,25. In addition to the above-mentioned methods, many studies have proved that the crystal plane structure of electrode materials has a significant effect on the electrochemical properties. Islam et al. reported that the (010) plane of LiFePO4 is a favorable plane for fast Li+ transport26. Wei et al. found that the electrochemical performance of lithium rich material Li(Li0.17Ni0.25 Mn0.58)O2 with (010) and (100) planes have been greatly increased, exhibiting not only a high reversible capacity but also an excellent cycle stability27. Huang et al. found the facet-dependent electrochemical properties of Co3O4 toward heavy metal ions and found that the Co3O4 nanoplates with (111) facet performed better electrochemical sensing capability than the Co3O4 nanocubes with (001) facet28. Not long ago, we also reported the facet-dependent electrochemical capability of Co3O4 as anode material for Li-ion batteries and proved the Co3O4 octahedron with exposed (111) plane exhibited more excellent electrochemical properties than that of Co3O4 cube with exposed (001) plane and Co3O4 truncated octahedron with exposed (001) and (111) planes29. Therefore, studies on the crystal plane controllable synthesis of nanomaterials are of great interest and are actively being pursued. So, controlling the exposed crystal plane of Fe2O3 might also be an effective strategy to further improve the electrochemical performance of Fe2O3 as anode materials for lithium-ion batteries.
In this article, we successfully synthesized two kinds of Fe2O3 with exposed different crystal plane, including nanorods with (001) and (010) plane and nanosheets with the (001) plane. Interestingly, when used as anode materials in lithium-ion batteries, Fe2O3 nanosheets exhibit better cycle performance and rate capabilities than that of Fe2O3 nanorods. To be specific, the discharge capacity of Fe2O3 nanosheets could stabilize at 865 mAhg−1 at the rate of 0.2C (1 C = 1000 mAg−1) and 570 mAhg−1 at the rate of 1.2 C over 80 cycles, which increased by 90% and 79% compared with 456 mAhg−1 and 318 mAhg−1 of Fe2O3 nanorodes. Herein, the outstanding electrochemical performance of Fe2O3 nanosheets can be attributed to the highly exposed (001) planes. Crystal structure have revealed that the (001) plane possesses larger packing density than that of (010) plane, and the crystal effect is the crucial reason for the differences of electrochemical performance30. On the other hand, potentiostatic intermittent titration (PITT) results show that Fe2O3 nanosheets have higher diffusion coefficient of Li+ (DLi) and are more favorable for the diffusion of lithium ion.
To the best of our knowledge, we, for the first time, combined electrochemical experiment and crystal structure analysis to elucidate exposed crystal plane-electrochemical properties relationship of Fe2O3 as anode for rechargeable lithium ion batteries. Our results indicate the superior electrochemical performances of Fe2O3 nanosheets can be attributed to (1) the larger packing density of Fe3+ and O2− of (010) plane and (2) the higher diffusion coefficient of Li+ (DLi) of Fe2O3 nanosheets during discharge-charge process. Furthermore, our results provide a idea which we can design and synthesize electrode materials with high performances according to their structure features in future.
Results
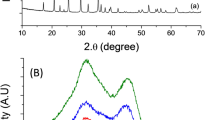
In Fig. 1, the indexed X-Ray Diffraction (XRD) patterns of Fe2O3 samples show that the diffraction peaks match well with the standard PDF card (JCPDS no. 86–2368), indicating the purity of the products and the two kinds of Fe2O3 belong to the same space group. The exposed facets of nanosheets and nanorodes have been determined by high resolution transmission electron microscopy (HRTEM) characterization in Fig. 2c–h. The clear lattice spacing and fast Fourier transform selected-area electron diffraction (FFT-SAED) patterns indicate that Fe2O3 nanosheets and nanorods are single crystalline. Figure 2c shows the TEM image of a Fe2O3 nanosheet, and the corresponding SAED pattern is shown in Fig. 2d. It can be clearly seen that the exposed crystal facet is perpendicular to the (3000), (0300) and (0030) facets of Fe2O3 nanosheets, and the interlayer spacings of 0.252 nm inserted in Fig. 1c correspond to the (110) plane of the Fe2O3. Thus it can be concluded that the exposed facets of the nanosheets are (001). Figure 2e and f show the similar interlayer spacings and SAED pattern compared with the Fe2O3 nanosheets, which indicate that one of the exposed facets of the Fe2O3 nanorods are (001). Figure 2g shows the TEM image of another facet of Fe2O3 nanorodes and the corresponding SAED pattern is shown in Fig. 2h. The SAED pattern in Fig. 2h shows that the exposed crystal facet is perpendicular to the (300), (006) and (202) facets of the Fe2O3, and the interlayer spacings of 0.210 nm inserted in Fig. 2g correspond to the (202) plane of the Fe2O3.
TEM images of Fe2O3 nanosheets (a) and Fe2O3 nanorods (b); (c,d) The TEM image of a Fe2O3 nanosheet, inset shows the lattice fringes and the corresponding SAED pattern; (e,f) The TEM image of Fe2O3 nanorode with {001} plane, inset shows the lattice fringes and the corresponding SAED pattern; (g,h) The TEM image of Fe2O3 nanorode with {010} plane, inset shows the lattice fringes and the corresponding SAED pattern.
So another exposed facets of the nanorodes are (010). The structural models of Fe2O3 nanorod is displayed in Fig. 3c, the exposed (001) and (010) crystal facets can be clearly shown and the models of Fe2O3 nanosheet is showed in Fig. 3f. Figure 3a,d show the SEM images of Fe2O3 nanorodes and nanosheets, respectively. It can be seen that the average length of Fe2O3 nanorods is about 500 nm, the width and thickness is about 50 and 15 nm, respectively. The average diameter and thickness of Fe2O3 nanosheets is about 200 nm and 15 nm, respectively. The Fe2O3 nanosheets and nanorods of nanosize can reduce the diffusion length of Li+ ions and increase reactivity of the material, which are very favorable for excellent electrochemical performances.
Subsequently, the comparison galvanostatic discharge capacities of Fe2O3 nanorods and nanosheets in a potential range of 0.1–3.0 V (vs Li/Li+) at a rate of 0.2 C were comprehensively investigated and illustrated in Fig. 4a–c. It can be seen from Fig. 4a that Fe2O3 nanorod and Fe2O3 nanosheet electrodes deliverer approximately a discharge capacity of 1135 mAhg−1 and 1210 mAhg−1 in the first cycle, respectively. After that the discharge capacity decreases rapidly, and the reason can be ascribed to the change of structure during the initial charge-discharge process. From the beginning of the second cycle, two kinds of Fe2O3 electrodes exhibit good cycle stability until to the 20 cycles. Surprisingly, after 20 cycles, the discharge capacity of the Fe2O3 nanorods monotonically decline, while the discharge capacity of Fe2O3 nanosheets slightly increase. Similar phenomenon also has been found in the case of CoO and Co3O4 as well as other Fe2O3 reports in the literature, though a clear understanding has not been obtained31,32,33. As shown in Fig. 4a, the discharge capacity of the Fe2O3 nanosheets maintains at 865 mAhg−1 with a capacity retention of 95.3% after 80 cycles, in contrast, the discharge capacity of Fe2O3 nanorods maintains at 456 mAh/g with a capacity retention of 50.7% after 80 cycles.
(a) Comparison of cycling performance of the two kinds of Fe2O3 at the rate of 0.2 C; The charge/discharge curves of (b) Fe2O3 nanorods and (c) Fe2O3 nanosheets in the 1st, 2nd, 20th, 60th and 80th cycles at the rate of 0.2 C, respectively. (d) The rate performances of Fe2O3 nanorods and Fe2O3 nanosheets. (e) Plots of the specific discharge capacity vs. cycle number for the Fe2O3 nanostructure electrode at the rate of 1.2 C; The discharge curves of (f) Fe2O3 nanorods and (g) Fe2O3 nanosheets in the 1st, 20th, 60th, 100th and 150th cycles at the rate of 1.2 C, respectively.
Figure 4d shows the rate performance of Fe2O3 nanorods and nanosheets. Specifically, the discharge capacity of Fe2O3 nanorods at 0.2, 0.4, 0.8, 1.2, 1.6, 2.0 and 2.4 C are 896, 763, 627, 544, 478, 430 and 385 mAhg−1, respectively. The corresponding values for the Fe2O3 nanosheets were 966, 832, 734, 667, 628, 586 and 550 mAhg−1, respectively. By comparing the discharge capacity of the two samples, Fe2O3 nanosheets display higher capacity than Fe2O3 nanorods at various charge−discharge rates from 0.2 to 2.4 C. Meanwhile, as the growth of charge–discharge current density, the gap between the discharge capacities of the Fe2O3 nanorods and nanosheets samples became larger. For instance, the discharge capacity of Fe2O3 nanosheets increase by 8% compared with that of Fe2O3 nanorods at 0.2 C, while the discharge capacity increase by 43% at the rate of 2.4 C. In addition, it should be noted when the rate was returned back to the 0.2 C, Fe2O3 nanosheets still show higher discharge capacity than that of Fe2O3 nanorods. At the recovery rate of 0.2 C, both Fe2O3 nanosheets and nanorods display lower discharge capacity compared with the initial capacity at 0.2 C. The phenomenon is due to the destruction of crystal structure of Fe2O3 during discharge-charge cycle process.
In order to research the cycle stability under high current density, the Fe2O3 nanorods and nanosheets are tested at the rate of 1.2 C, as shown in Fig. 4e–g. Obviously, the electrode of Fe2O3 nanosheets shows much higher discharge capacity than that of Fe2O3 nanorods at high rate. Especially, the discharge capacity of Fe2O3 nanosheets can reach 719 mAhg−1 after 150 cycles. This value is 71% higher than that of Fe2O3 nanorods, which only shows 419 mAhg−1 after 150 cycles.
SEM and TEM images of Fe2O3 nanosheets and nanorods samples after extensive cycling are shown in Fig. 5. It can be clearly seen in Fig. 5a,c that Fe2O3 nanosheets keep relatively complete sheet structure after extensive charge-discharge cycling. Similar to nanosheets, Fe2O3 nanorods also show well virgulate shape which can be seen in Fig. 5b,d. In addition, there is no significant change of the Fe2O3 particle size after charge-discharge cycling.
It is reported that the Brunauer–Emmett–Teller (BET) surface areas of electrode materials play a improtant role on the electrochemical performance of lithium ions batteries34. Our nitrogen-sorption analysis reveals that the BET specific surface areas of Fe2O3 nanorods and Fe2O3 nanosheets were 26.81 and 18.25 m2/g, respectively (Fig. 6). The BET specific surface areas of Fe2O3 nanorods is larger than that of Fe2O3 nanosheets, whereas, the Fe2O3 nanosheets exhibit better electrochemical properties compared with Fe2O3 nanorods. So it can be concluded that the effect of specific surface areas of electrodes on the difference of electrochemical properties between Fe2O3 nanosheets and nanorodes can be overlooked.
Evidently, the electrochemical performances of lithium ion batteries are related to the intrinsic crystal structure35. So the crystal structure of Fe2O3 is analyzed. For Fe2O3 samples, the (001) plane has been found possessing the larger packing density, in which Fe3+ and O2− ions pack layer by layer. Specifically, the packing densities of the Fe3+ and O2− are 9.11 nm−2 and 13.8 nm−2, respectively. In contrast, the packing densities of the (010) facets for ions are 2.89 nm−2 and 5.78 nm−2. Due to the high atomic density, more Fe3+ ions participate in the reaction, and lead to a high specific capacity28. The detailed crystal structure of Fe2O3 have been displayed in Fig. 7. Meanwhile, it can be seen from the model of Fe2O3 nanosheets and nanorodes in Fig. 7, that the proportion of (001) plane in nanosheet is almost 100%, while in nanorodes is about 23%. And the mainly exposed crystal plane is (010) facet in nanorodes, in which the proportion of (010) plane is about 77%. The results indicate that the Fe2O3 samples which exposed more (001) plane show a superior electrochemical capability.
The surface atomic configurations in (a) the (001) plane and schematic hematite structure projected along {001}, (b) the (010) plane and schematic hematite structure projected along {010}. The Fe2O3 Crystallographic Information File (CIF) was taken from the NIST/FIZ FindIt Inorganic Crystal Structure Database.
Figure 8 shows Nyquist plots of the two kinds of Fe2O3 electrode measured at the open circuit potential and an equivalent circuit proposed to fit the spectra. As can be seen from Table 1, the charge transfer resistances (Rct) for Fe2O3 nanosheets (53 Ω) is much smaller than that obtained from the Fe2O3 nanorodes (179 Ω) electrode. The electrochemical impedance spectroscopy (EIS) data indicates that Fe2O3 nanosheets possesses smaller lithium ion migration resistance and is more conducive to the rapid migration of lithium ions.
For the sake of confirming DLi in electrode materials, PITT measurement was performed. Figure 9a–c display the PITT results of Fe2O3 samples before discharge-charge cycle. It can be seen the DLi of Fe2O3 nanosheets is about one time higher than that of Fe2O3 nanorods. Figure 9d–f display the PITT results of Fe2O3 samples after a circle of discharge-charge cycle at the current density of 200 mA/g. It is obviously that DLi of Fe2O3 nanosheets are higher than that of Fe2O3 nanorods. For instance, the DLi average value of Fe2O3 nanosheets is 2.2 × 10−10 cm2 s−1 which increased by 15.7%, compared to 1.9 × 10−10 cm2 s−1 of Fe2O3 nanorods. The improved kinetic parameters DLi, indicate that Fe2O3 nanosheets possess higher lithium diffusion coefficient. For this reason, Fe2O3 nanosheets show better rate capability, as shown in Fig. 4d. Figure 4d show that Fe2O3 nanosheets with (001) planes possess higher discharge capacity not only at the low rate of 0.2 C but also at the high rate of 1.2 C. Additional, the Fe2O3 nanosheets with (001) planes exhibit better cycle stability and rate ability. Generally, the Fe2O3 nanosheets with (001) plane exhibit better electrochemical properties than that of the Fe2O3 nanorods with (010) and (001) planes.
PITT curves of (a) Fe2O3 nanosheets and (b) Fe2O3 nanorods and (c) diffusion coefficients of Li+ (DLi) before discharge-charge cycle; Current-time transient plots of PITT for a potential step of 1.0–0.8 V after one cycle, (d) I vs. t and (e) ln(I) vs. t; (f) DLi of Fe2O3 nanorods and Fe2O3 nanosheets during discharge processe.
In conclusion, we successfully synthesized two kinds of morphology of single crystal Fe2O3 with exposed different crystal plane, including nanorods with (001) and (010) plane and nanosheets with the (001) plane. Fe2O3 nanosheets exhibit better cycle performance and rate capabilities than that of Fe2O3 nanorods. The reasons can be attributed to that (1) the larger packing density of Fe3+ and O2− of (010) plane and (2) the higher diffusion coefficient of Li+ (DLi) of Fe2O3 nanosheets during discharge-charge process. Our studies indicate that the crystal structure has a very important influence on the electrochemical performances, which may be helpful for developing high performance lithium ion batteries.
Methods
Materials synthesis
The Fe2O3 nanorods were synthesized by FeOOH nanorods template. To prepare FeOOH nanorods precursors, 1.9 mmol of FeCl3·6H2O was put into 15 ml of deionized water to form a homogeneous solution, then added 15 ml of 0.8 M NaOH solution under stirring, quickly. After stirring for 10 min, the total solution was transferred into a 50 ml Teflon-lined stainless steel autoclave, sealed and heated at 180 °C for 4 h. The result product was collected by centrifugation, washed with deionized water and ethanol, then was dried at 80 °C and calcined at 250 °C for 2 h. The Fe2O3 nanosheets were synthesized based on the previous work28. 5.1 mmol of FeCl3·6H2O was dissolved in 35 ml of anhydrous ethanol, and 38.4 mmol of CH3COONa·3H2O was rapidly added into the solution with stirring. After about 5 min of stirring, all of the reactants were transferred into a 50 ml Teflon-lined stainless steel autoclave, sealed and heated at 200 °C for 22 h. The result product was collected by centrifuge, washed with deionized water and ethanol. Then dried at 80 °C and calcined at 250 °C for 2 h.
Characterization
XRD measurements were performed on a Persee XD2 X-ray diffractometer with Cu-Kα radiation (λ = 1.5418). The size and morphology of all of the samples were measured with a S-4800 HITACHI scanning electron microscope (SEM) and a JEM-2100 transmission electron microscope (TEM). The specific surface areas of the powders were collected by a Gemini V Brunauer-Emmett-Teller (BET).
Electrochemical Measurement
For electrochemical studies, working electrode was fabricated with mixing active material, acetylene black and polyvinylidene fluoride (PVDF) with weight ratio 2:1:1 using N-methylpyrrolidone (NMP) as solvent. The slurry was fully ground and pasted onto copper foil, and then the loaded copper foil was dried in a vacuum oven at 120 °C for 12 h. Lithium metal, celgard 2300 membrane and 1 M LiPF6 solution in DMC/EC (1: 1 in volume) were used as counter electrode, separator and electrolyte respectively to assemble coin cells in an Ar-filled glove box. The galvanostatic charge/discharge performance of the cells were tested on a battery testing system (BTS-5 V 5 mA, Neware) with the voltage between 0.1 and 3.0 V at the current density of 200, 400, 800, 1200, 1600, 2000, 2400 mA/g. The electrochemical spectroscopy (EIS) was tested with an (PGSTAT302N, Metrohm-Autolab) instrument using an amplitude of 5 mV and a frequency range from 100 KHz to 0.1 Hz. The PITT tests were also performed on the same instrument with EIS.
Additional Information
How to cite this article: Chen, M. et al. The Effect of Crystal Face of Fe2O3 on the Electrochemical Performance for Lithium-ion Batteries. Sci. Rep. 6, 29381; doi: 10.1038/srep29381 (2016).
References
Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon, J. M. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Mater 8, 500–506 (2009).
Luo, J. et al. Rationally Designed Hierarchical TiO2@Fe2O3 Hollow Nanostructures for Improved Lithium Ion Storage. Adv Energy Mater 3, 737–743 (2013).
Ji, X., Herle, S., Rho, Y. & Nazar, L. F. Carbon/MoO2 composite based on porous semi-graphitized nanorod assemblies from in situ reaction of tri-block polymers. Chem Mater 19, 374–383 (2007).
Chen, W., Li, S., Chen, C. & Yan, L. Self-Assembly and Embedding of Nanoparticles by in Situ Reduced Graphene for Preparation of a 3D Graphene/Nanoparticle Aerogel. Adv Mater 23, 5679 (2011).
Sun, X. et al. Three-Dimensionally “ Curved” NiO Nanomembranes as Ultrahigh Rate Capability Anodes for Li- Ion Batteries with Long Cycle Lifetimes. Adv Energy Mater 4 (2014).
Ji, X., Evers, S., Lee, K. T. & Nazar, L. F. Agitation induced loading of sulfur into carbon CMK-3 nanotubes: efficient scavenging of noble metals from aqueous solution. Chem Commun 46, 1658–1660 (2010).
Su, D., Dou, S. & Wang, G. Mesocrystal Co3O4 nanoplatelets as high capacity anode materials for Li-ion batteries. Nano Res 7, 794–803 (2014).
Lee, H. S. et al. An organometallic approach for microporous organic network (MON)-Co3O4 composites: enhanced stability as anode materials for lithium ion batteries. Chem Commun 48, 94–96 (2012).
Li, L., Guo, Z., Du, A. & Liu, H. Rapid microwave-assisted synthesis of Mn3O4-graphene nanocomposite and its lithium storage properties. J Mater Chem 22, 3600–3605 (2012).
Ding, Y.-L., Wen, Y., van Aken, P. A., Maier, J. & Yu, Y. Rapid and Up-Scalable Fabrication of Free-Standing Metal Oxide Nanosheets for High-Performance Lithium Storage. Small 11, 2011–2018 (2015).
Park, G. D., Lee, J.-H., Lee, J.-K. & Kang, Y. C. Effect of esterification reaction of citric acid and ethylene glycol on the formation of multi-shelled cobalt oxide powders with superior electrochemical properties. Nano Res 7, 1738–1748 (2014).
Rui, K., Wen, Z., Lu, Y., Jin, J. & Shen, C. One-Step Solvothermal Synthesis of Nanostructured Manganese Fluoride as an Anode for Rechargeable Lithium-Ion Batteries and Insights into the Conversion Mechanism. Adv Energy Mater 5 (2015).
Qiu, H.-J., Liu, L., Mu, Y.-P., Zhang, H.-J. & Wang, Y. Designed synthesis of cobalt-oxide-based nanomaterials for superior electrochemical energy storage devices. Nano Res 8, 321–339 (2015).
Li, G. et al. Controllable Synthesis of 3D Ni(OH)2 and NiO Nanowalls on Various Substrates for High-Performance Nanosensors. Small 11, 731–739 (2015).
Chen, J., Xu, L. N., Li, W. Y. & Gou, X. L. alpha-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv Mater 17, 582 (2005).
Sarradin, J., Guessous, A. & Ribes, M. Synthesis and characterization of lithium intercalation electrodes based on iron oxide thin films. J Power Sources 62, 149–154 (1996).
Larcher, D. et al. Effect of particle size on lithium intercalation into alpha-Fe2O3 . J Electrochem Soc 150, A133–A139 (2003).
Cao, K. et al. 3D Hierarchical Porous alpha-Fe2O3 Nanosheets for High-Performance Lithium-Ion Batteries. Adv Energy Mater 5 (2015).
Morales, J., Sanchez, L., Martin, F., Berry, F. & Ren, X. L. Synthesis and characterization of nanometric iron and iron-titanium oxides by mechanical milling: Electrochemical properties as anodic materials in lithium cells. J Electrochem Soc 152, A1748–A1754 (2005).
Larcher, D. et al. Combined XRD, EXAFS, and Mossbauer studies of the reduction by lithium of alpha-Fe2O3 with various particle sizes. J Electrochem Soc 150, A1643–A1650 (2003).
Zhang, L., Wu, H. B. & Lou, X. W. Iron-Oxide- Based Advanced Anode Materials for LithiumIon Batteries. Adv Energy Mater 4 (2014).
Jain, G., Balasubramanian, M. & Xu, J. J. Structural studies of lithium intercalation in a nanocrystalline alpha-Fe2O3 compound. Chem Mater 18, 423–434 (2006).
Wang, B., Chen, J. S., Wu, H. B., Wang, Z. Y. & Lou, X. W. Quasiemulsion-Templated Formation of alpha-Fe2O3 Hollow Spheres with Enhanced Lithium Storage Properties. J Am Chem Soc 133, 17146–17148 (2011).
Wang, B., Wu, H. B., Zhang, L. & Lou, X. W. Self-Supported Construction of Uniform Fe3O4 Hollow Microspheres from Nanoplate Building Blocks. Angew Chemi Int Edit 52, 4165–4168 (2013).
Islam, M. S., Driscoll, D. J., Fisher, C. A. J. & Slater, P. R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem Mater 17, 5085–5092 (2005).
Wei, G.-Z. et al. Crystal Habit-Tuned Nanoplate Material of LiLi1/3−2x/3NixMn2/3−x/3O2 for High-Rate Performance Lithium-Ion Batteries. Adv Mater 22, 4364 (2010).
Yu, X.-Y. et al. Facet-dependent electrochemical properties of Co3O4 nanocrystals toward heavy metal ions. Sci Rep 3 (2013).
Xiao, X. et al. Facile Shape Control of Co3O4 and the Effect of the Crystal Plane on Electrochemical Performance. Adv Mater 24, 5762–5766 (2012).
Lu, J. et al. Hematite nanodiscs exposing (001) facets: synthesis, formation mechanism and application for Li-ion batteries. J. Mater Chem A 1, 5232–5237 (2013).
Grugeon, S., Laruelle, S., Dupont, L. & Tarascon, J. M. An update on the reactivity of nanoparticles Co-based compounds towards Li. Solid State Sci 5, 895–904 (2003).
Chen, J. S. et al. Shape-Controlled Synthesis of Cobalt-based Nanocubes, Nanodiscs, and Nanoflowers and Their Comparative Lithium-Storage Properties. Acs Appl Mater Inter 2, 3628–3635 (2010).
Xu, J.-S. & Zhu, Y.-J. Monodisperse Fe3O4 and gamma-Fe2O3 Magnetic Mesoporous Microspheres as Anode Materials for Lithium-Ion Batteries. Acs Appl Mater Inter 4, 4752–4757 (2012).
Wang, Y., Takahashi, K., Shang, H. M. & Cao, G. Z. Synthesis and electrochemical properties of vanadium pentoxide nanotube arrays. J Phys Chem B 109, 3085–3088 (2005).
Wu, C., Yin, P., Zhu, X., OuYang, C. & Xie, Y. Synthesis of hematite (alpha-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J Phys Chem B 110, 17806–17812 (2006).
Acknowledgements
This work was supported by the Beijing Nova Program (Z141103001814065), the Youth Innovation Promotion Association CAS (2016152), National Science Foundation for Young Scholars of China (21201177), the State Key Project of Fundamental Research (2014CB931900 and 2012CB932504).
Author information
Authors and Affiliations
Contributions
X.X. designed the experiments. M.C. and E.Z. performed the experiments, D.C. supervised the experiments, X.X., M.C. and E.Z. collected and analyzed the data, and wrote the paper. Z.H. gave suggestion to revise the manuscript. All authors analyzed data, discussed the results, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, M., Zhao, E., Yan, Q. et al. The Effect of Crystal Face of Fe2O3 on the Electrochemical Performance for Lithium-ion Batteries. Sci Rep 6, 29381 (2016). https://doi.org/10.1038/srep29381
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29381
This article is cited by
-
New insights into improving electrochemical performances of LiNi0.5Mn0.5O2 cathode material by Li2ZrO3 coating and Zr4+ doping
Ionics (2019)
-
Improving the cycling performance, rate capacity, and thermal stability of LiCoO2 by doping high-valence ions into the Li+ site
Journal of Solid State Electrochemistry (2018)
-
Formation of iron oxide nanoparticles for the photooxidation of water: Alteration of finite size effects from ferrihydrite to hematite
Scientific Reports (2017)
-
Improvement in the electrochemical performance of a LiNi0.5Mn0.5O2 cathode material at high voltage
Journal of Applied Electrochemistry (2017)
-
Mg doping improving the cycle stability of LiNi0.5Mn0.5O2 at high voltage
Journal of Solid State Electrochemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.