Abstract
Nutraceuticals have been proposed to exert positive effects on human health and confer protection against many chronic diseases. A major bioactive component of soy-based foods is lunasin peptide, which has potential to exert a major impact on the health of human consumers worldwide, but the biochemical features of dietary lunasin still remain poorly characterized. In this study, lunasin was purified from a soy-based food product via strong anion exchange solid phase extraction and then subjected to top-down mass spectrometry analysis that revealed in detail the molecular diversity of lunasin in processed soybean foods. We detected multiple glycated proteoforms together with potentially toxic advanced glycation end products (AGEs) derived from lunasin. In both cases, modification sites were Lys24 and Lys29 located at the helical region that shows structural homology with a conserved region of chromatin-binding proteins. The identified post-translational modifications may have an important repercussion on lunasin epigenetic regulatory capacity. Taking together, our results demonstrate the importance of proper chemical characterization of commercial processed food products to assess their impact on consumer’s health and risk of chronic diseases.
Similar content being viewed by others
Introduction
Many epidemiological studies have demonstrated that nutritional modification can reduce the prevalence of major chronic disorders including diabetes and coronary heart diseases1,2,3. The health benefits of nutritional modification have been widely attributed to the influence of bioactive dietary compounds known as ‘nutraceuticals’, whose range of biological effects are only now being fully uncovered. While nutraceutical peptides are commonly found in many different foodstuffs including eggs and plants4,5,6, to the best of our knowledge their potential to influence human health and immunity remain poorly understood. This is in part due to the lack of biochemical characterization of dietary peptides, which limits attempts to predict their likely impact on human cells and tissues in vivo.
The biological activities of proteins and peptides are regulated by enzymatic and spontaneous post-translational modifications (PTMs) to amino acid side chains7. These PTMs change protein structure and function, resulting in the molecular diversification of individual gene products8. Common PTMs include the enzymatic addition of monosaccharides or extended sugar chains to the core protein (glycosylation) and the non-enzymatic addition of glycans caused by Maillard reactions between reducing sugars and amino functional groups (glycation). Covalent addition of sugar moieties to proteins has previously been documented to yield food products with antioxidant and antibiotic properties9,10. However, oxidation of Maillard reaction derivatives can also result in the formation of advanced glycation end products (AGEs), which contribute to the pathophysiology of major human disorders, including diabetes11 and Alzheimer’s disease12, by increasing oxidative stress13 and inflammation14, which both can promote cancer15.
In biological systems, production of AGEs occurs under hyperglycemic conditions as a response to cellular stress16. In particular, the AGEs Nε-carboxy-methyl-lysine (CML) and Nε-carboxy-ethyl-lysine (CEL) have been the subject of intensive study due to their ability to interact with the human cell receptor for AGEs (RAGE)17. However, synthesis of AGEs is not restricted to in vivo environments, since these compounds can also be formed during food production. The concentration and diversity of AGEs varies between different foodstuffs, being more abundant in animal-derived products and heat-processed foods18,19,20,21, while techniques such as roasting, frying and searing can also promote AGEs formation18,22. It is clear therefore that diet is a major component of human exposure to AGEs23 and consumption of AGEs will vary significantly between different populations around the world.
For generations, Asian populations have consumed diets that are high in soy, which is thought to contribute to the low relative risk of disorders such as osteoporosis and cardiovascular disease in this group24,25,26. In contrast, soybean has only recently been introduced into Western diets, where it has been consumed in a largely processed state. Despite the virtues historically attributed to soybean intake mainly credited to the presence of isoflavones and their effect on breast cancer27,28, multiple studies have reported null association of soybean intake and risk of breast cancer29,30,31, or even harmful effect (Hirose, et al.32 showed an increased risk of breast cancer with consumption of atsuage, (deep fried tofu) in postmenopausal women). Similarly, harmful associations were also observed between soybean intake and other types of cancers. A clear example is the epidemiological study performed by Sun, et al.33 with Chinese Singaporeans to evaluate the relation between the intake of soybean and the risk of bladder cancer, where 329,848 person-years of follow-up were accumulated. From that study a 2.3-fold increase in cancer risk was observed for the highest quartile of total soy intake (≥92.5 g/1000 kcal) after controlling for smoking habits and education. Based on this set of divergent studies, we could then affirm that the effect of soybean intake on health is still far to be fully elucidated. Presence and interaction of other nutraceuticals have to be considered when studying the effect of soybean intake on health33. Therefore thorough understanding the impact on health can only be achieved after comprehensive chemical characterization of soybean nutraceuticals33.
A key nutraceutical of soybean is the peptide lunasin, which is found in varying concentrations in different soy-based foods and has been reported to exert a wide range of biological effects34,35,36,37, emphasizing its strong epigenetic regulatory capacity (as reviewed by Hernández-Ledesma and de Lumen38), we therefore sought to use a top-down proteomics strategy to identify and characterize novel proteoforms of lunasin from commercial soybean food products, with the ultimate aim of shedding new light on the likely biological effects of these proteoforms following human consumption. Top-down proteomics - first introduced by McLafferty and co-workers - employs state of the art mass spectrometry (MS) technology to characterize PTMs in intact proteins39,40,41,42. This approach is uniquely capable of detecting multiple proteoforms in complex samples using fragmentation by electron capture dissociation (ECD), collision activated dissociation (CAD) and electron transfer dissociation (ETD)43,44. In the current report, we used an Orbitrap Elite mass spectrometer to identify that dietary lunasin exists as a heterogeneous mixture of glycated and glycoxidated variants likely to exert distinct biological effects in vivo. The top-down proteomic approach used in this study also allowed us to characterize the modification sites to uncover lunasin diversity in a soy-based foodstuff. These data improve our current understanding of the nutraceutical content of soy-based foods and will assist future analyses of how lunasin ingestion impacts on human health and risk of disease.
Results and Discussion
Identification of lunasin in soybean products
The soybean-derived peptide lunasin is thought to exert a range of biological effects that could be modified by protein structural changes during commercial processing. We therefore sought to determine the structural diversity of lunasin peptides in soy-based foodstuffs to investigate their potential impact on human health. To do this, we used a top-down proteomics approach to study the characteristics of lunasin peptide derived from two different dietary sources – raw soybeans and commercial soybean beverage powder. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis revealed that while the analyzed raw soybean extract contained little or no lunasin, the soybean beverage powder extract displayed a cluster of peptides in the 5000 Da mass range, suggesting the presence of multiple lunasin variants in this product (Fig. 1A,B). Moreover, the cluster of peaks detected in soybean beverage powder extract displayed a laddering mass-shift of 162 Da, indicating the presence of PTMs in these peptides.
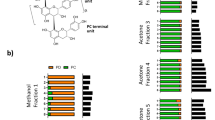
Analysis of soybean product extract by linear-mode MALDI-TOF MS.
(A) Spectrum of raw soybean extract. No peaks were detected in the 5000–6000 m/z mass range. (B) Spectrum of commercial soybean beverage powder extract. Multiple peaks with laddering mass-shift of 162.05 Da were identified in 5000–6000 m/z. (C) Lunasin-containing fractions obtained by reversed-phase high-performance liquid chromatography (RP-HPLC). (D) Spectrum of purified lunasin obtained by strong anion exchange solid phase extraction (SAX-SPE).
Consistent with previous reports, our data indicated that raw soybean extract contained only trace levels of lunasin, whereas the same peptide is relatively abundant and appears structurally diverse in commercial soy-based foods45. This discrepancy may be due to the use of different soybean genotypes46, and/or the impact of environmental factors during plant development36. In addition, the amount of lunasin present in soybean seeds increases with maturation and decreases during sprouting47. While it is common for commercially processed soybean to be subjected to periods of prolonged soaking, which could perhaps promote lunasin degradation in the seeds, our analysis indicated that dried raw soybean was not enriched in lunasin compared with the soaked beans (data not shown).
Purification of lunasin from soybean beverage powder
To confirm the presence of lunasin in soybean beverage powder, we next tested two different strategies for purifying the peptide from crude extract; reverse phase high pressure liquid chromatography (RP-HPLC) and strong anion exchange solid phase extraction (SAX-SPE). For RP-HPLC purification, lunasin separation from other proteinaceous compounds was optimized by modifying the HPLC gradient. MALDI-TOF MS analysis of the fractions collected revealed that lunasin was eluted over several different fractions and partially co-eluted with other proteins. Fraction numbers 32 and 33 contained the purest lunasin (Fig. 1C). For SAX-SPE purification, we achieved efficient isolation of lunasin using salt-containing phases (Fig. 1D). Optimization of the SAX-SPE strategy was performed by testing elution efficacy using increasing concentrations of NaCl (100–500 mM), Tris-HCl (150 mM) (Fig. 2). We observed that lunasin was completely eluted when using a buffer containing 100 mM NaCl, 150 mM Tris-HCl. It has been reported that other anionic sepharose exchange stationary phases showed an optimal elution of lunasin at 200–300 mM NaCl48. Our use of lower salt concentrations during elution improved the purity of the recovered lunasin.
Optimization of the elution step for lunasin purification by strong anion exchange solid phase extraction (SAX-SPE).
MALDI-MS spectra of samples eluted sequentially using varying concentrations of salt (100–500 mM NaCl, 150 mM Tris-HCl). Lunasin and its modified variants were completely eluted at 100 mM NaCl, 150 mM Tris-HCl.
While both purification techniques were capable of isolating unmodified lunasin as well as structural variants of this peptide, isolation by SAX-SPE delivered better results than did RP-HPLC. Indeed, since SAX-SPE is also relatively simple, enables rapid sample purification and requires only small volumes of solvents and could easily be scaled up to allow processing of large sample volumes by increasing the size of the solid phase extraction cartridge. In addition, this method yielded a total of 0.56 mg of lunasin/g of soybean beverage powder (protein quantification performed by Bradford assay) which was comparable to the scalable purification presented by Seber et al.49 where 0.44 mg of lunasin/g of soybean white flake were obtained.
Characterization of lunasin by top-down mass spectrometry
Top-down proteomics represents a powerful tool for the characterization of proteins and peptide proteoforms by providing complete amino acid sequences and PTM profiles39,40,41,42, whereas bottom-up strategies depend on enzymatic digestion steps that disrupt the links between peptides and their parent proteoforms41,50. We therefore applied a top-down approach to characterize the lunasin proteoforms we isolated from the commercial soybean food product. Prior to MS analysis, lunasin peptide was reduced and the Cys residues converted into positively charged pseudo-Lys by alkylation in bromoethylamine (BrEA), thereby achieving a mass increase of 2 × 43.04 Da and an addition of two positive charges to improve fragmentation by ETD43. While we initially intended to dissolve the sample in 50% acetonitrile (ACN), 0.1% formic acid (FA) for analysis by top-down nanospray ionization tandem MS (NSI-MS/MS) (in-line with reports that acidified ~50% organic solvent is optimal for NSI top-down studies42), we instead observed that spectrum quality and signal strength were greater when the sample was dissolved in low organic solvent. This is likely due to the fact that lunasin contains a poly-Asp carboxyl tail at the C-terminal site of the sequence and this region of dense negative charge may limit solubility in high organic solvent. We therefore used 3% ACN, 0.1% FA during sample preparation, which enabled us to successfully detect and fragment unmodified lunasin via high-energy collision dissociation (HCD) (Fig. 3A).
Lunasin is structurally defined as a 43-amino acid peptide that contains nine Asp residues at the C-terminal end51. However, our analysis of derivatized lunasin in soybean beverage powder revealed that the most abundant variant was a 44-amino acid peptide with a monoisotopic mass of 5225.36 Da and an extra C-terminal Asn residue (SKWQHQQDSCRKQLQGVNLTPCEKHIMEKIQGRGDDDDDDDDDN), consistent with the findings of Seber et al.49. We next proceeded to characterize lunasin structure in the soybean beverage powder extract by fragmenting the purified derivatized peptide using HCD and ETD by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS)52,53. ETD fragmentation typically generates c- and z-series ions, while collision-induced dissociation leads to the generation of b- and y-series ions54, hence we combined data from both of these complementary modes to robustly determine the amino acid sequence and PTM profile of lunasin peptide. Fragmentation of parent ions, which were predominantly ions at six charged state, revealed the presence of multiple lunasin proteoforms that were mainly generated by heterogeneous glycation. These post-translationally modified proteoforms were found to co-exist with the unmodified lunasin, indicating that not all the lunasin in commercial soy-based foods was altered by processing. The negatively charged poly-Asp-carboxyl tail of lunasin reduced the efficiency of ETD in the vicinity of this region of the peptide backbone (Fig. 3B). We detected distinct proteoforms of lunasin that exhibited different degrees of glycation mainly at residue Lys 24 and Lys 29. Figure 4 shows the MS spectrum of lunasin displaying a single glycation, which represented near 50% of total glycated lunasin (Fig. 5), while Supplementary information of annotated MS/MS spectra list 1 shows the spectra of lunasin proteoforms displaying either two or three sugar moieties, which represented the 30% and 14% of total glycated lunasin (Fig. 5). We were also able to detect a lunasin proteoform corresponding to the addition of four sugar moieties (4 × 162.05 Da), about 6% of the total of glycated lunasin and we detected also multiple non-glycated proteforms derived from the 43-amino acid lunasin (Fig. 5), but it was not possible to successfully sequence these species by MS/MS due to its low abundance in the sample.
Further top-down characterization of lunasin also revealed the occurrence of alternative side chain modifications (Table 1), including oxidation (Met), dihydroxy (Lys), dehydration (Asp/Asn), deamidation (Asn/Gln), methyl esterification (Asp), carbamylation (Lys), acetylation (Lys) and pyro-glutamate conversion (N-terminal Gln) (annotated MS spectra are shown in Supplementary information of annotated MS/MS spectra list 1). In general, these PTMs were detected at lower concentrations than glycations, except for oxidation which was more frequently observed (based on the relative intensity of the parent ions shown in the full MS spectrum, Fig. 5; and the number of spectra identified, Table 1). Certain peptide/protein PTMs can occur via spontaneous chemical reaction, such as oxidation, deamidation, dehydration and pyro-glutamate conversion. Other PTMs may occur naturally in plants e.g. methyl esterification of aspartyl residues in seeds mediated by the protein-repair enzyme L-isoaspartyl methyltransferase55. While it remains poorly understood how peptide modification results in the addition of dihydroxylysine (+31.99 Da), previous studies have identified this PTM in the primitive vertebrate antimicrobial peptide styelin D56 and in an antibiotic dipeptide derived from bacteria57. The molecular diversities generated via enzymatic or non-enzymatic PTMs are likely to significantly modify the bioactivities of lunasin.
It is established that glycation can modulate the bioactivities of specific peptides. For example, the insect-derived antibiotic peptide drosocin enhances its bioactivity almost 100-fold when it becomes glycosylated10 and glycated pea albumin exhibits increased capacity to modulate the composition of gut bacteria during culture in vitro58. Our data now suggest that nutraceutical peptides in soy-based foodstuffs also exhibit multiple glycated variants with potential to exert a range of biological effects in vivo. Nonetheless, the complex system of secondary reactions that may follow the simple initial reaction between sugars and proteinaceous giving rise to the final production of AGEs situates glycated peptides in a controversial position.
Advanced glycation end products derived from lunasin
While consumption of glycated bioactive peptides may confer significant health benefits, dehydration, condensation and atom rearrangement of early glycation products led to the formation of AGEs59. Dietary AGEs are readily absorbed via gut and can accumulate in body tissues where they contribute to the progression of several different disorders including diabetes, atherosclerosis and kidney disease60,61,62,63. We therefore probed for the presence of the well-characterized AGEs CEL and CML17 in the commercial soybean derivative studied here (Table 2).
AGEs from food are generally characterized by fluorimetric assays, whether by enzyme-linked immunosorbent assay based on an anti-CML monoclonal antibody18 or by fluorescence spectroscopy couple to a high-performance liquid chromatography64. The use of the top-down proteomic platform allowed us to go further in the characterization of AGEs derived from lunasin providing modification site information as well as CEL/CML identification.
We detected a total of six different lunasin-derived AGEs in the commercial soybean beverage powder, where four of them were derived from the 43-amino acid parent peptide. In contrast, we detected only two low abundant AGE-modified sequences derived from the 44-amino acid lunasin (see Supplementary information of annotated MS/MS spectra list 2 for the annotated spectra). The most abundant AGE derived from lunasin (based on spectral count) contained both CML and CEL modifications at Lys 24 and Lys 29, respectively. AGEs derived from lunasin present in the commercial soybean beverage powder were presumably generated spontaneously during food processing which involved thermal processing of the product, although water based culinary methods tend to be milder methods for the generation of AGEs in foods65 (technical parameters of the production process were not available).
Longitudinal study of lunasin glycated and glycoxidated proteoforms
We evaluated the occurrence of glycation/glycoxidation in lunasin peptide from commercial soybean beverage powder by performing a longitudinal study. To achieve this aim we profiled lunasin isolated from two independent batches of product manufactured in a three months interval. We observed that glycation and glycoxidation sites remained constant at residues Lys 24 and Lys 29 of lunasin whereas no glycation/glycoxidation was observed at residues Lys 2 and Lys 12 (Table 2 and Fig. 6A). Intriguingly, Lys 24 and Lys 29 are located at the helical region (EKHIMEKIQG) that shows structural homology with a conserved region of chromatin-binding proteins38 (Fig. 6B). Because this region is involved in targeting of H3-H4 histones66, it might be a highly exposed region of lunasin sequence. We hence explain the fixed localization of glycation/glycoxidation at Lys 24 and Lys 29 as dependent of the peptide conformation while the direct effect of production process is evidenced on the highly diverse glycated (mono-, di- and tri-hexose) and glycoxidated (CML/CEL) products detected (Fig. 6C). Presence of glycation/glycoxidation in that key region of the sequence may then have an important repercussion on lunasin epigenetic regulatory capacity, thus further studies have to be carried out to elucidate the impact of PTMs.
Glycated/glycoxidated modification sites in lunasin sequence.
(A) Occurrence of glycation and glycoxidation at the four Lys present in lunasin sequence. N.D. means not detected. (B) Description of distinct features present in lunasin sequence and localization of glycated/glycoxidated sites. Modified Lys residues (underlined in red) are both located at the chromatin-binding protein homologous region of lunasin (shaded in blue). (C) Analysis of the diversity of glycation and glycoxidation species detected at the residues Lys 24 and Lys 29. Distribution was calculated considering both analyzed batch and based on total spectral counts.
We further compared the presence of glycated and glycoxidated lunasin from both batches of product. This analysis revealed that the amount of AGEs in commercial soybean beverage powder ranged between 68.9 and 183.7 μg/g. Content of AGEs in foods depends mainly on food matrix and food processing18,64. Nonetheless, hazardousness of AGEs not only depends on the amount of AGEs consumed, but it also depends on which molecules and which sites are modified. Besides, non-modified lunasin ranged between 0.22 and 0.23 mg/g and glycated lunasin ranged between 27.1 and 38.8 μg/g. (Table 3). We hypothesize that these variations could be consequence of the complexity of the chemical reactions that took place during food processing.
Materials and Methods
Soybean products
Raw soybeans and commercial soybean beverage powder produced from non-genetically modified soybeans were obtained from a local supermarket in Singapore. The soybean beverage powder comprised listed ingredients of soybeans with bean coats removed and calcium carbonate added. The packaging indicated that no sugar was added to the product. Soybean beverage powder did not include other proteins from different sources. Production process described by the manufacturer combined steaming, coat removal, freeze-drying and grinding steps.
Preparation of soybean extract
Extraction
Soybean extract was prepared according to the protocol described by Seber et al.49, except for minor modifications. In brief, a total of 10 g raw soybeans were soaked overnight in distilled water and then blended for 5 min together with 100 mL extraction buffer (75.5 mM sodium phosphate, 68.4 mM sodium chloride, 10 mM sodium metabisulfite, 20 mM ascorbic acid, pH 7.4). The mixture was then centrifuged at 4000 × g for 30 min at 4 °C. In parallel, a total of 10 g commercial soybean beverage powder was combined with 100 mL extraction buffer, shaken for 30 min and then centrifuged at 4000 × g for 30 min at 4 °C. The supernatants were collected and filtered through Whatman™ grade 54 and grade 42 paper filters to remove any suspended material. All the extraction process was maintained at 4 °C.
Defatting
To remove lipids, the soybean extracts were combined with 100 mL hexane and shaken for 2 h, after which the hexane was removed by decanting and discarding the lower layer. The upper layer was retained and subjected to a second round of hexane-based defatting as described above, before being recovered and centrifuged at 5000 × g for 10 minutes at 4 °C. The resultant liquid was then filtered through 0.45 and 0.22 μm filters and stored at −20 °C.
Purification of lunasin from soybean beverage powder by strong anion exchange solid phase extraction (SAX-SPE)
Lunasin was isolated from other positively charged species by SAX-SPE (SAX Hypersep, 50 mg, Thermo Scientific, Bremen, Germany). The phases used in the extraction were 150 mM Tris-HCl buffer at pH 9 (phase A) and phase A with 0.1 M NaCl (phase B). The sample was loaded into the cartridge at pH 8.5 (for better retention of analytes in the stationary phase) and elution was performed using 1 mL phase B.
Chromatographic purification of lunasin from soybean beverage powder
Large proteins were removed from the soybean beverage extract by passing the samples though an Amicon 30 kDa-molecular weight cut off filter (Merck Millipore, MA, USA). Lunasin peptide was then isolated from the filtrate by RP-HPLC using a Luna column (3.6 μm, 100 mm × 4.6 mm, Phenomenex Inc, Torrance, CA, USA). Water and ACN were used as mobile phases A and B, respectively. Separation was performed using a 90-min gradient as follows: 5% B for 5 min, 5–45% B for 60 min, 45–100% B for 3 min, 100% B for 7 min and then returned to initial conditions over 0.5 min and kept isocratic for 14.5 min thereafter. A total of 400 μL soybean beverage extract was injected and fractions were collected every minute.
Cys-to-pseudoLys derivatization
SAX-purified peptides were reduced and alkylated as previously described67. Briefly, purified peptides were incubated with 30 mM dithiotreitol and 60 mM BrEA in 200 mM Tris-HCl buffer (pH 8.6) for 1 h at 55 °C. Reduction and BrEA alkylation of disulfide bonds converted Cys into pseudoLys, thereby increasing the mass of each Cys by 43.04 Da with the addition of a positive charge. Desalting was carried out using a C18 Sep-Pak column (Waters, Sep-Pak C18, 100 mg sorbent, Milford, MA., USA). Elution was performed using 1 mL 75% ACN, 0.1% FA. The eluted peptide solution was then dried overnight at room temperature in a vacuum concentrator (Eppendorf, Hamburg, Germany).
MALDI-TOF MS
Preparation of soybean extract, lunasin purification and Cys-to-pseudoLys derivatization were monitored using an Applied Biosystems 4800 MALDI-MS analyzer. The linear acquisition mode was used in the 1000 to 15,000 Da range with a focusing mass of 7000 Da. The matrix used was saturated α-cyano-4-hydroxycinnamic acid in 75% ACN with 0.1% trifluoroacetic acid. Desalting of samples was performed with C18 zip-tips (Millipore Corp., Billerica, MA, U.S.A.) when required. Each MALDI spot comprised 0.5 μL desalted sample and 0.5 μL matrix solution.
Top-down analysis by NSI-MS/MS
Purified peptide was dissolved in 3% ACN, 0.1% FA and then sprayed onto the detector using a Thermo Finnigan Dynamic NSI source (Thermo Scientific Inc., Bremen, Germany) with Proxeon NanoES spray capillaries (Thermo Scientific Inc). The Top-Down NSI-MS/MS analysis of purified lunasin was performed using an Orbitrap Elite mass spectrometer (Thermo Scientific Inc.) manually tuned using the isolated peptide. The capillary temperature was set to 200 °C and the spray voltage was set to 1.50 kV. Spectra were acquired in positive mode using a mass range from 200–2000 m/z and a resolving power of 120.000 (at 400 m/z). Precursor ion target was set to 1 × 106 charges for full MS and MS/MS experiments. Maximum injection time for full MS and MS/MS was set to 200 ms. Between 20–50 μscans were averaged for each spectrum. The most abundant lunasin ions at six charged state (874 m/z) were isolated with 1.5 m/z isolation window and then fragmented by HCD using 27% normalized collision energy. Data were collected manually in positive mode using LTQ Tune Plus software (Thermo Scientific Inc.) The automatic gain control (AGC) for full MS and MS/MS was set to 1 × 106.
Top-down analysis by LC-MS/MS
Lunasin characterization by LC-MS/MS was performed using an Orbitrap Elite mass spectrometer coupled to a Dionex UltiMate 3000 UHPLC system (Thermo Scientific Inc., Bremen, Germany). The sample was sprayed using a Michrom Thermo CaptiveSpray nanoelectrospray ion source (Bruker-Michrom Inc., Auburn, USA) and the separation was performed using a reversed phase Acclaim PepMap RSL column (75 μm ID × 15 cm, 2 μm particles, Thermo Scientific). Mobile phase A was 0.1% FA in water and mobile phase B was 90% ACN, 0.1% FA. Separation of peptides was performed in a 60-min gradient of 3% B for 1 min, 3–30% B for 31 min, 30–40% B for 10 min, 40–98% B for 5 min, 98% B for 5 min and then reverted to initial conditions over 30 s and kept isocratic for 7.5 min thereafter. Data acquisition was performed in positive mode using LTQ Tune Plus software alternating between full MS and MS/MS. Preliminary data acquisition was performed using 150–2000 m/z, 60.000 resolution (at 400 m/z) with 3 μscan averaged per spectrum. For subsequent injections, we used 120.000 resolution (at 400 m/z) and 10 μscan in order to isolate the cluster of peptides at six charged state. The AGC for full MS and MS/MS was set to 1 × 106 and the reagent AGC was 1 × 105. The 5 most intense ions were isolated with a 1.5 Da mass isolation window and then fragmented by HCD using 27% normalized collision energy or by ETD using reaction times over 80 ms.
Longitudinal study of lunasin proteoforms
Two batches of commercial soybean beverage powder purchased from local supermarket and manufactured in three months interval were processed as described above. From both batches, lunasin was purified and glycations and glycoxidations were profiled.
Data Analysis
Data from top-down NSI-MS/MS was analyzed using MASH Suite (version 1.0.0.23928, UW-Madison, U.S.A.)68 and further validated by manual inspection. Peak deconvolution for manual data analysis was performed using the Xtract algorithm (Thermo Scientific Inc.). Data from top-down LC-MS/MS was analyzed by ProSightPTM69 and PEAKS studio (version 7.0, Bioinformatics Solutions, Waterloo, Canada)70 against Glycine max 2S albumin pre-protein sequence. In all cases, 10 ppm MS and 0.05 Da MS/MS tolerances were used for data analysis. EA (Cys) was included as a fixed modification and additional EA was set as a variable modification to account for possible polymerization of the alkylating agent. For AGE product analysis, CML (Lys) and CEL (Lys) were added as variable modifications. A stringent false discovery rate of 0.1% was set for all searches. All sequences identified were further validated by manual inspection.
Data Deposition
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium71 via the PRIDE partner repository with the dataset identifier PXD003064.
Conclusions
Here in this study, the molecular diversity of lunasin from a commercial soybean derived foodstuff has been successfully depicted by applying a top-down proteomics strategy. The use of this approach allowed us to investigate the presence of PTMs and to identify for the first time AGEs derived from lunasin together with glycated proteoforms. Existence of PTMs in the helical region that shows structural homology with a conserved region of chromatin-binding proteins is likely to critically impact the epigenetic regulatory capacity of lunasin. Our results thus provide novel and valuable molecular details of lunasin that have to be considered when studying its mechanisms of action and health effects. The molecular characterization of lunasin presented in this study also demonstrated the need to conduct thorough chemical characterization of putative nutraceuticals in order to assess how best to exploit these compounds for the improvement of human health via dietary modifications.
Additional Information
How to cite this article: Serra, A. et al. Commercial processed soy-based food product contains glycated and glycoxidated lunasin proteoforms. Sci. Rep. 6, 26106; doi: 10.1038/srep26106 (2016).
References
Lukens, J. R. et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516, 246–249, doi: 10.1038/nature13788 (2014).
Martinez-Gonzalez, M. A. et al. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Europ. J. Nutr. 41, 153–160, doi: 10.1007/s00394-002-0370-6 (2002).
Sala-Vila, A., Estruch, R. & Ros, E. New Insights into the Role of Nutrition in CVD Prevention. Curr. Cardiol. Rep. 17, doi: 10.1007/s11886-015-0583-y (2015).
Mine, Y. & Shahidi, F. Nutraceutical Proteins and Peptides in Health and Disease (CRC Press, 2005).
Liu, J., Jin, Y., Lin, S., Jones, G. S. & Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 175, 258–266, doi: 10.1016/j.foodchem.2014.11.142 (2015).
Malaguti, M. et al. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 15, 21120–21135, doi: 10.3390/ijms151121120 (2014).
Shahidi, F. & Wanasundara, P. K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 32, 67–103, doi: 10.1080/10408399209527581 (1992).
Seo, J. & Lee, K. J. Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J. Biochem. Mol. Biol. 37, 35–44 (2004).
Joubran, Y., Mackie, A. & Lesmes, U. Impact of the Maillard reaction on the antioxidant capacity of bovine lactoferrin. Food Chem. 141, 3796–3802, doi: http://dx.doi.org/10.1016/j.foodchem.2013.06.096 (2013).
Lele, D. S., Talat, S., Kumari, S., Srivastava, N. & Kaur, K. J. Understanding the importance of glycosylated threonine and stereospecific action of Drosocin, a Proline rich antimicrobial peptide. Europ. J. Med. Chem. 92, 637–647, doi: 10.1016/j.ejmech.2015.01.032 (2015).
Basta, G., Schmidt, A. M. & De Caterina, R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardio. Res. 63, 582–592, doi: 10.1016/j.cardiores.2004.05.001 (2004).
Perrone, L. & Grant, W. B. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J. Alzheimer’s Dis. 45, 965–979, doi: 10.3233/jad-140720 (2015).
Traverso, N. et al. Malondialdehyde, a lipoperoxidation-derived aldehyde, can bring about secondary oxidative damage to proteins. J. Gerontol. A 59, B890–895, doi: 10.1093/gerona/59.9.B890 (2004).
Anderson, M. M., Requena, J. R., Crowley, J. R., Thorpe, S. R. & Heinecke, J. W. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest. 104, 103–113, doi: 10.1172/jci3042 (1999).
Gebhardt, C. et al. RAGE signaling sustains inflammation and promotes tumor development. J. Exp. Med. 205, 275–285, doi: 10.1084/jem.20070679 (2008).
Brownlee, M., Vlassara, H. & Cerami, A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann. Int. Med. 101, 527–537 (1984).
Xue, J. et al. Advanced Glycation End Product Recognition by the Receptor for AGEs. Structure 19, 722–732, doi: 10.1016/j.str.2011.02.013 (1984).
Goldberg, T. et al. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 104, 1287–1291, doi: 10.1016/j.jada.2004.05.214 (2004).
Goldin, A., Beckman, J. A., Schmidt, A. M. & Creager, M. A. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114, 597–605, doi: 10.1161/CIRCULATIONAHA.106.621854 (2006).
Ansari, N. A. & Dash, D. Amadori Glycated Proteins: Role in Production of Autoantibodies in Diabetes Mellitus and Effect of Inhibitors on Non-Enzymatic Glycation. Aging Dis. 4, 50–56 (2013).
Uribarri, J. et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 110, 911–916, e912, doi: 10.1016/j.jada.2010.03.018 (2010).
O’Brien, J. & Morrissey, P. A. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit. Rev. Food Sci. Nutr. 28, 211–248, doi: 10.1080/10408398909527499 (1989).
Delgado-Andrade, C., Tessier, F. J., Niquet-Leridon, C., Seiquer, I. & Pilar Navarro, M. Study of the urinary and faecal excretion of Nepsilon-carboxymethyllysine in young human volunteers. Amino acids 43, 595–602, doi: 10.1007/s00726-011-1107-8 (2012).
He, J. et al. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann. Intern. Med. 143, 1–9, doi: 10.7326/0003-4819-143-1-200507050-00004 (2005).
Kim, K. et al. Inhibitory effects of black soybean on platelet activation mediated through its active component of adenosine. Thrombosis Res. 131, 254–261, doi: 10.1016/j.thromres.2013.01.002 (2013).
Wei, P., Liu, M., Chen, Y. & Chen, D.-C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 5, 243–248, doi: http://dx.doi.org/10.1016/S1995-7645(12)60033-9 (2012).
Cho, Y. A. et al. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Europ. J. Clin. Nutr. 64, 924–932, doi: 10.1038/ejcn.2010.95 (2010).
Zhang, C. et al. Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci. 101, 501–507, doi: 10.1111/j.1349-7006.2009.01376.x (2010).
Key, T. J. et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Brit. J. Cancer 81, 1248–1256, doi: 10.1038/sj.bjc.6690837 (1999).
Trock, B. J., Hilakivi-Clarke, L. & Clarke, R. Meta-Analysis of Soy Intake and Breast Cancer Risk. J. Natl. Cancer Inst. 98, 459–471, doi: 10.1093/jnci/djj102 (2006).
Yuan, J. M., Wang, Q. S., Ross, R. K., Henderson, B. E. & Yu, M. C. Diet and breast cancer in Shanghai and Tianjin, China. Brit. J. Cancer 71, 1353–1358, doi: 10.1038/bjc.1995.263 (1995).
Hirose, K. et al. Soybean products and reduction of breast cancer risk: a case-control study in Japan. Brit. J. Cancer 93, 15–22, doi: 10.1038/sj.bjc.6602659 (2005).
Sun, C. L. et al. Dietary soy and increased risk of bladder cancer: the Singapore Chinese Health Study. Cancer Epidem. Biomar. 11, 1674–1677 (2002).
Chang, H. C. et al. Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol. Immunother. 63, 283–295, doi: 10.1007/s00262-013-1513-8 (2014).
Jeong, H. J., Jeong, J. B., Kim, D. S. & de Lumen, B. O. Inhibition of Core Histone Acetylation by the Cancer Preventive Peptide Lunasin. J. Agric. Food Chem. 55, 632–637, doi: 10.1021/jf062405u (2007).
Wang, W., Dia, V. P., Vasconez, M., de Mejia, E. G. & Nelson, R. L. Analysis of soybean protein-derived peptides and the effect of cultivar, environmental conditions and processing on lunasin concentration in soybean and soy products. J. AOAC Int. 91, 936–946 (2008).
Galvez, A. F. & de Lumen, B. O. A soybean cDNA encoding a chromatin-binding peptide inhibits mitosis of mammalian cells. Nature Biotech. 17, 495–500, doi: 10.1038/8676 (1999).
Hernández-Ledesma, B. & de Lumen, B. O. Lunasin: A Novel Cancer Preventive Seed Peptide. Perspec. Med. Chem. 2, 75–80, doi: 10.1016/j.peptides.2008.11.002. (2008).
Mortz, E. et al. Sequence tag identification of intact proteins by matching tanden mass spectral data against sequence data bases. Proc. Natl. Acad. Sci. USA 93, 8264–8267 (1996).
Kelleher, N. L. et al. Efficient sequence analysis of the six gene products (7-74 kDa) from the Escherichia coli thiamin biosynthetic operon by tandem high-resolution mass spectrometry. Protein Sci. 7, 1796–1801, doi: 10.1002/pro.5560070815 (1998).
Kelleher, N. L. et al. Top Down versus Bottom Up Protein Characterization by Tandem High-Resolution Mass Spectrometry. J. Am. Chem. Soc. 121, 806–812, doi: 10.1021/ja973655h (1999).
Sze, S. K., Ge, Y., Oh, H. & McLafferty, F. W. Top-down mass spectrometry of a 29-kDa protein for characterization of any posttranslational modification to within one residue. Proc. Natl. Acad. Sci. USA 99, 1774–1779, doi: 10.1073/pnas.251691898 (2002).
Zubarev, R. A. et al. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 72, 563–573, doi: 10.1021/ac990811p (2000).
Sze, S. K., Ge, Y., Oh, H. & McLafferty, F. W. Plasma electron capture dissociation for the characterization of large proteins by top down mass spectrometry. Anal. Chem. 75, 1599–1603, doi: 10.1021/ac020446t (2003).
Cavazos, A., Morales, E., Dia, V. P. & De Mejia, E. G. Analysis of lunasin in commercial and pilot plant produced soybean products and an improved method of lunasin purification. J Food Sci. 77, C539–545, doi: 10.1111/j.1750-3841.2012.02676.x (2012).
Gonzalez de Mejia, E., Vasconez, M., de Lumen, B. O. & Nelson, R. Lunasin concentration in different soybean genotypes, commercial soy protein and isoflavone products. J. Agric. Food Chem. 52, 5882–5887, doi: 10.1021/jf0496752 (2004).
Park, J. H., Jeong, H. J. & de Lumen, B. O. Contents and bioactivities of lunasin, bowman-birk inhibitor and isoflavones in soybean seed. J. Agric. Food Chem. 53, 7686–7690, doi: 10.1021/jf0506481 (2005).
Dia, V. P., Wang, W., Oh, V. L., Lumen, B. O. d. & de Mejia, E. G. Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity. Food Chem. 114, 108–115, doi: http://dx.doi.org/10.1016/j.foodchem.2008.09.023 (2009).
Seber, L. E. et al. Scalable purification and characterization of the anticancer lunasin peptide from soybean. Plos one 7, e35409, doi: 10.1371/journal.pone.0035409 (2012).
Gault, J. et al. Complete posttranslational modification mapping of pathogenic Neisseria meningitidis pilins requires top-down mass spectrometry. Proteomics 14, 1141–1151, doi: 10.1002/pmic.201300394 (2014).
Odani, S., Koide, T. & Ono, T. Amino acid sequence of a soybean (Glycine max) seed polypeptide having a poly(L-aspartic acid) structure. J. Biol. Chem. 262, 10502–10505 (1987).
Nielsen, M. L., Savitski, M. M. & Zubarev, R. A. Improving protein identification using complementary fragmentation techniques in fourier transform mass spectrometry. Mol. Cell. Prot. 4, 835–845, doi: 10.1074/mcp.T400022-MCP200 (2005).
Horn, D. M., Zubarev, R. A. & McLafferty, F. W. Automated de novo sequencing of proteins by tandem high-resolution mass spectrometry. Proc. Natl. Acad. Sci. USA 97, 10313–10317, doi: 10.1073/pnas.97.19.10313 (2000).
Zubarev, R. A. Electron-capture dissociation tandem mass spectrometry. Curr. Op. Biotech. 15, 12–16, doi: http://dx.doi.org/10.1016/j.copbio.2003.12.002 (2004).
Mudgett, M. B., Lowenson, J. D. & Clarke, S. Protein repair L-isoaspartyl methyltransferase in plants. Phylogenetic distribution and the accumulation of substrate proteins in aged barley seeds. Plant Physiol. 115, 1481–1489, doi: http://dx.doi.org/10.1104/pp.115.4.1481 (1997).
Taylor, S. W., Craig, A. G., Fischer, W. H., Park, M. & Lehrer, R. I. Styelin D, an extensively modified antimicrobial peptide from ascidian hemocytes. J. Biol. Chem. 275, 38417–38426, doi: 10.1074/jbc.M006762200 (2000).
Baldwin, J. E., Claridge, T. D. W., Kee-Chuan, G., Keeping, J. W. & Schofield, C. J. Revised structures for Tü 1718B and valclavam. Tetrahedron Lett. 34, 5645–5648, doi: http://dx.doi.org/10.1016/S0040-4039(00)73905-0 (1993).
Swiatecka, D., Kostyra, H. & Swiatecki, A. Impact of glycated pea proteins on the activity of free-swimming and immobilised bacteria. J. Sci. Food Agric. 90, 1837–1845, doi: 10.1002/jsfa.4022 (2010).
Takeuchi, M. et al. Assessment of the Concentrations of Various Advanced Glycation End-Products in Beverages and Foods That Are Commonly Consumed in Japan. Plos one 10, e0118652, doi: 10.1371/journal.pone.0118652 (2015).
Maier, H. M. et al. Dietary advanced glycation end-products exacerbate oxidative stress in patients with diabetic foot ulcers. Diabetes Res. Clin. Met. 3, doi: 10.7243/2050-0866-3-2 (2014).
Lin, R. Y. et al. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 168, 213–220, doi: http://dx.doi.org/10.1016/S0021-9150(03)00050-9 (2003).
Zheng, F. et al. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Met. Res. Rev. 18, 224–237, doi: 10.1002/dmrr.283 (2002).
Koschinsky, T. et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 94, 6474–6479 (1997).
Chen, G. & Scott Smith, J. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 168, 190–195, doi: http://dx.doi.org/10.1016/j.foodchem.2014.06.081 (2015).
Uribarri, J. & Tuttle, K. R. Advanced Glycation End Products and Nephrotoxicity of High-Protein Diets. Clin. J. Am. Soc. Nephrol. 1, 1293–1299, doi: 10.2215/cjn.01270406 (2006).
Kyle, S., James, K. A. & McPherson, M. J. Recombinant production of the therapeutic peptide lunasin. Microb. Cell Fact. 11, 28, doi: 10.1186/1475-2859-11-28 (2012).
Serra, A. et al. A high-throughput peptidomic strategy to decipher the molecular diversity of cyclic cysteine-rich peptides. Sci. Rep. 6, 23005, doi: 10.1038/srep23005 (2016).
Guner, H. et al. MASH Suite: a user-friendly and versatile software interface for high-resolution mass spectrometry data interpretation and visualization. J. Am. Soc. Mass Spec. 25, 464–470, doi: 10.1007/s13361-013-0789-4 (2014).
LeDuc, R. D. et al. ProSight PTM: an integrated environment for protein identification and characterization by top-down mass spectrometry. Nucleic Acids Res. 32, W340–345, doi: 10.1093/nar/gkh447 (2004).
Han, X., He, L., Xin, L., Shan, B. & Ma, B. PeaksPTM: Mass spectrometry-based identification of peptides with unspecified modifications. J. Proteome Res. 10, 2930–2936, doi: 10.1021/pr200153k (2011).
Vizcaino, J. A. et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotech. 32, 223–226, doi: 10.1038/nbt.2839 (2014).
Acknowledgements
This work is in part supported by grants from the NTU iFood (grant #: S006), the Singapore Ministry of Education (Tier 1: RGT15/13) and NTU-NHG Ageing Research Grant (ARG/14017). We thank Vincent for his collaboration on this study during his Undergraduate Research Experience on CAmpus (URECA).
Author information
Authors and Affiliations
Contributions
A.S. and R.S.-E.S.-T. performed experiments. A.S. and X.H. performed lunasin extraction. A.S. and X.G. wrote the manuscript. J.P.T. edited the manuscript. S.K.S. conceived the idea, supervised the experiments, wrote and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Serra, A., Gallart-Palau, X., See-Toh, RE. et al. Commercial processed soy-based food product contains glycated and glycoxidated lunasin proteoforms. Sci Rep 6, 26106 (2016). https://doi.org/10.1038/srep26106
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26106
This article is cited by
-
Novel Method for the Production, Purification, and Characterization of Recombinant Lunasin: Identification of Disulfide Cross-Linked Dimers
International Journal of Peptide Research and Therapeutics (2022)
-
System-wide molecular dynamics of endothelial dysfunction in Gram-negative sepsis
BMC Biology (2020)
-
The synergy between natural polyphenol-inspired catechol moieties and plant protein-derived bio-adhesive enhances the wet bonding strength
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.