Abstract
Doxorubicin (Dox), one of the most effective chemotherapy drug for cancer treatment, is limited by its severe side effects and chemoresistance. Dox induces DNA damage and leads to significant proteomic changes in the cancer cells, which makes the ubiquitin-proteasome system a potential target to enhance the efficacy of Dox therapy. The unsuccessful clinical trials of proteasome inhibitor PS-341 (bortezomib) in solid tumors led to the invention of MLN9708 (ixazomib), an orally bioavailable next-generation proteasome inhibitor with improved pharmacokinetic and pharmacodynamic features. In this preclinical study, we used eight human breast cancer cell lines, which represent the major molecular subtypes of breast cancer, to validate the cytotoxic effects of MLN9708, alone and in combination with Dox. We found that MLN9708 had cytotoxic effects, induced autophagy and MKP-1 expression, and enhanced Dox-induced apoptosis in these cell lines. MLN9708 also enhanced Dox-induced JNK and p38 phosphorylation and inhibited Dox-induced IκBα degradation. Our in vitro results suggest that MLN9708 has antitumor effects in breast cancer and can sensitize breast cancer cells to Dox treatment. This promising combination may be an effective and feasible therapeutic option for treating breast cancer and warrants clinical validation.
Similar content being viewed by others
Introduction
Doxorubicin (Dox)-containing adjuvant cytotoxic chemotherapy is considered the standard of care for breast cancer, according to the 2015 National Comprehensive Cancer Network guidelines1,2. As an anthracycline antibiotic, Dox works in all phases of the cell cycle. This topoisomerase II poisoning regimen has been widely used in anticancer therapies. Dox interferes with DNA synthesis, induces DNA damage, produces reactive oxygen species, and destroys membrane structure in the treated cells3,4,5,6. However, severe side effects, such as life-threatening cardiotoxicity, strictly limit Dox dosage7. Thus, other reagents or small molecules that can enhance the therapeutic effects of Dox are highly desirable and are being actively assessed in the laboratory and in the clinical setting8.
Studies show that the cytotoxic effects of Dox cause significant ubiquitin-proteasome system-mediated proteomic changes which are vital for cell survival in the treated cells9,10. The proteasome (multicatalytic proteinase complexes in eukaryotic cells) is responsible for the regulation and degradation of most intracellular proteins, some of which mediate cell-cycle progression and apoptosis, such as cyclins, caspases, and nuclear factor of κB (NF-κB)11. The NF-κB family of transcription factors plays critical roles in controlling inflammation, the immune response, and anti-apoptotic responses12,13. Inhibiting the activation of NF-κB promotes cell death, which has become a promising anticancer strategy14. Several studies have verified that inhibiting the proteasome can suppress the degradation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB), which inhibits NF-κB nuclear translocation and activation15,16. The proteasome system also plays an important role in the regulation of DNA damage response and is highly involved in the DNA repair process17,18. Additionally, because of their genetic instability and rapid proliferation, cancer cells tend to be more dependent on the proteasome than normal cells for the removal of aberrant intracellular proteins10,11. Therefore, functional inhibition of proteasome activity may disturb numerous cellular activities and lead to cancer cell death.
The first generation proteasome inhibitor PS-341 (bortezomib) has been approved by the United States Food and Drug Administration (FDA) for the treatment of many hematological malignancies. However, the results from clinical trials indicate that PS-341 and PS-341–containing therapies are not effective for the treatment of solid tumors including breast cancer due to the inability of PS-341 to penetrate into tumors and achieve therapeutically relevant concentrations in tumor19,20,21,22.
MLN9708 (ixazomib), the next-generation proteasome inhibitor, has been shown to have potent anticancer activity in both hematologic and solid tumor xenograft models with better pharmacokinetic and pharmacodynamic features than PS-34123. MLN9708 can be orally administrated, which is more convenient for clinical practice. Accumulating evidence indicates that MLN9708 could be a possible therapy for the treatment of solid tumors including breast cancer24,25.
Until now, the potential therapeutic effects of MLN9708 on breast cancer remain unknown23. In this preclinical study, by using a panel of breast cancer cell lines including T47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549 (representing ER/PR+/−, HER2+, or triple negative, respectively) (Table 1)26,27,28, we examined the cytotoxic effects of MLN9708 and whether MLN9708 could sensitize breast cancer cells to Dox-induced apoptosis.
Results
MLN9708 suppresses the proliferation of breast cancer cells
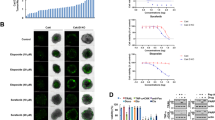
To assess the antitumor effect of MLN9708 on breast cancer cells, we selected eight breast cancer cell lines (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549), which represent the major molecular subtypes of breast cancer (Table 1)26,27,28. All cells were incubated with medium alone (control) or were treated with MLN9708 at the indicated concentrations (0.001 μM–10 μM) for 72 h and were subjected to a Cell Counting Kit-8 (CCK-8) assay. MLN9708 reduced the viability of all types of breast cancer cells in a dose-dependent manner (Fig. 1a). The cytotoxic effect of MLN9708 was confirmed by morphological images of the cells after treatment for 72 h (Fig. 1b). Since the median inhibitory concentration (IC50) values of MLN9708 were around the doses of 0.1 μM and 0.3 μM within all cell lines, we only show data from samples treated with these two doses.
(a) Cytotoxic effect of MLN9708 on breast cancer cells. Eight human breast cancer cell lines (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were incubated with medium alone or were treated with MLN9708 (0.001 μM, 0.003 μM, 0.01 μM, 0.03 μM, 0.1 μM, 0.3 μM, 1 μM, 3 μM, or 10 μM) for 72 h and then were subjected to a Cell Counting Kit-8 (CCK-8) assay. The absorbance of each well was measured at 450 nm, and the cell viability curve was plotted. Data were represented as means ± standard deviations (SD). Median inhibitory concentration (IC50) values of MLN9708 in breast cancer cell lines were listed. (b) Photographs of treated cells (magnification, ×200). (c) Colony formation of breast cancer cells treated with MLN9708. Cells were seeded in 6-well plates (5 × 103 per well), were incubated with medium alone or with MLN9708 (0.1 μM or 0.3 μM) for 72 h, and then were cultured in drug-free medium for 2 weeks. The cell colonies were fixed, were stained with crystal violet, and were photographed.
To validate the effect of MLN9708 on cell growth, we performed cell colony formation assays. The cells were incubated with medium alone or were treated with MLN9708 at concentrations of 0.1 μM or 0.3 μM for 72 h and then were cultured in drug-free medium for 2 weeks. MLN9708-treated cells had remarkably less proliferation potenital than the control groups (Fig. 1c). These data indicate that MLN9708 had a potent inhibitory effect on breast cancer proliferation, regardless of molecular subtype.
MLN9708 impairs the anchorage-independent growth of breast cancer cells
Cancer cells can grow colonies by forming a three-dimensional sphere in soft agar, a process called anchorage-independent growth. To assess whether MLN9708 could inhibit the anchorage-independent growth of breast cancer cells, we performed soft agar assays. Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were cultured with MLN9708 (0.1 μM or 0.3 μM) for 3 weeks. Then the visible colonies were fixed and stained. Untreated cells were used as controls. Because HCC1954 and SK-BR-3 cells did not form visible colonies in soft agar assay, data from these cell lines are not shown. The MLN9708-treated groups had fewer colonies than the control groups in the tested cell lines (Fig. 2a). MLN9708 significantly inhibited colony formation in a dose-sensitive manner in the tested cells (Fig. 2b).
(a) Cell anchorage-independent growth ability was assessed by soft agar assay. Six breast cancer cell lines (T-47D, MCF7, MDA-MB-361, MDA-MB-468, MDA-MB-231, and BT-549) were incubated with MLN9708 (0 μM, 0.1 μM, or 0.3 μM) in soft agar plates for 3 weeks. Cells were then stained with crystal violet and were photographed. (b) The colonies were counted, and the data were plotted. Data were represented as means ± standard deviations (SD). *P < 0.05, **P < 0.01, and ***P < 0.001 (analysis of variance and Dunnett multiple comparison post-test).
MLN9708 induces apoptosis in breast cancer cells
Studies have reported that MLN9708 induces apoptosis in many types of tumors, including multiple myeloma and lymphoma29,30. To determine whether MLN9708 induces apoptosis in human breast cancer cells, we treated the eight breast cancer cell lines with MLN9708 at different concentrations (0 μM, 0.03 μM, 0.1 μM, 0.3 μM, or 1 μM) for 24 h. Cells were then harvested and subjected to immunoblotting assays. Since MCF7 cells are Caspase 3 deficient, we measured Caspase 7 levels for this cell line. We found that MLN9708 induced poly (ADP-ribose) polymerase (PARP) and Caspase 3 (or Caspase 7) cleavage in the tested cell lines in a dose-dependent manner (Fig. 3a–h). These results suggest that MLN9708 alone triggered apoptosis in breast cancer cells.
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with MLN9708 (0.03 μM, 0.1 μM, 0.3 μM, or 1 μM) for 24 h. Untreated cells were used as controls. Whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with antibodies against PARP and Caspase 3 (or Caspase 7) to detect apoptosis. β-actin was used as a loading control.
MLN9708 induces autophagy activation in breast cancer cells
Because studies have proposed induction of autophagy as a resistance mechanism to proteasome inhibitor treatment in breast cancer cells31,32, we performed immunoblotting analysis to determine how MLN9708 treatment affects the autophagy machinery. Cells were treated with MLN9708 (0.1 μM) for 24 h and then were incubated with drug-free medium for 24 h, 48 h, or 72 h. The results show that MLN9708 induced autophagy marker LC3A/B-I/II expression in the cell lines tested (Fig. 4a–h). The response of individual cell lines to the addition or withdrawal of MLN9708 varied (Fig. 4a–h). The results suggest that autophagy machinery plays an important role in proteasome inhibitor treatment resistance in breast cancer cells.
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with MLN9708 (0.1 μM) for 24 h and then were cultured with drug-free medium for 24 h, 48 h, or 72 h. Then whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with antibodies against LC3A/B-I/II to detect autophagy. β-actin was used as a loading control.
MLN9708 induces MKP-1 expression in breast cancer cells
Because several studies have suggested that the buildup of mitogen-activated protein kinase phosphatase-1 (MKP-1) may account for the chemoresistance of cancer cells to proteasome inhibitors33,34,35, we assessed the effect of MLN9708 on the induction of MKP-1 expression in the eight breast cancer cell lines. Cells were cultured in medium alone or with MLN9708 (1 μM) for 4 h, 8 h, 16 h, or 24 h. We found that MLN9708 induced MKP-1 expression (Fig. 5a–h).
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with MLN9708 (1 μM) for 4 h, 8 h, 16 h, or 24 h. Untreated cells were used as controls. Then whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with antibodies against MKP-1. β-actin was used as a loading control.
MLN9708 enhances the cytotoxic effect of Dox on breast cancer cells
To determine whether MLN9708 enhances the cytotoxic effects of Dox in breast cancer cells, we cultured the cells in increasing concentrations of Dox alone or in combination with MLN9708 (0.1 μM [0.3 μM for BT-549 cells]) for 48 h and assessed the cell viability using CCK-8 assay. Cells in the combination groups had much lower viability than cells treated with Dox alone (Fig. 6a–h). These findings imply that MLN9708 sensitized breast cancer cells to Dox-mediated cytotoxicity.
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with Dox at the indicated concentrations with or without MLN9708 (0.1 μM [0.3 μM for BT-549 cells]) for 48 h. Cell viability was then measured by Cell Counting Kit-8 (CCK-8) assay. Data were represented as means ± standard deviations (SD). *P < 0.05, **P < 0.01, and ***P < 0.001 (t-test).
MLN9708 enhances Dox-induced apoptosis in breast cancer cells
To explore whether MLN9708 enhances Dox-induced cell apoptosis in breast cancer cells, we treated the cells with Dox alone (1 μM) or in combination with MLN9708 (0.1 μM) for 16 h or 24 h. Untreated cells were used as controls. Immunoblotting results demonstrated that MLN9708 enhanced Dox-induced PARP and Caspase 3 (or Caspase 7) cleavage in all subtypes of breast cancer cells tested (Fig. 7a–h). These findings indicate that MLN9708 intensified Dox-induced apoptosis in breast cancer cells.
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with Dox (1 μM) alone or in combination with MLN9708 (0.1 μM) for 16 h or 24 h. Untreated cells were used as controls. Then whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with antibodies against PARP and Caspase 3 (or Caspase 7) to detect apoptosis. β-actin was used as a loading control.
MLN9708 inhibits Dox-induced IκBα degradation
Both the NF-κB and mitogen-activated protein kinases (MAPK) pathways are important for regulating cell proliferation and survival36,37,38. Studies have shown that Dox induces NF-κB and MAPK activation and that proteasome inhibitors inhibit the activation of NF-κB39,40,41. To clarify this interaction, we assessed the effects of the combination of MLN9708 with Dox on the activity of NF-κB and MAPK in the eight breast cancer cell lines. Cells were cultured with Dox alone or in combination with MLN9708 (1 μM) for 2 h, 3 h, or 4 h. Untreated cells were used as controls. MLN9708 enhanced Dox-induced c-Jun N-terminal kinase (JNK) and p38 phosphorylation but suppressed Dox-induced IκBα degradation (Fig. 8a–h).
(a–h) Breast cancer cells (T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549) were treated with Dox (20 μM) alone or in combination with MLN9708 (1 μM) for 2 h, 3 h, or 4 h. Untreated cells were used as controls. Then whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with antibodies against p-JNK, JNK, p-p38, p38, and IκBα. β-actin was used as a loading control.
Discussion
Studies have revealed that inhibiting the proteasome activity disturbs the regulation and degradation of most intracellular proteins, including cyclins, cell-cycle–dependent kinase inhibitors, and proapoptotic proteins18. Moreover, the buildup of incorrectly folded proteins and proteins with a short half-life (mostly with regulatory functions) leads to cell destruction16. In this study, we examined the cytotoxic effects of MLN9708, the next-generation proteasome inhibitor, in breast cancer cells. We found that MLN9708 exhibited general antitumor activity in eight breast cancer cell lines.
Breast cancer is a heterogeneous group of diseases and is classified into several major subtypes, including the luminal type that expresses estrogen receptor, the HER2–enriched type, and basal-like breast cancers that comprise most triple-negative tumors26,27. Each subtype may have a specific response to different drugs. We investigated the general effects of MLN9708 on a panel of eight breast cancer cell lines, which represent the major subtypes of breast cancer (ER/PR+/−, HER2+, or triple-negative). We found that MLN9708 was cytotoxic in breast cancer cells in a dose-dependent manner (Fig. 1) but with varying efficacy. The IC50 values of MLN9708 in the luminal subtypes (T-47D, MCF7, and MDA-MB-361) were less than 0.1 μM, indicating that luminal (ER+) cancer cells are sensitive to MLN9708. This observation suggests that MLN9708 could be a complementary single agent for current endocrine therapy with reasonable toxicity. Triple-negative cells (MDA-MB-468, MDA-MB-231, and BT-549) had higher IC50 values and varied more dramatically than the other two major subtypes, indicating that MLN9708 would have less efficacy as a single-agent regimen for these patients than for those with other subtypes (Fig. 1). The IC50 values of HER2 + cells (SK-BR-3 and HCC1954) were intermediate (ranging from 0.05 μM to 0.5 μM), suggesting that patients with HER2 + breast cancer might benefit from MLN9708-containing therapy. IC50 values are dependent on the conditions under which they are measured, such as the number of cells in the sample and treatment duration. Our analysis roughly reflects the sensitivity of cell lines to MLN9708 treatment at 5,000 cells per well for 72 h.
Tumor heterogeneity characterizes cancer cells that display distinguishable phenotypic features, including cellular morphology, gene expression profile, metabolism, motility, and proliferation potential42,43. Although the IC50 values of MLN9708 on T-47D and BT-549 cells were different, the colony formation of these two cell lines treated with MLN9708 (0.1 μM or 0.3 μM) was similar (Fig. 1). We also found that MCF7, MDA-MB-361, and SK-BR-3 cells had similar MLN9708 IC50 values (Fig. 1a), but their colony formation results varied, especially when treated with 0.1 μM of MLN9708 (Fig. 1c). These differences might be due to variation of the diversity of cancer cell lines in cell size, morphology, doubling time, etc. The numbers of living cells from different cell lines differed after treatment with MLN9708 for 72 h. In addition, two weeks later with drug-free medium culture, the proliferation rate of the cell lines also varied. All of these factors contributed to the variations of the colony formation potential of the breast cancer cells. Regardless, MLN9708 inhibited the proliferation, colony formation, and anchorage-independent growth of breast cancer cells in a dose-dependent manner (Figs 1 and 2).
Dox treatment interferes with many intracellular biological reactions and simultaneously generates countless molecules, such as unfolded or misfolded proteins, peptides, and lipoproteins. To maintain normal cellular function and viability, the proteasome will degrade most of this cellular waste after ubiquitination10,11,15. When the degradation or removal process of this cellular waste is prolonged, cells die. As expected, several studies have demonstrated that proteasome inhibition led to the inhibition of the general DNA damage response and other vital processes17,18,44,45,46. These findings theoretically support the ability of proteasome inhibitors to sensitize cancer cells to other cytotoxic agents. Although the mechanism of the effects of two cytotoxic agents on cancer cells is difficult to be determined owing to the complexity of the cellular response, it is reasonable and feasible to use the end results, i.e., the killing of cancer cells, as an indicator to identify effective treatment regimens.
As shown in our studies, MLN9708 enhanced Dox-induced cytotoxicity (Fig. 6) and Dox-induced apoptosis in breast cancer cells that represent the major subtypes of breast cancer (Fig. 7). In addition to sensitizing breast cancer cells to Dox, MLN9708 also might lower the effective dose of Dox and achieve similar or better therapeutic effects with fewer side effects.
JNK and p38 are the master mediators for chemotherapeutic drug-induced cell death47,48. When we used JNK and p38 to assess cellular stress leading to apoptosis40, we found that MLN9708 enhanced Dox-induced phosphorylation of JNK and p38 (Fig. 8). The activation of NF-κB plays a pivotal role in promoting oncogenesis and resistance to chemotherapy49,50,51,52,53. NF-κB was reported to be activated in response to genotoxic stresses, such as Dox or VP16 treatment, and inhibition of NF-κB activation enhanced the effect of cytotoxic agents-induced cell death39,49,50. In support of these previous findings, we found that MLN9708 inhibited Dox-induced IκBα degradation (Fig. 8), which might block the translocation of NF-κB to the nucleus and the activation of NF-κB-dependent anti-apoptotic gene expression.
Although our study demonstrated that MLN9708 has potent cytotoxicity, potential mechanisms of chemoresistance to MLN9708 should be considered. Activation of autophagy has been proposed as a resistance mechanism to proteasome inhibitor MG132 and PS-341 treatment in breast cancer cells31,32, which suggests that combination with an autophagy inhibitor would be beneficial. We also observed that MLN9708 induced autophagy (Fig. 4). Compared with the other cell lines, cell lines with higher MLN9708 IC50 values (HCC1954, MDA-MB-231, and BT-549) had a high level of basal autophagy, which supports the findings of Gavilán et al.31. Other studies have suggested that the buildup of MKP-1 may account for chemoresistance of cancer cells to proteasome inhibitors33,34,35. Supporting these studies, we observed that MLN9708 induced buildup of MKP-1 (Fig. 5), which suggests that combining MLN9708 with appropriate regimens against chemoresistance could improve outcomes in cancer therapy.
Based on the previous studies with proteasome inhibitors and Raymond’s proteotoxic crisis hypothesis10,18,54, together with our observations in this study, we propose a working model regarding the role of proteasome inhibitors in cancer cells (Fig. 9). In this model, the balanced relationship between intracellular proteins and the functional proteasome in living cells is called proteostasis (Fig. 9a). When the cells are treated with a proteasome inhibitor, proteostasis is difficult to be maintained due to the impairment of the proteasome, which leads to increased accumulation of intracellular proteins and creates a crisis of proteostasis (Fig. 9b). DNA damage-inducing stimuli or other treatments greatly change the qualities, quantities, or distribution of intracellular proteins, which challenges the working capacity of the proteasome (Fig. 9c). The combination of a proteasome inhibitor with conventional cytotoxic drugs further challenges the processing ability of the proteasome, which ultimately leads to cell death (Fig. 9d). Our working model simplifies the possible therapeutic mechanisms of combining proteasome inhibitors with other conventional cytotoxic agents.
(a) The balance between intracellular proteins and the functional proteasome in living cells. (b) Loss of balance in cells treated with proteasome inhibitor MLN9708. (c) Doxorubicin (Dox)-induced changes of intracellular proteins challenge the working capacity of the proteasome. (d) Combination of MLN9708 with Dox further stresses the cells and causes apoptosis.
In conclusion, our findings demonstrate for the first time that the second-generation proteasome inhibitor MLN9708 is cytotoxic to various breast cancer cells (ER/PR+/−, HER2+, or triple-negative) and can induce cell death. MLN9708 enhances the anticancer effects of Dox by upregulating Dox-induced activation of JNK and p38 and inhibiting Dox-induced NF-κB activation. Our findings suggest that this combination strategy might be beneficial in treating breast cancer and warrant further investigation and clinical validation.
Materials and Methods
Cell lines and cell culture
Human breast cancer cell lines T-47D, MCF7, MDA-MB-361, SK-BR-3, HCC1954, MDA-MB-468, MDA-MB-231, and BT-549 were obtained from the ATCC (Manassas, VA, USA). MCF7, MDA-MB-361, SK-BR-3, and MDA-MB-231 cells were cultured in Dulbecco modified Eagle medium (Lonza, Basel, Switzerland), and T-47D, HCC1954, MDA-MB-468, and BT-549 were maintained in Roswell Park Memorial Institute-1640 medium (Lonza); all media were supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), penicillin (100 units/mL), and streptomycin (100 mg/mL). All cells were cultured at 37 °C in a humidified atmosphere of 5% carbon dioxide.
Antibodies and reagents
Antibodies against IκBα (#9242), phospho-SAPK/JNK (Thr183/Tyr185; #9251), SAPK/JNK (#9252), phospho-p38 MAPK (Thr180/Tyr182; #9211), p38 MAPK (D13E1) XP rabbit (#8690), PARP (46D11) rabbit (#9532), Caspase 3 (#9662), Caspase 7 (D2Q3L; #12827), LC3A/B (D344C) XP rabbit (#12741), anti-mouse IgG (#7076), and anti-rabbit IgG (#7074) were purchased from Cell Signaling Technology (Danvers, MA, USA). Dox (#D1515) and antibody against β-actin (#A2228) were obtained from Sigma-Aldrich. Antibody against MKP-1 (M-18) was purchased from Santa Cruz Biotechnology (#sc-1102, Dallas, TX, USA). MLN9708 (#A4007) was purchased from ApexBio (Houston, TX, USA) and was prepared according to the manufacturer’s recommendations.
Cytotoxicity assay
Cell cytotoxicity assays were performed using CCK-8 (Dojindo Molecular Technologies, Rockville, MD, USA) following the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates (5 × 103 cells per well). After 24 h of incubation at 37 °C, cells were either allowed to grow in medium alone (control) or in medium containing increasing concentrations of MLN9708, Dox, or a combination of the two agents. After 48 h or 72 h, cells were observed and photographed via optical microscope (magnification, ×200). CCK-8 (10 μL) was added to each well, and the cells were incubated for 2 h. The absorbance of each well was measured at 450 nm, and the data were plotted for the cell viability curve. Each experiment was performed in triplicate.
Colony formation assay
Cells were seeded in 6-well plates (5 × 103 cells per well). After 48 h, cells were incubated with medium alone or with MLN9708 at 0.1 μM or 0.3 μM for 72 h and then were cultured in drug-free medium for 2 weeks. Then cells were fixed and stained with methanol/crystal violet for 10 min and were photographed. Each experiment was performed in triplicate.
Anchorage-independent growth assay
Cell anchorage-independent growth ability was assessed by soft agar assay. In 6-well plates, the bottom layer in each well was composed of semi-solid 0.5% agar (2 mL). For the top layer, cells were mixed with 0.3% agar (1.5 mL) in each well at a density of 5 × 103 cells per well and were mixed with MLN9708 (0.1 μM or 0.3 μM). Untreated cells were used as controls. Cells grew at 37 °C for 3 weeks until the colonies were visible to the naked eye. Colonies were then stained with crystal violet for 2 h and were photographed. The colonies were counted by Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA), and the data were plotted. Each experiment was performed in triplicate.
Immunoblotting
For immunoblotting, after each treatment, cells were washed twice with ice-cold phosphate-buffered saline solution and were spun down. The cell pellets were dissolved in lysis buffer for 30 min (Tris-HCl [pH 7.4, 50 mM]; sodium chloride [150 mM]; EDTA [1 mM]; 1% NP-40; 0.25% sodium deoxycholate; phenylmethylsulfonyl fluoride [1 mM]; benzamidine [1 mM]; leupeptin [10 μg/mL]; dithiothreitol [1 mM]; sodium fluoride [10 mM]; sodium orthovanadate [0.1 mM]; and phosphatase inhibitor cocktail 2 and 3 [p5726 and p0044, Sigma-Aldrich]). The solutions were centrifuged at 13,000 rpm for 15 min, and the supernatants were collected as cell lysates. The cell lysates were subjected to 10% or 15% SDS-PAGE and were transferred to polyvinylidene fluoride membranes. The samples were subjected to immunoblotting with primary antibodies and with horseradish peroxidase–conjugated antibodies against rabbit or mouse IgG. The membranes were developed using the ECL Western blotting system (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (La Jolla, CA, USA). All values are presented as means ± standard deviation. P values less than 0.05, 0.01, or 0.001 were considered to be statistically significant. The Student t-test (two-tailed) or analysis of variance (the Dunnett multiple comparison post-test) was used to analyze the difference between the drug treatment groups and the control group.
Additional Information
How to cite this article: Wang, H. et al. Next-generation proteasome inhibitor MLN9708 sensitizes breast cancer cells to doxorubicin-induced apoptosis. Sci. Rep. 6, 26456; doi: 10.1038/srep26456 (2016).
References
Hortobagyi, G. N. Developments in chemotherapy of breast cancer. Cancer 88, 3073–3079 (2000).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer (Version 3.2015) (2015).
Tacar, O., Sriamornsak, P. & Dass, C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 65, 157–170 (2013).
Swift, L. P., Rephaeli, A., Nudelman, A., Phillips, D. R. & Cutts, S. M. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 66, 4863–4871 (2006).
Pang, B. et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun. 4, 1908 (2013).
Yang, F., Kemp, C. J. & Henikoff, S. Doxorubicin enhances nucleosome turnover around promoters. Curr Biol. 23, 782–787 (2013).
Yamaguchi, N. et al. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. Eur J Cancer. 51, 2314–2320 (2015).
Mohammad, N. et al. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-beta-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci. Rep. 5, 11853 (2015).
Hammer, E. et al. Proteomic analysis of doxorubicin-induced changes in the proteome of HepG2cells combining 2-D DIGE and LC-MS/MS approaches. Proteomics 10, 99–114 (2010).
Deshaies, R. J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 12, 94 (2014).
Adams, J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 4, 349–360 (2004).
Ghosh, S. & Karin, M. Missing pieces in the NF-kappaB puzzle. Cell. 109 Suppl, S81–96 (2002).
Hayden, M. S. & Ghosh, S. Signaling to NF-kappaB. Genes Dev. 18, 2195–2224 (2004).
Orlowski, R. Z. & Baldwin, A. S. Jr. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 8, 385–389 (2002).
Voorhees, P. M., Dees, E. C., O’Neil, B. & Orlowski, R. Z. The proteasome as a target for cancer therapy. Clin Cancer Res. 9, 6316–6325 (2003).
Kubiczkova, L., Pour, L., Sedlarikova, L., Hajek, R. & Sevcikova, S. Proteasome inhibitors-molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 18, 947–961 (2014).
Stone, H. R. & Morris, J. R. DNA damage emergency: cellular garbage disposal to the rescue? Oncogene. 33, 805–813 (2014).
Chen, S. et al. Genome-wide siRNA screen for modulators of cell death induced by proteasome inhibitor bortezomib. Cancer Res. 70, 4318–4326 (2010).
Yang, C. H. et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 17, 813–817 (2006).
Engel, R. H. et al. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 25, 733–737 (2007).
Schmid, P. et al. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 19, 871–876 (2008).
Trinh, X. B. et al. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancer. Oncol Rep. 27, 657–663 (2012).
Kupperman, E. et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 70, 1970–1980 (2010).
Smith, D. C. et al. Phase 1 study of ixazomib, an investigational proteasome inhibitor, in advanced non-hematologic malignancies. Invest New Drugs. 33, 652–663 (2015).
Chattopadhyay, N. et al. KRAS Genotype Correlates with Proteasome Inhibitor Ixazomib Activity in Preclinical In Vivo Models of Colon and Non-Small Cell Lung Cancer: Potential Role of Tumor Metabolism. PloS One 10, e0144825 (2015).
Neve, R. M. et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10, 515–527 (2006).
Holliday, D. L. & Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 13, 215 (2011).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature. 406, 747–752 (2000).
Chauhan, D. et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 17, 5311–5321 (2011).
Gu, J. J. et al. MLN2238, a proteasome inhibitor, induces caspase-dependent cell death, cell cycle arrest, and potentiates the cytotoxic activity of chemotherapy agents in rituximab-chemotherapy-sensitive or rituximab-chemotherapy-resistant B-cell lymphoma preclinical models. Anticancer Drugs. 24, 1030–1038 (2013).
Gavilan, E. et al. Breast cancer cell line MCF7 escapes from G1/S arrest induced by proteasome inhibition through a GSK-3beta dependent mechanism. Sci Rep. 5, 10027 (2015).
Milani, M. et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 69, 4415–4423 (2009).
Shi, Y. Y., Small, G. W. & Orlowski, R. Z. Proteasome inhibitors induce a p38 mitogen-activated protein kinase (MAPK)-dependent anti-apoptotic program involving MAPK phosphatase-1 and Akt in models of breast cancer. Breast Cancer Res Treat. 100, 33–47 (2006).
Small, G. W. et al. Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Mol Pharmacol. 66, 1478–1490 (2004).
Moutzouris, J. P. et al. Proteasomal inhibition upregulates the endogenous MAPK deactivator MKP-1 in human airway smooth muscle: mechanism of action and effect on cytokine secretion. Biochim Biophys Acta. 1803, 416–423 (2010).
Hayden, M. S. & Ghosh, S. Shared principles in NF-kappaB signaling. Cell. 132, 344–362 (2008).
Shostak, K. & Chariot, A. NF-kappaB, stem cells and breast cancer: the links get stronger. Breast Cancer Res. 13, 214 (2011).
Whyte, J., Bergin, O., Bianchi, A., McNally, S. & Martin, F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 11, 209 (2009).
Liang, L. et al. TAK1 ubiquitination regulates doxorubicin-induced NF-kappaB activation. Cell Signal. 25, 247–254 (2013).
Deavall, D. G., Martin, E. A., Horner, J. M. & Roberts, R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012, 645460 (2012).
Dick, L. R. & Fleming, P. E. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 15, 243–249 (2010).
Heppner, G. H. Tumor heterogeneity. Cancer Res. 44, 2259–2265 (1984).
Marusyk, A. & Polyak, K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 1805, 105–117 (2010).
Dantuma, N. P., Groothuis, T. A., Salomons, F. A. & Neefjes, J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 173, 19–26 (2006).
Gudmundsdottir, K., Lord, C. J. & Ashworth, A. The proteasome is involved in determining differential utilization of double-strand break repair pathways. Oncogene. 26, 7601–7606 (2007).
Levy-Barda, A. et al. Involvement of the nuclear proteasome activator PA28gamma in the cellular response to DNA double-strand breaks. Cell Cycle. 10, 4300–4310 (2011).
Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 9, 537–549 (2009).
Dhillon, A. S., Hagan, S., Rath, O. & Kolch, W. MAP kinase signalling pathways in cancer. Oncogene. 26, 3279–3290 (2007).
Jin, H. S. et al. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 69, 1782–1791 (2009).
Fan, Y. et al. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis. 18, 1224–1234 (2013).
O’Leary, D. P., Wang, J. H., Cotter, T. G. & Redmond, H. P. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut. 62, 461–470 (2013).
Corliss, J. Broadening recruitment for minorities, the elderly. Cancer Discov. 1, 461 (2011).
Aggarwal, B. B. & Sung, B. NF-kappaB in cancer: a matter of life and death. Cancer Discov. 1, 469–471 (2011).
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Acknowledgements
This work was supported by the University Cancer Foundation via the Institutional Research Grant Program at The University of Texas MD Anderson Cancer Center (to Hong Zhang). Hao Wang was funded by the China Scholarship Council (#201308230130). We thank Jill Delsigne at the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, and Kristine L Yang for language editing.
Author information
Authors and Affiliations
Contributions
H.W., Y.C. and H.Z. developed the study concept and design. H.W., Y.Y., Z.J., W.M.C., Z.W. and J.D. performed experiments and collected data. H.W., Y.Y., W.M.C., Y.Z., Y.C. and H.Z. analyzed the data. H.W., Y.C. and H.Z. drafted the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, H., Yu, Y., Jiang, Z. et al. Next-generation proteasome inhibitor MLN9708 sensitizes breast cancer cells to doxorubicin-induced apoptosis. Sci Rep 6, 26456 (2016). https://doi.org/10.1038/srep26456
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26456
This article is cited by
-
Long non-coding RNAs as the critical regulators of PI3K/AKT, TGF-β, and MAPK signaling pathways during breast tumor progression
Journal of Translational Medicine (2023)
-
Nanomedicine for autophagy modulation in cancer therapy: a clinical perspective
Cell & Bioscience (2023)
-
DNMT2/TRDMT1 gene knockout compromises doxorubicin-induced unfolded protein response and sensitizes cancer cells to ER stress-induced apoptosis
Apoptosis (2023)
-
Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors
Journal of Experimental & Clinical Cancer Research (2022)
-
Molecular mechanisms of apoptosis and autophagy elicited by combined treatment with oridonin and cetuximab in laryngeal squamous cell carcinoma
Apoptosis (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.