Abstract
In clinical practice, it is necessary to define an optimal choice from many different therapeutic regimens. This study aimed to assess the efficacy and safety of neoadjuvant endocrine therapy (NET) for breast cancer patients. Randomized clinical trials were included. Nine studies comprising 2133 patients were included in the final analysis. Network meta-analysis showed that everolimus plus letrozole was more easily accepted by patients than exemestane (≥20wks) (odds ratio (OR): 856697.02, 95% confidence intervals (95%CI): 1.88 to 87242934...); exemestane (≥20wks) had worse acceptability than letrozole (OR: 0.00, 95%CI: 0.00 to 0.98). Letrozole produced a better clinical objective response (COR) than tamoxifen (OR: 1.99, 95%CI: 1.04 to 3.80). The incidence of fatigue between the anastrozole plus gefitinib group and the everolimus plus letrozole group was significantly different (OR: 0.08, 95%CI: 0.01 to 0.83). The exemestane (<20wks) plus celecoxib group had fewer hot flushes than others. Ranking showed the everolimus plus letrozole was most likely rank first in comparisons of COR and acceptability, and had a 64% possibility to rank first after stochastic multi-criteria acceptability analysis. In conclusion, our study showed that letrozole plus everolimus is the most effective treatment for postmenopausal, hormone receptor-positive breast cancer in the neoadjuvant setting.
Similar content being viewed by others
Introduction
Breast cancer is a common malignant disease worldwide. Surgery, systemic therapy and radiotherapy, as the main treatment modalities, have significantly improved the prognosis of breast cancer1. Neoadjuvant endocrine therapy (NET), with the advantage of downsizing the tumor before surgery, provides a therapeutic alternative for patients with hormone receptor-positive (HR-positive), postmenopausal breast cancer2. Recently, many randomized clinical trials (RCTs) concerning NET have emerged and its clinical application is gradually gaining recognition. Based on the available research conclusions, more than 90% of experts voted for the use of NET in patients with HR-positive breast cancer during the 13th St. Gallen International Breast Cancer Conference3. Although some research results for NET have been reported, it is difficult to integrate information on the relative efficacy of all tested regimens because most individual trial compared only a few treatments; it is impossible to involve all therapeutic regimens in one trial4. Thus, a summary of these trials may be needed. Network meta-analysis not only synthesizes information from different trials and combines direct and indirect evidence on the relative effectiveness of the treatments, but also can tell us which regimen is appropriate after comparisons of the benefits and risks based on the evidence5,6.
In this study, we assessed the efficacy and safety of NET systematically for postmenopausal, HR-positive, non-metastatic breast cancer by conducting direct and indirect comparisons from RCTs. We aimed to provide a useful summary of different treatment regimens that could be used to guide treatment decisions.
Results
Overview of the Literature Search and Study Characteristics
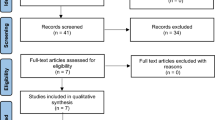
A total of 998 articles were identified in the original database search, of which 973 were discarded after reviewing the titles and abstracts because they clearly did not meet the criteria for inclusion. The remaining full texts were read and six papers were excluded because they derived from two trials. Two papers were repetitive and one was reserved. Another eleven studies were discarded because six studies provided results from either a too small sample size or obviously inadequate information; the tumor size in one study was not assessed using calipers; only therapeutic effects of different dose of fulvestrant were reported in one study; two studies are still under way; and the last one was not a randomized trial. Finally, nine studies were identified and included (Fig. 1)7,8,9,10,11,12,13,14,15.
The Assessment of the Risk of Bias
The pooled risks of bias for the different studies included in this network analysis are presented in Supplementary Figure 1.
Results of Direct Comparisons
The nine studies comprised 2133 patients. The duration of treatment was from 12 to 24 weeks. An investigation into the optimal duration of exemestane was reported in one study8. To make a distinction, we defined exemestane (<20wks) if the duration of exemestane was less than 20 weeks, and exemestane (≥20wks) if the treatment duration was than 20 weeks. There were three arms in two studies, respectively. One study was about anastrozole plus different treatment protocols of gefitinib compared with anastrozole, and we considered anastrozole versus anatrozole plus gifitinib12. As a result, ten arms were assessed including, chemotherapy, tamoxifen, letrozole, anastrozole, exemestane (≥20wks), exemestane (<20wks), anastrozole plus tamxifen, letrozole plus everomilus, anatrozole plus gefitinib, and exemestane (<20wks) plus celecoxib. All patients were postmenopausal women diagnosed with non-metastatic breast cancer. All patients except for four were HR-positive15. Four studies reported the levels of HER27,8,9,11. Characteristics of the eligible studies are listed in Table 1.
The numbers of patients who achieved a clinical objective response (COR) and completed treatment were reported in nine studies. Eight studies provided information about fatigue and hot flushes, and seven studies reported the number of patients that received breast conserving surgery (BCS) after NET. Pathological complete response (pCR) was reported in four studies and only eight (1.1%) patients achieved pCR8,11,15. Direct comparisons were performed and are listed in Table 2. Forest plots are shown in Supplementary Figures 2–6. From the eligible studies, a network diagram of the studies comparing COR was done using Stata, and the result are shown in Fig. 2.
Each link represents at least 1 study and the widths of each link are proportional to the number of studies comparing the particular arms. The size of each node is proportional to the total sample size. CT = chemotherapy, Ana = anastrozole, Tam = tamoxifen, Gef = gefitinib, Let = letrozole, Exe (<20wks) = Exemestane (<20wks), Exe (≥20wks) = Exemestane ((≥20wks), Cel = Celecoxib, Eve = Everolimus.
From direct comparisons, we found that the COR rate in the letrozole group was significantly higher than that in the tamoxifen group (odds ratio (OR): 2.20, 95% confidence interval (95%CI): 1.41 to 3.44, p = 0.001) or the exemestane (<20wks) group (OR: 1.63, 95%CI: 1.01 to 2.64, p = 0.042). Significantly worse acceptability of letrozole was observed compared with letrozole plus everolimus (OR: 0.5, 95%CI: 0.28 to 0.87, p = 0.015); however, the incidence of fatigue in the letrozole group was remarkably lower than in the letrozole plus everolimus group (OR: 0.34, 95%CI: 0.13 to 0.89, p = 0.028). Besides, patients taking anastrozole suffered less fatigue than those taking tamoxifen (OR: 0.47, 95%CI: 0.22 to 0.98, p = 0.044). The incidence of hot flushes in the letrozole group was significantly higher than in the exemestane (<20wks) (OR: 2.47, 95%CI: 1.30 to 4.70, p = 0.006), exemestane (<20wks) plus celecoxib (OR: 8.44, 95%CI: 2.55 to 27.91, p = 0.0001) or chemotherapy (OR: 6.92, 95%CI: 1.29 to 37.29, p = 0.024) groups. More patients accepted BCS after taking anastrozole than among those taking tamoxifen (OR: 1.95, 95%CI: 1.26 to 3.02, p = 0.003) or letrozole (letrozole vs. anastrozole (OR: 0.39, 95%CI: 0.18 to 0.84, p = 0.016)).
Bayesian Network Meta-Analysis
To assess the consistency and inconsistency in the network meta-analysis, node-splitting analyses were performed. Which revealed no statistical differences between the direct and indirect evidence. From the eligible studies, indirect comparisons were then performed. The outcomes of indirect comparisons of COR, treatment completion (TC) and adverse events are shown in Tables 3 and 4.
Network meta-analysis showed that everolimus plus letrozole more easily accepted by patients than exemestane (≥20wks) (OR: 856697.02, 95%CI: 1.88 to 87242934…), and exemestane (≥20wks) was also had worse acceptability than letrozole (OR: 0.00, 95%CI: 0.00 to 0.98). There was a statistically significant difference between letrozole and tamoxifen group in the comparison of COR (OR: 1.99, 95%CI: 1.04 to 3.80). In addition, the incidence of fatigue between the anastrozole plus gefitinib group and the everolimus plus letrozole group showed a significant difference (OR: 0.08, 95%CI: 0.01 to 0.83). The incidence of hot flushes in the exemestane (<20wks) plus celecoxib group seem to be the lowest and four comparisons had statistically significant differences: anastrozole vs. exemestane (<20wks) + celecoxib (OR: 8.44, 95%CI: 1.53 to 48.18), anastrozole + tamoxifen vs. exemestane (<20wks) + celecoxib (OR: 13.11, 95%CI: 1.76 to 109.65), exemestane (<20wks) + celecoxib vs. letrozole (OR: 0.11, 95%CI: 0.02 to 0.47), and exemestane (>20wks) + celecoxib vs. tamoxifen (OR: 0.99, 95%CI: 0.02 to 0.52)). Furthermore, the incidence of hot flushes in the chemotherapy group was significantly lower than in another three treatment regimens (chemotherapy vs. letrozole (OR: 0.12, 95%CI: 0.01 to 0.76), chemotherapy vs. tamoxifen (OR: 0.11, 95%CI: 0.01 to 0.86), and anastrozole plus tamoxifen vs. chemotherapy (OR: 11.94, 95%CI: 1.14 to 171.80)).
Rankings for the outcomes of COR, TC, BCS and adverse events in the present analysis were also performed. The probabilities were calculated for a total of 100%, both within a rank over interventions and within an intervention over ranks. The top and second highest percentage within each intervention are shown in Table 5. Besides, a subgroup analysis was performed involving complete response (CR) and partial response (PR) (Supplementary Figures 7–13).
Rankings showed that everolimus plus letrozole had the highest probability to rank first in the comparisons of COR (62%), PR (45%) and acceptability (44%). Seven studies reported information about the BCS rate. From the limited data, we found that more patients could accept BCS after receiving anastrozole plus gefitinib (69%).
Stochastic Multi-criteria Acceptability Analysis (SMAA)
The SMAA benefit-risk analyses were based on evidence synthesis. The criteria were COR, TC, and the alternatives were treatment arms. Ranking for SMAA benefit-risk analysis is shown in Supplementary Figure 14. The first and second high percentages within each intervention over ranks are shown in Table 5.
SMAA benefit-risk analyses suggested that everolimus plus letrozole, having a 64% possibility to rank first, was the best treatment arm when considering COR and TC, and letrozole was the second choice.
Discussion
The use of neoadjuvant chemotherapy in the treatment of locally advanced breast cancer is well established. However, endocrine therapy, with lower toxicity, can be a valid alternative to chemotherapy in the treatment of hormone-sensitive tumors, particularly in postmenopausal women16. It can downsize tumors and provide an early measurement tool to evaluate response to endocrine therapy3. Here, we presented a meta-analysis of the efficacy of the available studies involving NET.
From the direct and indirect comparisons, we found that the letrozole group had a higher COR rate than the tamoxifen group. This was consistent with previous reports. For example, a study involving meta-analyses of two cohorts concerning adjuvant endocrine therapy demonstrated efficacy and superiority of aromatase inhibitors (AIs) when compared with tamoxifen17,18. In breast cancer, the PI3K/Ak/mTOR pathway is important in the clinical sensitivity of breast cancer to endocrine therapy. Everolimus, an mTOR inhibitor, can restore sensitivity to endocrine therapy19. A phase III randomized trial showed that everolimus combined with an AI could improve progression-free survival in patients with HR-positive, advanced breast cancer previously treated with non-steroidal AIs20. Ranking in this study also showed that everolimus plus letrozole might be the best choice for patients to reach COR and was more easily accepted.
In addition, we found that chemotherapy was the first choice for patients to obtain a CR. However, a phase 2 randomized trial of primary endocrine therapy versus chemotherapy did not show a significant difference for pCR (3% vs. 6%) and disease progression (9% vs. 9%) rates, respectively (p>0.05). Besides, the rate of BCS was slightly higher in the endocrine group (33% vs. 24%; p = 0.058)21. The sample size in the chemotherapy arm was small and pCR was not analyzed in this study; therefore, more trials will be needed to compare NET with chemotherapy.
Compared with chemotherapy, an important superiority of endocrine therapy is its lower toxicity. In the nine included studies, severe adverse events were rarely reported and the most common side effects were fatigue and hot flushes. Although everolimus plus letrozole produced a higher incidence of fatigue, it was still more easily accepted. Ranking also showed that everolimus plus letrozole had the highest probability to rank first for acceptance. Hot flushes were another common side effect. This study suggested that anastrozole plus tamoxifen had a 57% probability to rank first and exemestane (<20wks) plus celecoxib had a 47% probability to rank last. Therefore, when patients have severe hot flushes after receiving endocrine therapy, celecoxib, a non-steroidal anti-inflammatory drug (NSAID), is a good choice.
In this study, there was no significant difference between the exemestane (≥20wks) and exemestane (<20wks) groups in terms of reaching COR and complete treatment. Ranking also struggled to decide which one was better than the other. In addition, adverse events in the original study investigating optimal duration of exemestane therapy were not available8. Thus, it was hard to produce a comprehensive analysis. SMAA suggested that exemestane (≥20wks) ranked last and exemestane (<20wks) ranked sixth or seventh. A phase II study that investigated preoperative treatment with exemestane for 6 months in postmenopausal patients with HR-positive breast cancer showed a more beneficial effect for 6 months22. However, Hojo demonstrated that responses were equal during 4 or 6 months of exemestane treatment, and showed that 4-months of treatment with exemestane appeared to be warranted in postmenopausal patients because of its increased acceptability8. Therefore, the optimal duration of exemestane remains controversial.
This study provided an insight into the NET for HR-positive, postmenopausal breast cancer. However, it had some limitations. First, the number of studies and the patients included are relatively limited. Second, for the comparisons in the network meta-analysis, no direct evidence was available, and indirect comparisons might cause heterogeneity. Third, we did not consider the influence of diversity of ethnicity and the SMAA benefit-risk analysis only analyzed two criteria23. Finally, the indicator of this study is limited. The COR was restricted to being assessed using calipers, without considering other assessment methods, such as ultrasound and mammography. Therefore, future studies will be needed to assess more indicators and consider more influencing factors.
In conclusion, our study proved that letrozole plus everolimus is the most effective treatment for postmenopausal, HR-positive breast cancer in the neoadjuvant setting. In addition, when patients have hot flushes during the period of NET, NSAIDs, such as celecoxib, are recommended.
Methods
Search Strategy
Studies were identified by searching Embase database, the Cochrane library and PubMed with the following search terms: breast cancer or breast neoplasm or breast carcinoma; neoadjuvant or preoperative; endocrine therapy or hormonal therapy. The searches were limited to studies written in English with full text. There was no date restriction. In addition, we screened the references of all studies fulfilling the eligibility criteria in case we missed some relevant articles by the electronic searches.
Selection Criteria
Randomized trials that compared at least two arms of different treatment regimens involving NET in postmenopausal patients with HR-positive, non-metastatic breast cancer were considered. There were no dose and duration restrictions. All titles and abstracts were screened to exclude obviously unmatched articles and the remaining full texts were read for further identification. If multiple publications of the same trial were retrieved, only the most informative publication was included. Risk of bias in the studies was assessed by two authors (Wang and Zhou) for quality; appropriateness of allocation, blinding, and management of incomplete outcome data; the completeness of reporting of outcomes and other bias using the Cochrane Collaboration risk of bias tool24.
Data Extraction
A data extraction sheet based on Excel was developed. Data were extracted independently by two authors (Wang and Zhou) including: characteristics of trial participants (age, gender, menopausal status, HR and HER2 status, histological type, clinical tumor status, tumor grade and nodal status), the inclusion and exclusion criteria in each trial, type of intervention (type, dose, duration and frequency) and outcomes.
Definition of Outcomes
The primary outcome in this study was the number of patients that achieved COR. COR included CR and PR. They were defined according to UICC, WHO or RECIST criteria. The tool used for tumor assessment in the studies was restricted to calipers. Other endpoints were the number of patients who completed treatment and the number of patients with adverse events. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (Version 2.0 or 3.0) with no grade restrictions. The adverse events concerned in this study were fatigue and hot flushes. The numbers of patients who reached pCR and received BCS were also considered.
Statistical Methods
In the direct comparisons, OR was utilized for pooling effect sizes because most of the outcomes were dichotomous variables. If a direct comparison was based on two or more studies, statistical heterogeneity was calculated using the I2 statistic. Furthermore, we defined I2 above 50% as a large between-study heterogeneity. If there was no significant heterogeneity, data were pooled using the Mantel-Haenszel fixed effects model25. Results were reported with OR and 95%CI. All statistical tests were two-sided.
For comparisons between two interventions with both direct and indirect evidence, the consistency between these types of evidence was verified by the node-split analysis provided in the Aggregate Data Drug Information System (ADDIS), an open source evidence-based drug oriented strategy decision support system26. If there was no significant inconsistency, the relative effects of the interventions were analyzed using a consistency model based on a random-effects Bayesian model provided by the ADDIS software27,28. Benefit-risk analysis was performed using SMAA. The results of the analysis are presented as OR with 95% CI. Ranking for each treatment was performed by calculating the probability of each arm to achieve the best rank among all treatments. In addition, sensitivity analyses were considered.
Direct comparisons and risk of bias across studies were assessment by Stata, Version 11.2 (Stata Corp, College Station, TX, USA). Risk of bias in individual studies was assessed by Review Manager (RevMan), Version 5.3 (The Nordic Cochrane Centre: The Cochrane Collaboration, Copenhagen, Norway). Bayesian network meta-analyses and the node-splitting analyses were calculated by ADDIS,Version1.16.5. The reporting of this meta-analysis was done according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines29.
Additional Information
How to cite this article: Wang, W. et al. Network Meta-Analysis of the Effectiveness of Neoadjuvant Endocrine Therapy for postmenopausal, HR-Positive Breast Cancer. Sci. Rep. 6, 25615; doi: 10.1038/srep25615 (2016).
References
Acevedo, F., Herrera, M. E., Madrid, J. & Sanchez, C. Neoadjuvant endocrine therapy in breast cancer. Revista medica de Chile 141, 367–374 (2013).
Palmieri, C., Patten, D. K., Januszewski, A., Zucchini, G. & Howell, S. J. Breast cancer: current and future endocrine therapies. Molecular and cellular endocrinology 382, 695–723 (2014).
Charehbili, A. et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: A systematic review. Cancer treatment reviews 40, 86–92 (2014).
Nagayama, A. et al. Comparative effectiveness of neoadjuvant therapy for HER2-positive breast cancer: a network meta-analysis. Journal of the National Cancer Institute 106, doi: 10.1093/jnci/dju203 (2014).
Ades, A. E. et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics 24, 1–19 (2006).
Caldwell, D. M., Ades, A. E. & Higgins, J. P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj 331, 897–900 (2005).
Palmieri, C. et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast cancer research and treatment 148, 581–590 (2014).
Hojo, T. et al. Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: a randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. Breast (Edinburgh, Scotland) 22, 263–267 (2013).
Ellis, M. J. et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29, 2342–2349 (2011).
Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27, 2630–2637 (2009).
Chow, L. W., Yip, A. Y., Loo, W. T., Lam, C. K. & Toi, M. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. The Journal of steroid biochemistry and molecular biology 111, 13–17 (2008).
Smith, I. E. et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25, 3816–3822 (2007).
Cataliotti, L. et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer 106, 2095–2103, (2006).
Smith, I. E. et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. Journal of Clinical Oncology 23, 5108–5116 (2005).
Eiermann, W. et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 12, 1527–1532 (2001).
Viola, G., Sergi, D., Conti, F. & Lopez, M. [Neoadjuvant endocrine therapy for locally advanced breast cancer]. La Clinica terapeutica 158, 441–452 (2007).
Dowsett, M. et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 509–518 (2010).
Van Asten, K., Neven, P., Lintermans, A., Wildiers, H. & Paridaens, R. Aromatase inhibitors in the breast cancer clinic: focus on exemestane. Endocrine-related cancer 21, R31–49 (2014).
Gnant, M., Greil, R., Hubalek, M. & Steger, G. Everolimus in postmenopausal, hormone receptor-positive advanced breast cancer: summary and results of an austrian expert panel discussion. Breast care 8, 293–299 (2013).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England journal of medicine 366, 520–529 (2012).
Semiglazov, V. F. et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110, 244–254 (2007).
Barnadas, A. et al. Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: a phase II trial. British journal of cancer 100, 442–449 (2009).
Lahdelma, R. & Salminen, P. Prospect theory and stochastic multicriteria acceptability analysis (SMAA). Omega-Int J Manage S 37, 961–971 (2009).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928 (2011).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558 (2002).
Gupta, A. K. & Paquet, M. Network meta-analysis of the outcome ‘participant complete clearance’ in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. The British journal of dermatology 169, 250–259 (2013).
Veroniki, A. A., Vasiliadis, H. S., Higgins, J. P. & Salanti, G. Evaluation of inconsistency in networks of interventions. International journal of epidemiology 42, 332–345 (2013).
van Valkenhoef, G., Tervonen, T., Zwinkels, T., de Brock, B. & Hillege, H. ADDIS: A decision support system for evidence-based medicine. Decis Support Syst 55, 459–475 (2013).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj-Brit Med J 339, doi: 10.1136/Bmj.B2700 (2009).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81071753, 81172502, 81202077 and 81272916), the Natural Science Foundation of Jiangsu Province (BK20141023), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU (IRT-008), and a project Funded by the Priority Academic Program Development of Jiangsu Higher Education Insti-tutions (PAPD).
Author information
Authors and Affiliations
Contributions
Study concept and design: S.W. and W.W. Acquisition of data: W.W. and W.B.Z. Analysis and interpretation of data: W.W., C.H.L., W.B.Z., T.S.X., H.X. and S.W. Drafting of the manuscript: W.W. and W.B.Z. Critical revision of the manuscript for important intellectual content: W.W., C.H.L., W.B.Z., T.S.X., H.X. and S.W. Study supervision: S.W. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, W., Liu, C., Zhou, W. et al. Network Meta-Analysis of the Effectiveness of Neoadjuvant Endocrine Therapy for Postmenopausal, HR–Positive Breast Cancer. Sci Rep 6, 25615 (2016). https://doi.org/10.1038/srep25615
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25615
This article is cited by
-
Breast-conserving surgery is an appropriate procedure for centrally located breast cancer: a population-based retrospective cohort study
BMC Surgery (2023)
-
SMAA methods and their applications: a literature review and future research directions
Annals of Operations Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.