Abstract
Cardiovascular disease is one of the leading causes of death in patients with advanced renal disease. The objective of this study was to investigate impairments in heart rhythm complexity in patients with end-stage renal disease. We prospectively analyzed 65 patients undergoing peritoneal dialysis (PD) without prior cardiovascular disease and 72 individuals with normal renal function as the control group. Heart rhythm analysis including complexity analysis by including detrended fractal analysis (DFA) and multiscale entropy (MSE) were performed. In linear analysis, the PD patients had a significantly lower standard deviation of normal RR intervals (SDRR) and percentage of absolute differences in normal RR intervals greater than 20 ms (pNN20). Of the nonlinear analysis indicators, scale 5, area under the MSE curve for scale 1 to 5 (area 1–5) and 6 to 20 (area 6–20) were significantly lower than those in the control group. In DFA anaylsis, both DFA α1 and DFA α2 were comparable in both groups. In receiver operating characteristic curve analysis, scale 5 had the greatest discriminatory power for two groups. In both net reclassification improvement model and integrated discrimination improvement models, MSE parameters significantly improved the discriminatory power of SDRR, pNN20, and pNN50. In conclusion, PD patients had worse cardiac complexity parameters. MSE parameters are useful to discriminate PD patients from patients with normal renal function.
Similar content being viewed by others
Introduction
Cardiovascular disease is the leading cause of mortality and morbidity in patients with chronic kidney disease or end-stage renal disease (ESRD) undergoing either hemodialysis or peritoneal dialysis (PD)1,2. Sudden cardiac death is one of the most common causes of mortality, with an estimated rate of around 7% per year in patients with ESRD3. One explanation for this high rate is autonomic nervous system dysfunction3,4. Both parasympathetic damage and sympathetic nerve overactivity are common in ESRD patients5. Furthermore, sympathetic nerve overactivity is associated with mortality and cardiovascular outcomes in ESRD patients6.
Analysis of variations in heart rate oscillation, commonly known as heart rate variability (HRV), is commonly used to assess autonomic function in clinical studies due to its simple and noninvasive approach7. Initially, HRV was derived by linear methods such as Fourier transform by using certain frequency ranges to represent sympathetic and parasympathetic activities7. This concept has been proved by one large scale study showing that HRV parameters from linear method could predict outcomes in patients with cardiovascular disease8. In recent years, newer methods based on nonlinear and nonstationary signal modeling have been developed and successfully applied9. The concept of heart rhythm complexity analysis using nonlinear methods including detrended fractal analysis (DFA) or multiscale entropy (MSE) is based on the assumption that a healthy system exerts meaningful complex control over time to maintain operation in an ever-changing environment10,11. Conversely, decreased complexity of heart rate dynamics has been demonstrated in patients with various diseases such as heart failure, stroke, sepsis, primary aldosteronism and critical illnesses requiring extracorporeal life support12,13,14,15,16. Compared to traditional HRV parameters based on linear methodology, heart rhythm complexity analysis has been shown to have a better predictive power for prognosis in patients with cardiovascular disease16,17.
Only few studies evaluate heart rhythm complexity in ESRD patients18,19,20. All these studies have used DFA or approximate entropy anaylsis to derive heart rhythm complexity19,20. So far, no study has reported the results of MSE analysis in ESRD patients. Besides, most of the studies evaluate the prognostic prediction in ESRD patients without enrolling control groups. Since renal impairment might have direct or indirect effect (through cardiovascular disease) on autonomic nervous system, in this study, we plan to enroll a group of ESRD patients without cardiovascular disease and a group of subjects with normal renal function to evaluate the direct effect of ESRD on heart rhythm complexity.
Results
Patients
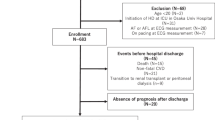
Sixty-five patients (39 men) undergoing PD without prior cardiovascular disease and 72 individuals (36 men) with normal renal function (control group) were enrolled in this study. The reason of PD implementation were variable primary glomerulonephritis in 47 patients , diabetic nephropathy in 9 patients, lupus nephritis in 3 patients, polycystic kidney disease in 2 patients and other causes in 4 patients.
The clinical data are shown in Table 1. The PD patients had significantly higher rates of using beta-blockers and calcium channel blockers than the controls. In addition, the PD patients had higher levels of serum fasting glucose and creatinine, and lower levels of sodium and potassium than the controls. The left ventricular ejection fraction was comparable in both groups.
Holter data
In linear analysis, the PD patients had a significantly lower standard deviation of normal RR intervals (SDRR), percentage of absolute differences in normal RR intervals greater than 20 ms (pNN20), the percentage of absolute differences in normal RR intervals greater than 50 ms (pNN50), low frequency (LF), high frequency (HF), and low/high frequency ratio than control participants (Table 2).
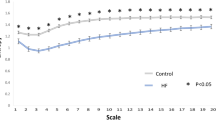
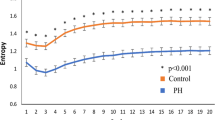
In DFA anaylsis, both DFA α1 and DFA α2 were comparable in both groups. The quantification of MSE parameters is shown in Fig. 1. The entropy over different time scales in the two group of patients is shown in Fig. 2. The PD patients had significant lower entropy in each time scale than control group . Of the nonlinear analysis indicators, scale 5, area under the MSE curve for scale 1 to 5 (area 1–5) and 6 to 20 (area 6–20) were significantly lower than those of the control group.
Four parameters of the MSE were assessed. The first was the linear-fitted slope between scales 1–5 (slope 1–5). The second was the entropy value of scale 5 (scale 5). The area under the curve between scale 1–5 (area 1–5) was used to represent complexity between short scales. For longer scales, the common profile of entropy gradually increased as the time scale increased and reached a plateau where information richness could be accumulated rapidly if the system responded well. We used the area under curve between scale 6–20 (area 6–20) to represent complexity between long scales.
Correlation between linear and MSE parameters
In analysis of all participants, scale 5, area 1–5 and area 6–20 were significantly correlated with both time and frequency domain parameters (Table 3). In contrast, slope 1–5 was only significantly correlated with frequency but not time domain parameters. In subgroup analysis, short time scales complexity parameters (scale 5 and area 1–5) were significantly correlated with pNN20 and HF in both groups. Long time scales complexity parameters (area 6–20) were also significantly correlated with very low frequency (VLF), LF and pNN20 in both groups. There were some differences in correlation patterns between the PD and control groups.
Comparisons of all linear and nonlinear parameters to differentiate the two groups
In receiver operating characteristic (ROC) curve analysis, scale 5 had the greatest discriminatory power for the two groups compared to all other linear and non-linear parameters (Fig. 3). The areas under the curve (AUC) of scale 5, SDRR, pNN50, PNN20, VLF, LF, HF, DFA α1, DFA α2, slope 1 to 5, area 1–5, area 6–20 were 0.806, 0.800, 0.667, 0.693, 0.584, 0.657, 0.603, 0.471, 0.485, 0.582, 0.786 and 0.771, respectively.
The advantage of adding MSE parameters to linear parameters to discriminate the two groups
In both net reclassification improvement (NRI) and integrated discrimination improvement (IDI) models, three MSE parameters (scale 5, area 1–5, and area 6–20) significantly improved the discriminatory power of SDRR, pNN20, and pNN50 (Table 4). Scale 5 was especially good in the models for pNN20 and pNN50.
Discussion
The major findings of this study were: 1) the PD patients had worse heart rhythm complexity than those with normal renal function; 2) in all linear and non-linear parameters, scale 5 and SDRR had the greatest single discriminatory power to detect the patients undergoing PD; 3) the combination of linear and non-linear improved the discriminatory power to differentiate PD patients from patients with normal renal function.
Traditional linear analysis of HRV is a useful tool to evaluate the autonomic system, and is commonly used to stratify the risk of patients with cardiovascular disease8,21. ESRD is characterized by a high prevalence of sudden cardiac death and autonomic nervous system dysfunction including parasympathetic damage and sympathetic nerve overactivity3,4,5. Therefore, HRV is also a useful tool to predict mortality and cardiovascular outcomes in ESRD patients22,23. In our results, the PD patients had significantly lower values of several linear parameters, which again reflect prominent autonomic dysfunction in ESRD patients.
DFA is a scaling analysis method to represent the correlation property of a signal. The physiological background of DFA has been demonstrated to be associated with a delicate interplay between sympathetic and vagal outflow24. The breakdown this correlation property occur only when sympathetic and vagal outflow are co-activated. For patients with ESRD, Suzuki et al. demonstrated the superiority of DFA analysis compared to traditional linear parameters to predict 5-year mortality rates in hemodialysis patients18. Furthermore, in the same study, the addition of scaling exponent α1 (a DFA parameter) to the clinical risk factors significantly improved the prediction of mortality. Although DFA is a predictor for clinical outcomes, in the current study, we showed that PD patients had comparable DFA parameters to the control patients. To our knowledge, no previous study has compared DFA parameters between patients with ESRD and those with normal renal function. Perhaps, ESRD patients without cardiovascular disease still maintain a certain degree of normal interaction (no co-activation) and thus preserve the nonlinear scaling behavior of heart rate dynamics.
Complexity is a concept that lies between periodicity and randomness. Heart rate dynamics is a complex system. The nonlinear methods viewed the healthy heart beat fluctuation as an output of an integrative control system with multiple interacting physiological processes operating at different time scales which emerge as complex dynamical patterns. When disease status developed, the system breakdown into either periodic-like14,16 (for example, heart failure) or random-like status25 (for example, atrial fibrillation). Recently, the MSE method, which was specifically developed to treat heterogeneous complexity, has shown the ability to extend the traditional entropy algorithm to quantify information richness over multiple time scales in physiological systems26. Slope 1–5, area 1–5, and scale 5 of MSE were the quantitative estimation of information richness over short timescales. The slope 1–5 may outline the structure of heart rate dynamics and negative slope was observed in patients with heart failure, atrial fibrillation or critical illness, indicating highly irregular but less information richness structure (i.e. uncorrelated fluctuations with the loss of feedback interactions)14,26. On the contrary, when the RR intervals of the individuals were entrained by respiration, the higher the respiratory modulated amplitude, the lower the entropy value expected. Since coarse-graining procedure over small timescales filtered out the periodic respiratory oscillations26, the sample entropies of heartbeat fluctuations increased over short timescales and slope 1–5, therefore, exhibited positive value. The area 1–5 in MSE probes the complexity structure of the heart rate dynamics and the scale 5 may give a powerful overall combined estimation of heart rate short-term complexity and the integrity of sinus arrhythmia26. The indexes of long-term scales were more controversial since several physiological mechanisms beneath the time scales. The important physiological mechanisms such as baroreflex and hormonal system can be the contributing factors15. For example, excess aldosterone not only cause cardiovascular damage27,28,29, but impaired both short-term and long-term scales of MSE15.
The usefulness of MSE is not limited to the cardiovascular system, and it has also been shown to be able to predict the outcomes of patients with severe trauma requiring treatment in an intensive care unit and across the diverse spectrum of traumatic injury30, the neurological outcomes of patients after stroke12, and the clinical consequences of sepsis13. However, no previous study has reported the results of MSE analysis in ESRD patients. In the present study, we found that PD patients had significantly decreased MSE parameters such as scale 5, area 1–5, and area 6–20 and these parameters significantly improved the predictive power of linear parameters. This demonstrates the additive effects of linear and non-linear parameters to differentiate PD patients from patients with normal renal function.
In the present study, scale 5 had the best discriminatory power in both groups. Similar findings have also been reported in heart failure and older patients11. Costa et al. found the largest separation between heart failure patients and healthy subjects is obtained for scale 5. Interesting, the strongest separation was also obtained for scale 5 for separation from elder from young subjects11. Taken together with our findings, this implies that scale 5 may be a good marker to detect a loss of complexity by age or disease status, although the underling mechanism is unclear. However, a marker that can differentiate patients from healthy participants may not be the same as a marker that can predict prognosis. For example, in heart failure patients, instead of short time scale parameter, long time scale parameters (area 6–20) have been shown to have the best prognostic predictive power16. Further studies are needed to elucidate whether scale 5 is also a prognosis marker in PD patients.
Patients with ESRD carry a higher risk of developing cardiovascular disease including vasculopathy and cardiomyopathy secondary to pressure and volume overload1,2,3. These could cause a loss of complexity in heart rate dynamics in patients with ESRD. However, in the present study, we enrolled patients without obvious cardiovascular disease therefore, the mechanism leading to deterioration of MSE are not secondary to it. Furthermore, we enrolled PD patients since the fluid status of hemodialysis patients vary a lot day by day and might interfere the measurements of HRV28. Even though the direct effect of uremic on autonomic nervous function is not clear, uremic toxins with a molecular weight of 300–12000, so called middle molecules were suggested to be the major etiology31. Studies also demonstrated that PD and hemodialysis with dialyzer membrane highly permeable to middle molecules could dramatically reduce the prevalent of uremic neuropathy32.
There are several limitations to this study. First, we only enrolled PD patients, and further studies are needed to validate whether our findings can be applied to hemodialysis patients. Second, this cross-sectional study aimed to investigate impairments in heart rhythm complexity in ESRD patients, and whether MSE parameters can predict the clinical prognosis is unclear.
In conclusion, the PD patients had impaired cardiac complexity. Scale 5 in MSE studies had the greatest discriminatory power to differentiate PD patients from patients with normal renal function. In addition, MSE parameters significantly improved the discriminatory power of linear parameters.
Methods
Patients
In this prospective, cross-sectional study, we enrolled 65 Taiwanese patients who received PD with conventional glucose-based lactate-buffered solutions (UltraBag; Baxter Healthcare SA, Singapore) and 72 participants (control group) who visited for health check-up at National Taiwan University Hospital.
The PD patients were enrolled prospectively for a cohort study. Some study results regarding echocardiography or laboratory data were published33,34. The inclusion criteria for patients group in this study were 1) received PD for more than 6 months; 2) no history of cardiovascular disease such as atrial fibrillation, significant valvular heart disease, coronary artery disease, myocardial infarction, heart failure, cerebrovascular events, or peripheral artery disease.
The participants in control group were prospectively enrolled for this study. The inclusion criteria were 1) estimated glomerular filtration rate >60 ml/min according to the Chinese Modification of Diet in Renal Disease Study equation35; 2) no history of cardiovascular disease such as atrial fibrillation, significant valvular heart disease, coronary artery disease, myocardial infarction, heart failure, cerebrovascular events, or peripheral artery disease.
A medical history of each participant including demographics and medications was carefully recorded and biochemical parameters were measured at the first evaluation. All patients underwent 24-h ambulatory ECG Holter recording (ZymedDigiTrak Plus 24-Hour Holter Monitor Recorder and Digitrak XT Holter Recorder 24 Hour, Philips, Amsterdam, Netherlands). Standard transthoracic echocardiography (iE33 xMATRIX Echocardiography System, Philips, Amsterdam, Netherlands) was performed in each patient. This study was approved by the Institutional Review Board of National Taiwan University Hospital, and all subjects provided written informed consent including for storage of their information in the hospital database and usage for research. The methods in the study were carried out in accordance with the approved guidelines.
Data pre-processing
An epoch of four hours of daytime RR intervals (between 9AM and 5PM) was selected for analysis36. The selected ECGs were automatically annotated using an automatic algorithm and carefully corrected by an experience technician.
Time and frequency domain analysis
SDRR was calculated and taken to represent the overall variability of autonomic modulation. Two pNNx parameters (pNN50 and pNN20) were calculated as the percentage of absolute differences in normal RR intervals greater than x ms. The threshold of x ms can filter out the RR changes with larger amplitudes, and better evaluate the function of the autonomic system37. The frequency domain parameters, high frequency (HF; 0.15–0.4 Hz), low frequency (LF; 0.04–0.15Hz), and very low frequency (VLF; 0.003–0.04) power, were also calculated by fast Fourier transformation according to the recommendations of the European Society of Cardiology and the North American Society of Pacing Electrophysiology7.
Nonlinear methods
The parameters derived from nonlinear methods were used to quantify the important characteristics of the physiological systems beyond variability such as scale-invariant (fractal behavior) underlying the signals that vary with time26,38.
DFA analysis
DFA can be used to evaluate the fractal behavior beneath the RR dynamics originating from well-regulated, interconnected systems38. The external trends related to environmental interference were fit linearly over different scales (e.g. 1, 2, 3… and n beats) and removed from the integrated time series. Fluctuations in each scale were calculated by summing up the detrended integrated time series. The slope (α exponent) of the logarithmic plot of fluctuations against time scales were computed and taken to indicate the fractal correlation property of the time series.
Short-term RR intervals are predominately modulated by respiratory sinus arrhythmia in normal subjects causing drastic changes in the slope between short- and long-term timescales. Crossover can be quantified by the α exponents of RR dynamics over short (α1; 4–11 beats) and long (α2; 11–64 beats) timescales to better probe the fractal property of the physiological system38.
MSE analysis
Instead of merely estimating the predictability of a time series with a single scale, MSE provides meaningful information richness embedded in different timescales by the degree of predictable sequential changes over the timescale26. The time series of different time scales were reconstructed using a coarse-graining process (i.e. averaging the non-overlapping n consecutive beats to form the new time series) and quantified by sample entropy39. The calculated entropy can then be used to represent the function of scale to assess the complex structure of the physiological signals, and the profile of the MSE curve can be used to assist the clinical categorization of several diseases26. In this study, four different parameters were calculated from the MSE profile: the entropy value of scale 5 (scale 5), the summation of entropy values of scales 1–5 (area 1–5) or 6–20 (area 6–20) to quantify the complexity of RR dynamics exhibited in short and long timescales. The linear-fitted slope of scale 1–5 (slope 5) was also calculated to characterize the regulatory behavior of the underlying system over a short timescale (Fig. 1).
To avoid underestimation of entropy due to external non-stationarity, the RR dynamics were detrended by removing any trend longer than 2 hours before performing MSE. The empirical mode decomposition (EMD) method, which is based on Hilbert-Huang transformation, was used to adaptively extract the trends and subtract them from the original RR interval signals40, since EMD algorithm can better approximate hidden trends in complex time series40,41,42.
Echocardiography
Standard transthoracic echocardiography (iE33 xMATRIX Echocardiography System, Philips, Amsterdam, Netherlands) was performed in each patient. The echocardiographic measurements included two-dimensional, M-mode and Doppler ultrasound recordings. Left ventricular dimension, interventricular septum and posterior wall thicknesses, and left ventricular ejection fraction (M-mode) were measured via a parasternal long axis view.
Statistical analysis
Continuous variables were presented as median (25th–75th percentile). Comparisons of continuous data between the PD and control groups were made using the Mann-Whitney U-test. Differences between proportions were assessed using the chi-square test or Fisher’s exact test. Correlation tests were performed using Spearman’s correlation tests.
In order to compare the ability of different Holter parameters to differentiate the PD patients from the control patients, we used area under the ROC curve analysis with a logistic regression models. We used C-statistics to describe the discrimination of the models before and after adding non-linear parameters43,44,45.
NRI and IDI models were used to assess improvements in prediction using two different logistic regression models44, with 0.2 and 0.4 used as the cutoff points. NRI is equal to sum of the increasing probability for survivors and decreasing probability for non-survivors subtracted by the decreasing probability for and increasing probability for non-survivors after adopting the updated model. IDI is defined as the average improvement of survival probability for all patients after adopting the updated model. All statistical analyses were performed using R software (http://www.r-project.org/), version 2.15.2. Statistical significance was set at p < 0.05.
Additional Information
How to cite this article: Lin, Y.-H. et al. Heart rhythm complexity impairment in patients undergoing peritoneal dialysis. Sci. Rep. 6, 28202; doi: 10.1038/srep28202 (2016).
References
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108, 2154–2169 (2003).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. American journal of kidney diseases: the official journal of the National Kidney Foundation 32, S112–119 (1998).
Herzog, C. A., Mangrum, J. M. & Passman, R. Sudden cardiac death and dialysis patients. Seminars in dialysis 21, 300–307 (2008).
Koomans, H. A., Blankestijn, P. J. & Joles, J. A. Sympathetic hyperactivity in chronic renal failure: a wake-up call. Journal of the American Society of Nephrology: JASN 15, 524–537 (2004).
Vita, G. et al. Uremic autonomic neuropathy studied by spectral analysis of heart rate. Kidney international 56, 232–237 (1999).
Zoccali, C. et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105, 1354–1359 (2002).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Tsuji, H. et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855 (1996).
Seely, A. J. & Macklem, P. T. Complex systems and the technology of variability analysis. Critical care 8, R367–384 (2004).
Yuan, H. K. et al. Acute increase of complexity in the neurocardiovascular dynamics following carotid stenting. Acta Neurol Scand 123, 187–192 (2011).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of complex physiologic time series. Physical review letters 89, 068102 (2002).
Tang, S. C. et al. Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke. Journal of neurology, neurosurgery, and psychiatry 86, 95–100 (2015).
Ahmad, S. et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 4, e6642 (2009).
Lin, Y. H. et al. Multi-scale symbolic entropy analysis provides prognostic prediction in patients receiving extracorporeal life support. Critical care 18, 548 (2014).
Lin, Y. H. et al. Reversible heart rhythm complexity impairment in patients with primary aldosteronism. Scientific reports 5, 11249 (2015).
Ho, Y. L., Lin, C., Lin, Y. H. & Lo, M. T. The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One 6, e18699 (2011).
Makikallio, T. H. et al. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. The American journal of cardiology 87, 178–182 (2001).
Suzuki, M. et al. Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clinical journal of the American Society of Nephrology: CJASN 7, 1454–1460 (2012).
Fukuta, H. et al. Prognostic value of nonlinear heart rate dynamics in hemodialysis patients with coronary artery disease. Kidney international 64, 641–648 (2003).
Kojima, M. et al. Loss of fractal heart rate dynamics in depressive hemodialysis patients. Psychosomatic medicine 70, 177–185 (2008).
La Rovere, M. T. et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107, 565–570 (2003).
Hathaway, D. K. et al. Autonomic dysregulation in patients awaiting kidney transplantation. American journal of kidney diseases: the official journal of the National Kidney Foundation 32, 221–229 (1998).
Hayano, J. et al. Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 14, 1480–1488 (1999).
Tulppo, M. P. et al. Physiological background of the loss of fractal heart rate dynamics. Circulation 112, 314–319 (2005).
Goldberger, A. L., Peng, C. K. & Lipsitz, L. A. What is physiologic complexity and how does it change with aging and disease? Neurobiology of aging 23, 23–26 (2002).
Costa, M., Goldberger, A. L. & Peng, C. K. Multiscale entropy analysis of biological signals. Physical review. E, Statistical, nonlinear, and soft matter physics 71, 021906 (2005).
Lin, Y. H. et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 221, 154–159, doi: S0021-9150(11)01133-6 (2012).
Lin, Y. H. et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J Hypertens 30, 1606–1613 (2012).
Lin, Y. H. et al. Reversal of myocardial fibrosis in patients with unilateral hyperaldosteronism receiving adrenalectomy. Surgery 150, 526–533 (2011).
Riordan, W. P., Jr., Norris, P. R., Jenkins, J. M. & Morris, J. A., Jr. Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. The Journal of surgical research 156, 283–289, doi: 10.1016/j.jss.2009.03.086 (2009).
Babb, A. L., Ahmad, S., Bergstrom, J. & Scribner, B. H. The middle molecule hypothesis in perspective. American journal of kidney diseases: the official journal of the National Kidney Foundation 1, 46–50 (1981).
Man, N. K. et al. One year follow-up of short dialysis with a membrane highly permeable to middle molecules. Proceedings of the European Dialysis and Transplant Association . European Dialysis and Transplant Association 10, 236–246 (1973).
Ho, L. C. et al. Visceral fat area is associated with HbA1c but not dialysate-related glucose load in nondiabetic PD patients. Scientific reports 5, 12811 (2015).
Wu, C. K. et al. Left ventricular diastolic dysfunction in peritoneal dialysis: a forgotten risk factor. Medicine 94, e819 (2015).
Kong, X. et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 28, 641–651 (2013).
Vanoli, E. et al. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation 91, 1918–1922 (1995).
Mietus, J. E., Peng, C. K., Henry, I., Goldsmith, R. L. & Goldberger, A. L. The pNNx files: re-examining a widely used heart rate variability measure. Heart 88, 378–380 (2002).
Peng, C. K., Havlin, S., Stanley, H. E. & Goldberger, A. L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87 (1995).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. American journal of physiology. Heart and circulatory physiology 278, H2039–2049 (2000).
Wu, Z., Huang, N. E., Long, S. R. & Peng, C. K. On the trend, detrending, and variability of nonlinear and nonstationary time series. Proceedings of the National Academy of Sciences of the United States of America 104, 14889–14894 (2007).
Peng, C. K., Costa, M. & Goldberger, A. L. Adaptive Data Analysis of Complex Fluctuations in Physiologic Time Series. Adv Adapt Data Anal 1, 61–70 (2009).
Lo, M. T., Novak, V., Peng, C. K., Liu, Y. & Hu, K. Nonlinear phase interaction between nonstationary signals: a comparison study of methods based on Hilbert-Huang and Fourier transforms. Physical review. E, Statistical, nonlinear, and soft matter physics 79, 061924 (2009).
Pencina, M. J., D’Agostino, R. B., Sr., D’Agostino, R. B., Jr. & Vasan, R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27, 157–172; discussion 207–112 (2008).
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21, 128–138 (2010).
Pencina, M. J. & D’Agostino, R. B. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 23, 2109–2123 (2004).
Acknowledgements
We thank Ms. Fen-Fun Hsien for medical technological support and the staff of the Second Core Lab of the Department of Medical Research in National Taiwan University Hospital for technical assistance. This study was supported by National Taiwan University Hospital (NTUH 104-S2696, UN103-065), Ministry of Science and Technology (MOST 103-2220-E-002 -011, MOST 103-2221-E-008-006-MY3). M.T.L. and C.L. gratefully acknowledge the support from Ministry of Science and Technology (Grant No. 104-3115-E-008 -001 and 104-2745-B-008 -001) and the joint foundations (Grant No 103CGH-NCU-A1, VGHUST103-G1-3-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.H.L., L.Y.L., K.Y.H. and J.W.H. conceived and designed the experiments. C.L., V.C.W., Y.H.H., L.Y.D.L. and M.T.L. analyzed the data. Y.H.L. wrote the paper. C.L. and C.K.P. made scientific comments on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, YH., Lin, C., Ho, YH. et al. Heart rhythm complexity impairment in patients undergoing peritoneal dialysis. Sci Rep 6, 28202 (2016). https://doi.org/10.1038/srep28202
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28202
This article is cited by
-
Heart rhythm complexity analysis in patients with inferior ST-elevation myocardial infarction
Scientific Reports (2023)
-
Real-time machine learning model to predict in-hospital cardiac arrest using heart rate variability in ICU
npj Digital Medicine (2023)
-
Heart rhythm complexity as predictors for the prognosis of end-stage renal disease patients undergoing hemodialysis
BMC Nephrology (2020)
-
Usefulness of heart rhythm complexity in heart failure detection and diagnosis
Scientific Reports (2020)
-
Preoperative Heart Rate Variability as Predictors of Vagus Nerve Stimulation Outcome in Patients with Drug-resistant Epilepsy
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.