Abstract
Hormone replacement therapy (HRT) is associated with risk of vascular disease. The association between atrial fibrillation (AF), vascular events and different HRTs, including estradiol and conjugated equine estrogens (CEE), has been controversial in previous studies. Thus, we conducted a retrospective cohort study to investigate these associations. Female patients (>45 years old) first diagnosed with menopause were enrolled from National Health Insurance Research Dataset (1998–2008). Cox regression analysis estimated risk of new-onset AF, stroke and major adverse cardiac events (MACE) after exposure to estradiol or CEE. Of 5489 females (mean age = 55 years) enrolled, 1815 treated with estradiol and 3674 treated with CEE. Incidence per 103 person-years of AF, stroke and MACE in CEE vs estradiol patients was 2.23 vs. 0.92, 14.0 vs. 9.09 and 15.55 vs. 10.47. As compared with patients treated with estradiol, those treated with CEE had a significantly higher incidence of AF, stroke and MACE. The adjusted hazard ratios for each category were 1.96, 1.30 and 1.26, respectively. The significant results remained similar, even after use of propensity-score-matched strategy. In conclusion, CEE was associated with a higher risk of AF, stroke and MACE than estradiol in menopausal females. Further exploration of underlying mechanisms is necessary.
Similar content being viewed by others
Introduction
Hormone replacement therapy (HRT) is widely used to treat menopausal symptoms, but studies including the Women’s Health Initiative (WHI) have indicated that HRT is associated with an increased risk of coronary heart disease (CHD), stroke and venous thromboembolic disease regardless of years of therapy since menopause1,2,3. In contrast, the results of a clinical trial showed that the risk of stroke was not significantly different between patients receiving conjugated estrogen plus progestin and those receiving a placebo4. Despite the uncertain effects and mechanisms of HRT on the risk of stroke, current evidence has indicated that HRT may still play a role in the incidence of stroke.
Women experiencing stroke have a higher prevalence of atrial fibrillation (AF) than men, but these gender differences remain largely unexplained5. In women >65 years6, AF is independently associated with a 22–25% increased risk of stroke and a 1.7-fold increased risk of all-cause mortality7. Clinically, AF is also a major risk factor contributing to ischemic stroke. However, to the best of our knowledge, there is no evidence that a higher AF incidence may result in a higher stroke incidence with HRT treatment. Furthermore, despite the importance of the relationship between AF and HRT, this relationship remains largely undescribed and controversial8,9. At present, there is still a lack of evidence regarding factors that may modulate the risks involved in HRT treatment, such as different estrogen and progestogen preparations and different doses and routes of administration. Thus, we evaluated the risk of AF, stroke and cardiovascular diseases (CVDs) in menopausal women in Taiwan receiving different types of HRT.
Results
Baseline characteristics
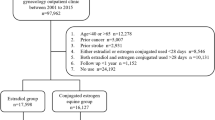
A total of 5489 females were enrolled in the final analysis from the 2000 National Health Insurance Research dataset (NHIRD; Fig. 1). Of these patients (mean age = 55 years), 1815 were treated with estradiol and 3674 were treated with conjugated equine estrogens (CEE; Table 1). Patients in the CEE group were older and had a higher prevalence of diabetes, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), liver disease and use of calcium channel blockers (CCBs) but a lower rate of sleep apnea and statin use than those in the estradiol group. The follow-up period in our patients was 7.8 [standard deviation (SD) = 3.02] years. The average drug exposure time was 0.51 (SD = 0.87) years in the estradiol group and 0.79 (SD = 1.29) years in the CEE group. The total average follow-up time in each group was 6.50 (SD = 3.04) and 8.28 (SD = 0.79) years in the estradiol and CEE groups, respectively. After matching by propensity score, we found that the baseline characteristics, including age, were comparable in two groups (Supplementary eTable 1).
AF and stroke endpoints
From a 10-year survey, 78 cases were noted with new-onset AF (11 in the estradiol and 68 in the CEE group). Furthermore, 512 subjects (105 in the estradiol and 407 in the CEE group) had a first-time stroke. The incident rate of AF was 0.92 and 2.23 per 103 person-years in the estradiol and CEE groups, respectively, whereas the incident rate of stroke was 9.09 and 14.0 per 103 person-years, respectively. Figure 2A,B depict the Kaplan–Meier (KM) curves for the AF and stroke incidences in the estradiol and CEE groups, respectively.
In the multivariate Cox proportional hazards models, age, valvular heart disease (VHD) and treatment with CEE were associated with AF (Table 2), whereas age, diabetes mellitus (DM), hypertension and treatment with CEE were associated with stroke (Table 3). Other comorbidities, concomitant drug use and income group did not associate with AF and stroke in our analysis, as shown in Tables 2 and 3. The adjusted hazard ratio (HR) for AF and stroke in patients treated with CEE versus estradiol was 1.96 [95% confidence interval (CI), 1.03–3.73, P = 0.042 and 1.30 (95% CI, 1.04–1.62, P = 0.021), respectively. The significant results remained similar, even after the matching by propensity score (Supplementary eTables 2 and 3).
Major adverse cardiac event (MACE) endpoints
In total, 606 subjects (125 in the estradiol and 481 in the CEE group) had MACEs during the follow-up period. The incidence of MACE in the CEE and estradiol groups was 15.55 and 10.47 per 103 person-years, respectively. KM curves showing the difference in the incidence of MACE between the groups are presented in Fig. 3. The incidence of MACE was increased in the CEE group as compared with that in the estradiol group.
Table 4 demonstrates that age, DM, hypertension and treatment with CEE were correlated with the incidence of MACE in a multivariate Cox proportional hazards model. The adjusted HR for MACE in the CEE group was 1.26 (95% CI, 1.03–1.54, P = 0.025) as compared with the estradiol group, which results were similar by the use of propensity-score-matched strategy (Supplementary eTable 4).
Regarding specific cardiac events, only the incidence of stroke was higher in the CEE group than that in the estradiol group; the adjusted HR of acute myocardial infarction (AMI) 2.23 (95% CI, 0.68–7.75, P = 0.179), percutaneous coronary intervention (PCI) 0.89 (95% CI, 0.43–1.85, P = 0.752), coronary artery bypass grafting (CABG) 1.19 (95% CI, 0.11–12.55, P = 0.887) and mortality 0.89 (95% CI, 0.49–1.65, P = 0.732) were not different between the groups, respectively.
Discussion
In the present study, we found that CEE use in postmenopausal Taiwanese women was associated with a higher incidence of AF than estradiol use in both before and after propensity-score-matched strategy. To the best of our knowledge, this is the first study to demonstrate that CEE use is associated with a higher AF rate than estradiol use in HRT of postmenopausal women. The incidence of stroke and MACE were also higher in the CEE group than in the estradiol group in our study. The main contribution to the higher MACE incidence was a higher stroke rate in the CEE group than in the estradiol group; however, acute myocardial infarction and mortality were not increased, which is comparable with the findings of other studies10. The higher AF incident rate may be a causal factor for the higher stroke incidence in the CEE group than in the estradiol group.
The association between HRT and CVD is controversial. Postmenopausal hormone use could decrease the risk for MACE in women without a previous heart disease even at low doses (0.3 mg) of oral conjugated estrogen daily. However, estrogen at daily doses of 0.625 mg or greater may increase the risk for stroke11. The combination of CEE at 0.625 mg/d and medroxyprogesterone acetate at 2.5 mg/d significantly increased the risk of CHD, stroke and pulmonary embolism but not all-cause mortality in WHI randomized controlled trials2. A secondary analysis of the same trials confirmed this trend; however, the difference was not significant, showing that HRT closer to menopause tended to reduce the risk for CHD and total mortality as compared to women more distant from menopause3. However, the risk of stroke was elevated regardless of years since menopause. With respect to different populations, the conclusion that HRT is associated with CVD also remains controversial3,12. In this study, we did not include the never-use HRT group for comparison due to the high possibility of non-comparable issue between the use and never-use HRT groups. Because this study was to compare the effect of different types of HRT use on the risk of AF, stroke, or MACE, rather than the comparison between the uses and never use HRT groups, we were unable to answer the relationship between HRT use and the risk of AF, stroke, or MACE.
New-onset AF was independently associated with AMI, CHF and stroke in a group of healthy women4. The all-cause mortality rate was also associated with new-onset AF but not new-onset paroxysmal AF in the same study. Wolf et al.6 found that among women >75 years with CVD, those with AF were significantly more likely to be admitted with a stroke than age-matched female patients without AF even after 3 years of follow-up. There was no similar trend in men with CVD in that study. AF in women associated with stroke and mortality is an important issue. A study by Chien et al. demonstrated that the incidence of AF in Taiwanese women were 0.76 per 103 person-years using community-based data13. In addition, another study also showed that the frequency of AF increased from 37 per million people 50–59 years old to 147 per million people 60–69 years old, 457 per million people 70–79 years old and 1631 per million people 80 years old or older using the NHIRD14. Our data are compatible with studies that found higher AF incidence (3.15 per 103 person-years or 1421 per million people) in menopausal women, particularly in the CEE group (2.23 per 103 person-years) as compared to the estradiol group (0.92 per 103 person-years)13,14. Similarly, our results show that stroke and MACE are also found at higher frequencies in menopausal women15. It may be important to use estradiol but not CEE for HRT in menopausal women to reduce the risk of AF and stroke. Furthermore, age is an important risk factor in CVD and AF16. Our data showed that patients in the CEE group (mean age = 55.73 years) were older than those in the estradiol group (mean age = 53.21 years). The older age of women in the CEE group may have contributed to the higher AF risk than that in the estradiol group. However, after adjusting for age in a Cox regression model with different outcomes, estradiol still showed a greater protective effect against AF, stroke and MACE than CEE exposure.
Research regarding the association between HRT and AF is limited and controversial. Perez et al.8 found that the incidence of AF was moderately elevated in women undergoing hysterectomy and those with an intact uterus receiving CEE, but not in women with an intact uterus receiving estrogen plus progestin. Bretler et al.9 demonstrated that HRT is associated with a decreased risk of new-onset AF in female AMI patients during the first year after discharge. To the best of our knowledge, there has been no analysis of the effects of AF in different types of HRT. Instead, overall HRT was associated with a decreased risk of AF, particularly in women ≥80 years old. In our study, the cumulative incidence rate of AF was lower in the estradiol group than in the CEE group, which shows that the effects on AF in different HRT are heterogeneous.
The main biological mechanism of AF in menopausal women using HRT remains unclear. Previous data has shown that CEE has longer-lasting metabolites in humans than transdermal estradiol. Moreover, CEE and its metabolites may be a cause of inflammation, whereas estradiol has a short half-life and is not proinflammatory17. Inflammation has a potentially important role in mediating systemic CVD18,19. Inflammation may be a cause of hypercoagulability of the blood and a trigger mechanism for AF; therefore, CEE may increase the inflammatory effect to cause AF. Atrial remodeling and oxidative stress have been suggested to play a role in the pathogenesis of AF20,21. Xie et al. also identified a link between oxidative stress22,23 and aberrant intracellular Ca (2+) release via the type 2 ryanodine receptor (RyR2) that promotes AF21. Despite the fact that some studies have demonstrated that HRT has beneficial effects on oxidative stress in postmenopausal women24,25, studies on the direct vascular effects of HRT remain sparse and the mechanism has not been completely elucidated. Moreover, the mechanisms linking HRT and oxidative stress or intracellular Ca (2+) to AF are not well understood. Evidence of AF in menopausal women using CEE or estradiol is currently unavailable and further research is warranted to examine the underlying mechanism.
Several limitations were present in this study. First, because all information about drugs prescription was from claim data, we assumed that all patients completely adhered to the physicians’ instructions. However, some potential bias cannot be completely ruled out because some patients may not have used their HRT prescriptions and some may have used over-the-counter HRT. This may have resulted in some measurement error in terms of HRT exposure, including the potential systematic underestimation of HRT exposure duration, misclassification of population into cohort, or exclusion of some target populations in this study. Because those misclassifications seemed to happen in the two groups, the random misclassification, which underestimated the significance, was likely26. Second, the diagnosis of AF and other comorbidities from the NHIRD coding may not always be accurate. However, we have validated these diagnoses in our previous study16,26. Previous studies have used the Longitudinal Health Insurance Database (LHID) 2000 to assess the epidemiology of AF or menopause27,28. Despite the fact that there was a bias from including the number of patients using LHID 2000 in different studies, reliability was higher and standardization of the methodology was similar among different studies27,28. Furthermore, claims data can be used to identify patients with AF and menopause because of a high positive predictive value26,29. Third, no information was available regarding laboratory data, blood pressure, hormone level (in the blood), or lifestyle, which may be potential confounders. Finally, the patients in our sample were Han Chinese; therefore, generalization of the results to other ethnic or racial groups must be made with caution. In conclusion, from a large national population database, CEE had a higher risk of AF, stroke and MACE than estradiol use for HRT in menopausal women in Taiwan. CEE was also an independent risk factor for AF, stroke and MACE in menopausal women receiving HRT. Further exploration of the underlying mechanisms is warranted.
Methods
Data sources
This population-based retrospective cohort study was carried out using information from the NHIRD in Taiwan, which contains encrypted computerized outpatient care claims, hospital inpatient care, ambulatory care, dental services and prescription drug records.
The LHID 2000 contains all the original claim data of 1,000,000 individuals randomly sampled from beneficiaries of the NHIRD in 2000, which maintains the registration data of everyone who was a beneficiary of the National Health Insurance (NHI) program during the period of 1996–2000. The NHI program enrolled about 23 million people, approximately 99% of the Taiwanese population. All registration and claim data of the 1,000,000 individuals collected by the NHI program constitute the LHID 2000. There was no significant difference in the gender distribution (χ2 = 1.74, df = 1, P = 0.187) between the patients in the LHID 2000 and the original NHIRD.
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-EXEMPT-20140064). Current NHIRD and hospital regulations and guidelines did not mandate informed consent in this retrospective cohort study. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the directives of the Declaration of Helsinki.
Study sample
We identified all female patients over the age of 45 years as those having either an outpatient visit or an inpatient hospitalization with menopause, International Classification of Diseases, Ninth Revision (ICD-9: 627), diagnosed between 1998 and 2008 from LHID 2000. The date of the first menopause diagnosis was assigned as the index date.
We excluded the patients who had not been prescribed HRT after the index date or had ever been prescribed HRT before the index date. Otherwise, patients prescribed with HRT one time only were also excluded from the study population. To evaluate the systemic effect of estrogen, we included patients taking an oral form of HRT after the index date only. Patients diagnosed with stroke, cancer, AF, amenorrhea (primary and secondary diagnostic codes), AMI, deep vein thrombosis and those receiving PCI and CABG surgery before the index date were excluded from our study population.
Hormone replacement therapy
Female patients diagnosed with menopause who received at least two prescriptions of HRT within the follow-up time were included in this study. Patients who were prescribed estradiol after discharge were categorized as estradiol users and those prescribed with CEE were divided into a CEE users group.
To decrease the immortal-time bias because of the delay of first-time prescribed HRT after index date, we selected patients prescribed with first-time HRT within 30 days after the index date only. Additionally, patients who received a prescription of both estradiol and CEE during follow-up were excluded to avoid interactions between estradiol and CEE in our study.
Comorbidities, prescribed medications and study endpoints
The comorbidities of patients in our study were identified by their diagnoses in the International Classification of Diseases, Ninth Revision (ICD9) codes of one in inpatient diagnosis and one in outpatient diagnosis 1 year before the index date. The comorbidities included hypertension, DM, hyperlipidemia, liver disease, COPD, CHF, sleep apnea, thyroid disease, aortic atherosclerosis, CKD, VHD and ischemic heart disease (excluding AMI). Income group was classified by the individual’s yearly gross income during a 1-year period before the index date. We defined the low income group according to an annual income of less than or equal to NT$894,574.00 or US$29,819.10, the national average annual household income in 2005 in Taiwan (Source: Directorate General of Budget, Accounting and Statistics, Executive Yuan, Taiwan). Alternatively, the high income group was defined as having an annual income of greater than this amount.
Data on claimed prescriptions included quantity, dispensing date, drug type and dose. The claimed data of medication prescribed in hospital and outpatient visits were used to determine medical treatment and duration. Use of concomitant drugs was identified according to claimed prescriptions one year before the index date. These prescribed drugs included diuretics, beta-receptor antagonists, angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), calcium channel blockers (CCB), alpha-receptor antagonists, nitrates, aspirin, amiodarone, warfarin, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, Cox-II inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), steroids and oral anti-diabetic drugs.
The endpoints in this study included first-time diagnosis of AF and MACE. MACE was defined as either stroke, AMI, PCI, CABG, or death. These endpoints were obtained by ICD-9 code in once hospital records or over twice diagnoses in outpatient claims. Information about mortality was also coded in hospital claims. Patients in this study were followed until a diagnosis of outcome or events were recorded, death occurred, or patients withdrew from the NHI. The collection period ended on 31 December, 2009. These endpoints were estimated separately over time and compared with estradiol and CEE exposure in our survey. The methods of determining the diagnostic code, protocols regarding medication administration and the diagnosis procedures related to complications utilized in this study were validated in our previous study16.
Statistics
All data are expressed as frequency (percentage) and mean ± SD. Continuous and categorical variables were compared between estradiol users and CEE users with Student’s t-test or the chi-square test, as appropriate.
Because the main interest of this study was HRT exposure and the risk of AF, each female participant was followed to accumulate person-time beginning from the index date to the newly-onset AF during an 11-year follow-up period. If patients were present to have other endpoints (stroke or MACE) or died from other causes before the onset of AF, they were censored to account for the competing risks attributable to other causes30. All cases with no endpoint or death occurring during follow-up were also censored.
Kaplan–Meier analysis and log-rank testing were used to examine the relationship of the cumulative incidence rate of outcomes between estradiol and CEE users. A Cox proportional hazards model was used to estimate HR of cardiovascular outcomes with estradiol and CEE after adjusting for other covariates. The covariates in the model included age, comorbidities and administration of medications.
To consider for the issue of potential non-comparable baseline characteristics between two groups of estradiol and CEE, we also used propensity-score-matched strategy to match the covariates, listed in Table 1, as 1:2 by using a ‘greedy’ matching algorithm, with a maximum calliper of 0.1, for analysis31,32. All statistical analysis were carried out using SAS software (version 9.3; SAS Institute, Inc., Cary, NC, USA). Statistical significance was inferred at a two-sided P-value of <0.05.
Additional Information
How to cite this article: Tsai, W.-C. et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci. Rep. 6, 24132; doi: 10.1038/srep24132 (2016).
References
Wassertheil-Smoller, S. et al. Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women. JAMA. 289, 2673–2684 (2003).
Rossouw, J. E. et al. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women. JAMA. 288, 321–333 (2002).
Rossouw, J. E. et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 297, 1465–1477 (2007).
Simon, J. A. et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS). Circulation. 103, 638–42 (2001).
Haast, R. A., Gustafson, D. R. & Kiliaan, A. J. Sex differences in stroke. J Cereb Blood Flow Metab. 32, 2100–2107 (2012).
Wolf, P. A., Mitchell, J. B., Baker, C. S., Kannel, W. B. & D’Agostino, R. B. Impact of atrial fibrillation on mortality, stroke and medical costs. Arch Intern Med. 158, 229–234 (1998).
Conen, D. et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 305, 2080–2087 (2011).
Perez, M. V. et al. Effects of Postmenopausal Hormone Therapy on Incident Atrial Fibrillation: The Women’s Health Initiative Randomized Controlled Trials. Circ Arrhythm Electrophysiol. 5, 1108–1116 (2012).
Bretler, D.-M. et al. Hormone Replacement Therapy and Risk of New-Onset Atrial Fibrillation after Myocardial Infarction - A Nationwide Cohort Study. PLoS ONE. 7, e51580 (2012).
Smith, N. L. et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 174, 25–34 (2014).
Grodstein, F. et al. A Prospective, Observational Study of Postmenopausal Hormone Therapy and Primary Prevention of Cardiovascular Disease. Ann Intern Med. 133, 933–941 (2000).
Su, I. H. et al. Risks and benefits of menopausal hormone therapy in postmenopausal Chinese women. Menopause. 19, 931–941 (2012).
Chien, K. L. et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 139, 173–180 (2010).
Lee, C. H. et al. Characteristics of hospitalized patients with atrial fibrillation in Taiwan: a nationwide observation. Am J Med. 120, 819e1–7 (2007).
Mikkola, T. S. et al. Estradiol-based postmenopausal hormone therapy and risk of cardiovascular and all-cause mortality. Menopause. 22, 976–983 (2015).
Tsai, W. C. et al. Areca nut chewing and risk of atrial fibrillation in Taiwanese men: a nationwide ecological study. Int J Med Sci. 10, 804–811 (2013).
Shifren, J. L. et al. A comparison of the short-term effects of oral conjugated equine estrogens versus transdermal estradiol on C-reactive protein, other serum markers of inflammation and other hepatic proteins in naturally menopausal women. J Clin Endocrinol Metab. 93, 1702–1710 (2008).
Sin, D. D. & Man, S. F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 107, 1514–1519 (2003).
Wang, Q., Tang, X. N. & Yenari, M. A. The inflammatory response in stroke. J Neuroimmunol. 184, 53–68 (2007).
D’Ascia, S. L. et al. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int J Clin Pract. 65, 1149–1155 (2011).
Jalife, J. & Kaur, K. Atrial remodeling, fibrosis and atrial fibrillation. Trends Cardiovasc Med. 25, 475–84 (2015).
Xie, W. et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 5, 11427 (2015).
Xie, W. et al. Imaging atrial arrhythmic intracellular calcium in intact heart. J Mol Cell Cardiol. 64, 120–123 (2013).
Ceravolo, G. S. et al. Conjugated equine estrogen treatment corrected the exacerbated aorta oxidative stress in ovariectomized spontaneously hypertensive rats. Steroids. 78, 341–346 (2013).
Chang, S. P. et al. Effects of hormonal replacement therapy on oxidative stress and total antioxidant capacity in postmenopausal hemodialysis patients. Ren Fail. 24, 49–57 (2002).
Cheng, C. L., Kao, Y. H., Lin, S. J., Lee, C. H. & Lai, M. L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 20, 236–242 (2011).
Wang, C. C. et al. Atrial fibrillation associated with increased risk of venous thromboembolism. A population-based cohort study. Thromb Haemost. 113, 185–192 (2015).
Liu, H. L., Lee, H. M. & Chung, Y. C. Dyspareunia and its comorbidities among Taiwanese women: analysis of the 2004–2010 Nationwide Health Insurance Database. J Sex Med. 12, 1012–1018 (2015).
Cheng, C. L., Chien, H. C., Lee, C. H., Lin, S. J. & Yang, Y. H. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 201, 96–101 (2015).
Noordzij, M. et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 28, 2670–2677 (2013).
Patorno, E., Glynn, R. J., Hernandez-Diaz, S., Liu, J. & Schneeweiss, S. Studies with many covariates and few outcomes: selecting covariates and implementing propensity-score-based confounding adjustments. Epidemiology. 25, 268–278 (2014).
Lin, F. C. et al. Early statin use and the progression of Alzheimer’s disease: A total population-based case-control study. Medicine (Baltimore). 94, e2143 (2015).
Acknowledgements
We are grateful for the data from the National Health Interview Survey Original Database provided by the Bureau of Health Promotion and Taiwan Bureau of National Health Insurance, Department of Health, National Health Research Institutes. The interpretation and conclusions in the present study do not represent those of Bureau of Health Promotion and Taiwan Bureau of National Health Insurance, Department of Health, National Health Research Institutes. The authors thank the help from the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital. This work was supported by the Ministry of Science and Technology [grant number 104-2320-B-037 −007] and [grant number 104–2320-B-037 −035].
Author information
Authors and Affiliations
Contributions
W.-C.T., P.-C.H., C.-S.C., S.-J.J. and C.-Y.C. designed the data collection instruments, H.-F.K., H.-M.S., T.-H.L. and W.-H.T. conceptualized and designed the study, W.-C.T. and C.-Y.C. drafted the initial manuscript and approved the final manuscript as submitted. W.-C.T., C.-Y.C., Y.-B.H., K.-T.L., S.-H.S., W.-T.L. and M.-T.W. carried out the initial analyses, critically reviewed the manuscript and approved the final manuscript as submitted.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tsai, WC., Haung, YB., Kuo, HF. et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci Rep 6, 24132 (2016). https://doi.org/10.1038/srep24132
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24132
This article is cited by
-
Sex-related differences of fatty acid-binding protein 4 and leptin levels in atrial fibrillation: an updated review
International Journal of Arrhythmia (2024)
-
Pharmacological activation of estrogenic receptor G protein-coupled receptor 30 attenuates angiotensin II-induced atrial fibrosis in ovariectomized mice by modulating TGF-β1/smad pathway
Molecular Biology Reports (2022)
-
Atrial Fibrillation in Women: from Epidemiology to Treatment
Current Cardiovascular Risk Reports (2022)
-
Conjugated equine estrogen used in postmenopausal women associated with a higher risk of stroke than estradiol
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.