Abstract
A series of 3-(3′-hydroxy-4′-methoxyphenyl)selenyl-5,6,7-trimethoxy-1H-indoles and 3-(3′-hydroxy-4′-methoxyphenyl)thio-5,6,7-trimethoxy-1H-indoles were obtained as a new class of combretastatin A-4 (CA-4) analogues via a convenient ultrasound (US)-assisted two-step process involving 3-selenenylation/sulfenylation followed by O-deallylation. With the assistance of US irradiation, both the reaction rates and yields of selenenylation, sulfenylation and O-deallylation could be significantly improved. A comparison of the reaction rates of O-deallylation and ester reduction demonstrated that O-deallylation was more sensitive to US irradiation. Finally, these products were evaluated for their antiproliferative activities, and most of them showed moderate to potent activities against three human cancer cell lines in vitro.
Similar content being viewed by others
Introduction
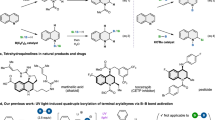
CombretastatinA-4 (CA-4, 1a, Fig. 1), a natural stilbene isolated from the South African tree Combretum caffrum, is a powerful inhibitor of tubulin polymerization that displays cytotoxic and antivascular activities by binding at the colchicine site of tubulin1. CA-4P (1b), the water-soluble pro-drug of CA-4, has shown promising results in human cancer clinical trials2. The structure-activity relationship (SAR) study of CA-4 indicated that the presence of a cis-double bond and 3,4,5-trimethoxyphenyl ring-A is essential for its anti-tubulin activity, and the introduction of a hydroxyl group to the C′-3 position in ring-B was found to strongly enhance its activity. Replacement of ring-A and ring-B with other aromatic or heteroaromatic systems, and swapping the positions of ring-A and ring-B are very effective strategies for designing CA-4 analogues3,4,5,6,7,8,9.
In the last few years, a number of CA-4 analogues with potent activities have been developed. Among these analogues, indole group-containing compounds (2, 3, 4) have been reported to show potent antitubulin and antiproliferative activities3,4,10,11. Recently, we reported the development of a set of 3-arylselenylindoles (5) as CA-4 analogues, which also exhibited potent activities in the tubulin polymerization inhibition and against three human cancer cell lines12. These results led us to start a pharmacophore exploration and optimization effort around the 3-arylselenyl-indoles and the bioisosteric 3-arylthio-indoles, moving the trimethoxyl groups to the indoline moiety, to afford the isomeric 5,6,7-trimethoxyindole derivatives with a general structure (6) as shown in Fig. 1.
Most protocols for the synthesis of 3-arylthioindoles and 3-arylselenylindoles are limited by the instability and the complex synthetic process of activated sulfur or selenium reagents13,14,15,16,17. A previously reported method for direct synthesis of 3-arylthioindoles and 3-arylselenylindoles using iron (III) and iodine as the catalysts suffered from long reaction time18. Previously, we improved this method by using microwave irradiation to shorten the reaction times and increase the yields12. Ultrasound (US) is becoming a widely used laboratory and industrial technique that offers a green energy source for organic synthesis and has been proven to be a very useful tool in enhancing the reaction rates in a variety of reacting systems under quite mild conditions (room temperature)19,20,21,22. It was found that the sonochemical effect of US waves mainly comes from acoustic cavitation, which provides enough kinetic energy to drive reactions to completion in shorter durations19,20. However, to the best of our knowledge, only a very limited number of studies have reported the synthesis of 3-arylselenyl- and 3-arylthio-indoles under US conditions23.
In our effort to search for antitubulin/antiproliferative agents, a new series of 3-arylselenyl- and 3-arylthio-indoles were designed as CA-4 analogues, in which 5,6,7-trimethoxy indole was employed as ring-A, 3′-hydroxyl-4′-methoxyphenyl was employed as ring-B and selenium or sulfur atoms were used as the substitutions for the cis-double bond of CA-4. In addition, several substituents were introduced to the N-1 and C-2 positions of the indole rings. All target compounds were obtained via an US-assisted two-step process involving 3-selenenylation/sulfenylation followed by a rapid selective O-deallylation. To verify the US efficiency in this two-step protocol, a comparative study between reactions performed under non-ultrasound (non-US) and US conditions was carried out. Finally, we evaluated the antiproliferative activities of the newly developed compounds against three human cancer cell lines. And the effects of the most potent compound, 10a, on the inhibition of tubulin assembly were tested in vitro by immunofluorescence studies.
Results and Discussion
Chemistry
Based on our previous study on the synthesis of 3-arylselenylindoles under microwave irradiation conditions12 (at 80 °C), 3-selenenylation/sulfenylation of indoles was tested by using US irradiation under quite mild conditions (at room temperature). The synthesis of 9a from 5,6,7-trimethoxy-2-methyl-1H-indole24,25,26,27 (7a) and 1,2-bis(3-(allyloxy)-4-methoxyphenyl) diselenide12 (8a) in the presence of 20 mol% of FeCl3 and 1 mol% of iodine was studied initially, and the results are summarized in Table 1. As shown in entry 1, 9a was isolated in only 67% yield when the reaction was refluxed in acetonitrile for 24 h by thermal heating (oil bath). In contrast, 9a was obtained in moderate yield (52%) by US irradiation at room temperature for 3 h (entry 2). A clear improvement in yield was observed when the reaction time was prolonged to 6 h (entry 3). However, further extending the reaction time under US conditions did not show apparent benefits (entry 4). In addition, only THF showed a comparable efficiency for this reaction after screening of the other solvents (entries 5–8). The optimized conditions were developed when acetonitrile was chosen as the solvent with the assistance of US irradiation for 6–9 h at room temperature.
Next, the optimized reaction conditions were used for the synthesis of compounds 9b–h (Table 2). By changing the methyl group at the C-2 position of compound 9a to hydrogen, many other indoles with various substrates at the N-1 position successfully underwent 3-selenenylation to furnish the desired products. The results in entries 1–4 clearly indicate that US conditions generated higher yields and much faster reaction rates than non-US conditions for the 3-selenenylation reaction. These reaction conditions were further utilized for the synthesis of thioindoles 9f–h using various indoles with disulphide28,29 (8b), which allowed the reactions to proceed smoothly and generate the desired products in moderate yields (entries 5–7).
Allyl groups are widely used in organic synthesis as important protecting groups of alcohols due to the availability and stability of the corresponding allyl derivatives. Many methodologies have been documented in terms of deprotection of allyl ethers30,31,32,33. In our case, an allyl group was used as the protecting group of the 3′-phenolic hydroxyl group. The O-deallylation reactions to furnish the 3′-phenolic hydroxyl substituted target compounds were initiated by using TiCl4, an efficient O-deallylation agent34, at −20 °C in dry dichloromethane. Unfortunately, 9a decomposed under these conditions and no deallylation product (10a) was observed.
Pd(PPh3)4/NaBH4 was reported to be a useful system for the deprotection of O-allyl under neutral conditions35,36. When we performed the O-deallylation reaction by following this procedure using 1 mol% of Pd(PPh3)4 and an excess of sodium borohydride (8 equivalents) at room temperature for 30 h, 10a could be isolated in 73% yield (Table 3, entry 2). To our delight, the reaction rates and yields were significantly improved by US irradiation. When this reaction was performed under US irradiation for 3 h, 10a was obtained in 64% yield (entry 4). Furthermore, the yield was increased to 90% after 3 h US irradiation with 10 mol% of Pd(PPh3)4 (entry 7). In contrast, a lower reaction yield and rate were obtained when the reaction was performed using the same amount of Pd(PPh3)4 under non-US conditions (entry 8).
Then, the optimized conditions were applied for the synthesis of compounds 10b–h (Table 4). Under US conditions, all the O-deallylations were performed successfully within a shorter reaction time and provided higher yields compared with reactions conducted under non-US conditions. The reducible functional groups, including amide, N-benzyl and halogen (Cl), showed good tolerance to these reaction conditions. However, when the deallylation of 9d and 9g were performed under US conditions for more than 5 h, compounds 11 and 12 were obtained as the predominant products respectively, in which the allyl ethers were cleaved and the ester groups were simultaneously reduced. When these reactions were carried out under non-US conditions at r.t. until the starting materials 9d and 9g were fully consumed (over 10 h), only compounds 11 and 12 were obtained, respectively. Thus, we believed that there was a significant rate difference between the O-deallylation and ester reduction reactions under US conditions.
To investigate the rate differences between the O-deallylation and the ester reduction, the reactions of 9d under non-US and US conditions were detected by high performance liquid chromatography (HPLC). As shown in Fig. 2(b), when the reaction was performed under non-US conditions for 120 min, 80% of 10d in the reaction mixture was observed along with 15% of 11 as the reduction product. Moreover, the lower reaction rate under non-US conditions required a reaction time of at least 10 h for the full consumption of 9d, and all the ester groups in 10d had been reduced to hydroxyl groups. However, under US conditions, substrate 9d was quantitatively converted into 10d within 80 min (95% yield). US irradiation for more than 7 h resulted in the full reduction of the ester group of 10d. Compared with the yields of 10d and 11 at 80 min under non-US conditions, US irradiation significantly increased the rate of O-deallylation. It is worth noting that this magnified rate difference between the US promoted O-deallylation and ester reduction made it possible to selectively synthesize the desired compounds as the predominant products.
To further illuminate the rate differences between the O-deallylation and reduction of ester under US conditions, an intermolecular competition experiment using an equimolar mixture of 9b and 7d was carried out under US and non-US conditions, respectively (Fig. 3). The results demonstrated that under US conditions, the O-deallylation rate of 9b was approximately 1.7 fold faster than that performed under non-US conditions within 30 min of the reaction, while the reduction of 7d showed no significant difference between the US and non-US conditions. These results were in agreement with those obtained from the intramolecular competition experiment under US conditions.
Antiproliferative activity assay
To evaluate the antiproliferative activity, the target compounds and reference compound CA-4 were screened against three human cancer cell lines (gastric adenocarcinoma SGC-7901 cells, mouth epidermal carcinoma KB cells and fibrosarcoma HT-1080 cells) using a standard MTT assay (Table 5). The reported IC50 values are the average of at least three independent experiments. Generally, the target compounds in this study were less potent than the ones with the trimethoxyl groups on the 3-arylselenyl ring in our previous study17, except 10a. The 3-arylselenylindoles were more active than the bioisosteric 3-arylthioindole analogues, but the exact reason for these observations needs to be further investigated. The most active compound, 10a, was found to exhibit activity comparable to that of CA-4 and showed IC50 values in the nanomolar range against all three of the cell lines. The introduction of various substituents to the N-1 position of the indole ring also tended to reduce potency when comparing the activities of 10b with 10c, 10d and 10e. In addition, removal of the methyl group at the C-2 position of 10a resulted in a loss of activity (10b). It reminds us that modification at the C-2 position may have a direct effect on the activity. Future study of the 3-arylselenylindoles will focus on the C-2 substituents variations including -Et, -OMe, -CHO and others.
Immunofluorescence studies
We further investigated the capacity of the most potent compound, 10a, to alter the microtubule network in HT-1080 cell by tubulin immunostaining and compared the observations to those of the reference compound CA-4 (Fig. 4). Confocal analysis showed that the microtubule network exhibited normal arrangement and organization (slim and fibrous) in the control cells. Exposure to 11 nM of CA-4 for 24 h led to a complete loss of microtubule formation (microtubules became short and wrapped around the nucleus) and strongly affected the cell shape (turned round); treatment with 14 nM of compound 10a produced results similar to those of CA-4-induced microtubule and cell shape changes. These results confirmed that 10a exerted similar effects to CA-4 on the microtubule network, suggesting that 10a is most likely targeting tubulin.
In summary, a convenient and efficient US-assisted two-step process was developed for the synthesis of novel combretastatin A-4 analogues, 3-(3′-hydroxy-4′-methoxyphenyl)selenyl-5,6,7-trimethoxy-1H-indoles and 3-(3′- hydroxy-4′-methoxyphenyl)thio-5,6,7-trimethoxy-1H-indoles, which involved US-promoted 3-selenenylation/sulfenylation followed by a O-deallylation. Compared with conventional methods used to prepare these compounds, US irradiation induced a remarkable acceleration in the reaction rates and generated the products in higher yields. Moreover, US irradiation significantly increased the O-deallylation rate, while having almost no evident influence on the reduction of the esters. This magnified rate difference made it possible to selectively synthesize O-deallylic or O-deallylic and ester reduced compounds as predominant products. The synthesized target compounds were also evaluated for their antiproliferative activities against three human cancer cell lines in vitro, and most of them exhibited moderate to potent activities. An immunofluorescence study of compound 10a revealed that its target was most likely tubulin. Further studies of seleniferous indoles substituted at the C-2 position are currently in process.
Methods
Reagents and equipment
All the solvents and chemical materials were commercially available, and were used without further purification. Silica gel GF254 and silica gel H (200–300 mesh) from Qingdao Haiyang Chemical Company were used for preparative thin-layer chromatography and column chromatography, respectively. US irradiation was performed in an ultrasonic cleaner (KQ-400KDE, made in Kunshan Ultrasonic Equipment Co., Ltd.) with frequency of 25 kHz and a nominal power of 400 W at 25–30 oC. The content of diselenide (8a) was analyzed by HPLC (HPLC-LC-20AT, Shimadzu) using SPD-20A UV as the detector and 80% methanol in water as mobile elution. 1H and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 (TMS as internal standard) using a Bruker Avance 300, 400, or 600 spectrometer (1H at 300, 400 or 600 MHz, 13C at 100 or 150 MHz). Chemical shifts δ are in ppm, and the following abbreviations are used: singlet (s), doublet (d), triplet (t), multiplet (m), broad singlet (brs). Mass spectra (MS) were determined on an Agilent 1100-sl mass spectrometer (ESI) from Agilent Co., Ltd. High resolution accurate mass determinations (HRMS) were run on a Bruker Micromass Time of Flight mass spectrometer (ESI). The IR spectra were obtained on a Bruker IFS 55 FT-IR spectrophotometer using KBr disks. Melting points were measured by an X-4 Micro-Melting point detector (Beijing Tech Instrument Co., Ltd.), without correction.
General experimental section
General procedure for synthesis of 3-arylselenylindoles and 3-arylthioindoles
Method A: Based on the reported method12, a mixture of the appropriate indole (0.6 mmol), 1,2-bis(3-(allyloxy)-4-methoxyphenyl)diselenide (8a) or 1,2-bis(3- (allyloxy)-4-methoxyphenyl) disulfide (8b) (0.35 mmol), FeCl3 (20 mol%) and I2 (1 mol%, 0.0001 g/mL in CH3CN) was placed in a 25 mL vessel. The reaction mixture was irradiated in the water bath of an ultrasonic cleaner at room temperature for 9 h. After the evaporation of the solvent, the residual crude product was purified by preparative thin-layer chromatography with n-hexane-AcOEt (v/v = 5:1) or pure CH2Cl2 and analyzed by MS, HRMS, 1H-NMR, 13C-NMR and IR. See the supplementary information for the characterization details.
Method B: Similar to method A, a mixture of the appropriate indole (0.6 mmol), 1,2-bis(3-(allyloxy)-4-methoxyphenyl)diselenide (8a) or 1,2-bis(3-(allyloxy)- 4-methoxyphenyl) disulfide (8b) (0.35 mmol), FeCl3 (20 mol%) and I2 (1 mol%, 0.0001 g/mL in CH3CN) was placed in a 25 mL vessel. The reaction mixture was refluxed at 80 °C for 20 h in an oil bath. After the evaporation of the solvent, the residual crude product was purified by preparative thin-layer chromatography with n-hexane-AcOEt (v/v = 5:1) or pure CH2Cl2.
General procedure for synthesis of 3′-hydroxyl-3-arylselenylindoles and 3′-hydroxyl-3-arylthioindoles
Method A: Based on the reported method36, allyl ether (0.2 mmol) dissolved in anhydrous THF (10 mL), Pd(PPh3)4 (0.02 mmol) and NaBH4 (0.06 g, 1.6 mmol) were added under a nitrogen atmosphere. The reaction mixture was placed in a water bath of an ultrasonic cleaner and irradiated for 3 h. The reaction mixture was filtered and the solvent was evaporated to leave a residue which was purified by preparative thin-layer chromatography with n-hexane-AcOEt (v/v = 4:1) and analyzed by MS, HRMS, 1H-NMR, 13C-NMR and IR. See the supplementary information for the characterization details.
Method B: Similar to method A, to a solution of allyl ether (0.2 mmol) in anhydrous THF (10 mL) under a nitrogen atmosphere, Pd(PPh3)4 (0.02 mmol) and NaBH4 (0.06 g, 1.6 mmol) were added. The reaction mixture was stirred at 25 °C for 15 h. The reaction mixture was filtered and the solvent was evaporated to leave a residue which was purified by preparative thin-layer chromatography with n-hexane-AcOEt (v/v = 4:1).
Antiproliferative activity assay
The in vitro antiproliferative activity assay was performed following a previously reported method12.
Immunofluorescence studies
Immunofluorescence studies were performed following a previously reported method with 14 nM of 10a, or 11 nM of CA-412.
Additional Information
How to cite this article: Wen, Z. et al. Ultrasound-promoted two-step synthesis of 3-arylselenylindoles and 3-arylthioindoles as novel combretastatin A-4 analogues. Sci. Rep. 6, 23986; doi: 10.1038/srep23986 (2016).
References
Pettit, G. R. et al. Inolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 45, 209–211 (1989).
Romagnoli, R. et al. Synthesis and biological evaluation of 2-(alkoxy carbonyl)-3-anilinobenzo[b]thiophenes and thieno[2,3-b]pyridines as new potent anticancer agents. J. Med. Chem. 56, 2606–2618 (2013).
Zhao, D. G. et al. Synthesis and structure - activity relationships of N-methyl- 5,6,7-trimethoxyl indoles as novel antimitotic and vascular disrupting agents. J. Med. Chem. 56, 1467–1477 (2013).
Lee, H. Y. et al. Concise syntheses of N-aryl-5,6,7-trimethoxyindoles as antimitotic and vascular disrupting agents: application of the copper-mediated Ullmann-type arylation. Org. Biomol. Chem. 9, 3154–3157 (2011).
Novoa, A. et al. Design, synthesis and antiproliferative activities of biarylolefins based on polyhydroxylated and carbohydrate scaffolds. Eur. J. Med. Chem. 46, 3570–3580 (2011).
Burja, B. et al. Pyrazolone-fused combretastatins and their precursors: synthesis, cytotoxicity, antitubulin activity and molecular modeling studies. Bioorg. Med. Chem. 18, 2375–2387 (2010).
Tsyganov, D. V. et al. 3-(5-)-Amino-o-diarylisoxazoles: regioselective synthesis and antitubulin activity. Eur. J. Med. Chem. 73, 112–125 (2014).
Romagnoli, R. et al. Synthesis and evaluation of 1,5-disubstituted tetrazoles as rigid analogues of combretastatin A-4 with potent antiproliferative and antitumor activity. J. Med. Chem. 55, 475–488 (2012).
Quintin, J., Roullier, C., Thoret, S. & Lewin, G. Synthesis and anti-tubulin evaluation of chromone-based analogues of combretastatins. Tetrahedron. 62, 4038–4051(2006).
Brancale, A. & Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 27, 209–238 (2007).
La Regina, G. et al. Toward highly potent cancer agents by modulating the C-2 group of the arylthioindole class of tubulin polymerization inhibitors. J. Med. Chem. 56, 123–149 (2013).
Wen, Z. et al. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 90, 184–194 (2015).
Raban, M. & Chern, L. Reactions of arenesulfenyl chlorides with indole. Carbon-13 and proton nuclear magnetic resonance spectra of 3-(arylthio)indoles. J. Org. Chem. 45, 1688–1691 (1980).
Matsugi, M. et al. Facile and efficient sulfenylation method using quinone mono-O, S-acetals under mild conditions. J. Org. Chem. 66, 2434–2441 (2001).
Tudge, M., Tamiya, M., Savarin, C. & Humphrey, G. R. Development of a novel, highly efficient halide-catalyzed sulfenylation. Org. Lett. 8, 565–568 (2006).
Nicolaou, K. C., Claremon, D. A., Barnette, W. E. & Seitz, S. P. N-Phenylselenophthalimide (N-PSP) and N-phenylselenosuccinimide (N-PSS). Two versatile carriers of the phenylseleno group. Oxyselenation of olefins and a selenium-based macrolide synthesis. J. Am. Chem. Soc. 101, 3704–3706 (1979).
Zhao, X., Yu, Z., Xu, T., Wu, P. & Yu, H. Novel Brфnsted acid catalyzed three-component alkylations of indoles with N-phenylselenophthalimide and styrenes. Org. Lett. 9, 5263–5266 (2007).
Fang, X., Tang, R., Zhong, P. & Li, J. Iron-catalyzed sulfenylation of indole with disulfides promoted by a catalytic amount of iodine. Synthesis. 24, 4183–4189 (2009).
Cravotto, G. & Cintas, P. Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scal applications. Chem. Soc. Rev. 35, 180–196 (2006).
Nasir Baig, R. B. & Varma, R. S. Alternative energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 41, 1559–1584 (2012).
Cintas, P., Palmisano, G. & Cravotto, G. Power ultrasound in metal-assisted synthesis: from classical Barbier-like reactions to click chemistry. Ultrason. Sonochem. 18, 836–841 (2011).
Thompson, L. H. & Doraiswamy, L. K. Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 38, 1215–1249 (1999).
Vieira, B. M. et al. Sonochemistry: an efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason. Sonochem. 27, 192–199 (2015).
Tanemura, K., Suzuki, T., Nishida, Y., Satsumabayashi, K. & Horaguchi, T. A mild and efficient method for the mononitration of aromatic compounds by cerium (III) ammonium nitrate in acetic anhydride. J. Chem. Research (S), 497–499 (2003).
Bianco, A., Passacantilli, P. & Righi, G. Improved procedure for the reduction of esters to alcohols by sodium borohydride. Synth. Commun. 18, 1765–1771 (1988).
Prakasham, A. P. et al. Synthesis and anticancer activity of 2-benzylidene indanones through inhibiting tubulin polymerization. Bioorg. Med. Chem. 20, 3049–3057 (2012).
Ando, S., Okamoto, Y., Umezawa, K. & Otsuka, M. Synthesis of the indole core structures of conophylline. J. Heterocyclic Chem. 45, 1803–1808 (2008).
Dos Santos, E. D. A. et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg. Med. Chem. Lett. 23, 4669–4673 (2013).
García Ruano, J. L., Parra, A. & Alemán J. Efficient synthesis of disulfides by air oxidation of thiols under sonication. Green Chem. 10, 706–711 (2008).
Konda, S. G., Humne, V. T. & Lokhande, P. D. Rapid and selective deallylation of allyl ethers and esters using iodine in polyethylene glycol-400. Green Chem. 13, 2354–2358 (2011).
Ploypradith, P., Cheryklin, P., Niyomtham, N., Bertoni, D. R. & Ruchirawat, S. Solid-supported acids as mild and versatile reagents for the deprotection of aromatic ethers. Org. Lett. 9, 2637–2640 (2007).
RajaRam, S., Chary, K. P., Salahuddin, S. & Iyengar, D. S. A mild chenoselective and rapid regeneration of alcohols from O-allyl ethers by LiCl/NaBH4 . Synthetic Commun. 32, 133–137 (2002).
Li, C. B. et al. A convenient and efficient deallylation procedure. Chinese Chem. lett. 14, 459–460 (2003).
Aristegui, S. R., El-Murr, M. D., Golding, B. T., Griffin, R. J. & Hardcastle, I. R. Judicious application of allyl protecting groups for the synthesis of 2-morpholin-4-yl-4oxo-4H-chromen-8-yl triflate, a key precursor of DNA-dependent protein kinase inhibitors. Org. Lett. 8, 5927–5929 (2006).
Beugelmans, R., Neuville, L., Bois-Choussy, M., Chastanet, J. & Zhu, J. Palladium catalyzed reductive deprotection of alloc : transprotection and peptide bond formation. Tetrahedron Lett. 36, 3129–3132 (1995).
Meroni, G., Ciana, P., Maggi, A. & Santaniello, E. A new synthesis of 2-cyano-6-hydroxybenzothizole, the key intermediate of D-luciferin, starting from 1,4-benzoquinone. Synlett. 16, 2682–2684 (2009).
Acknowledgements
This research was financially supported by National S&T Major Project (2012ZX09103101-060), by the Natural Science Foundation of Liaoning Province (2013010434-401), by the National Natural Science Foundation of China (81502932), and by the Program for Innovative Research Team of the Ministry of Education and Program for Liaoning Innovative Research Team in University.
Author information
Authors and Affiliations
Contributions
Z.W., X.L., D.Z., B.L., Y.W., M.J. and H.M. performed the experiments. Z.W., X.L., K.B., Y.W. and W.Z. reviewed, analyzed, and interpreted the data. Z.W., X.L., K.B. and W.Z. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wen, Z., Li, X., Zuo, D. et al. Ultrasound-promoted two-step synthesis of 3-arylselenylindoles and 3-arylthioindoles as novel combretastatin A-4 analogues. Sci Rep 6, 23986 (2016). https://doi.org/10.1038/srep23986
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23986
This article is cited by
-
Pyrazoles, isoxazoles, and 1,2,3-triazoles as analogs of the natural cytostatic combretastatin A-4: efficient routes of synthesis, tubulin inhibition, and cytotoxicity
Chemistry of Heterocyclic Compounds (2021)
-
Design, synthesis and structure-activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as novel tubulin inhibitors
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.