Abstract
Recently, CRISPR/Cas9 technology has emerged as a powerful approach for targeted genome modification in eukaryotic organisms from yeast to human cell lines. Its successful application in several plant species promises enormous potential for basic and applied plant research. However, extensive studies are still needed to assess this system in other important plant species, to broaden its fields of application and to improve methods. Here we showed that the CRISPR/Cas9 system is efficient in petunia (Petunia hybrid), an important ornamental plant and a model for comparative research. When PDS was used as target gene, transgenic shoot lines with albino phenotype accounted for 55.6%–87.5% of the total regenerated T0 Basta-resistant lines. A homozygous deletion close to 1 kb in length can be readily generated and identified in the first generation. A sequential transformation strategy—introducing Cas9 and sgRNA expression cassettes sequentially into petunia—can be used to make targeted mutations with short indels or chromosomal fragment deletions. Our results present a new plant species amenable to CRIPR/Cas9 technology and provide an alternative procedure for its exploitation.
Similar content being viewed by others
Introduction
During the past three decades, great efforts and achievements have been made in developing efficient tools for targeted genome modification in plants1,2. Before the CRISPR/ Cas9 system (CRISPR: clustered regularly interspaced short palindromic repeats; Cas9: CRISPR-associated protein 9) was introduced into plant research in 20133,4,5,6,7,8,9,10, three classes of sequence-specific nucleases had been used for plant genome engineering: zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and meganucleases11. Against this background, due to its versatility, design simplicity, high efficiency and low cost, the CRISPR/Cas9 technique has rapidly become a focus of attention over the past three years1,2,12,13,14,15.
The CRISPR/Cas9 system is composed of Cas9 nuclease and customizable sgRNA. The sgRNA guides Cas9 to recognize target DNA and create double strand breaks (DSBs) which trigger non-homologous end joining (NHEJ) and homologous recombination (HR) repair pathways, resulting in genome modifications16. Genome modification is based on the repair of DSBs11,17. Because previous reports show that the efficiency of DSB repair pathways differs between species and also cell types1,17, it is important to investigate the feasibility of the CRISPR/Cas9 system in each plant species of interest.
To date, the primary application of the CRISPR/Cas9 system in plants has been the creation of gene knock-outs13,15. It has been successfully used to induce genetic modification in plants including Arabidopsis3,6,7,8,18,19,20, Nicotiana benthamiana3,4,7,21, Nicotiana tabacum22, rice5,6,7,8,9,10,23,24, wheat5,25,26, Zea mays27, sorghum7, tomato28,29,30, potato31, sweet orange32, poplar33 and liverwort34. These reports show that CRISPR/Cas9-mediated targeted mutagenesis is efficient in plants. Analysis also suggests that the production of mutations by CRISPR/Cas9 in plants is highly specific20,23 and can be stably transmitted to subsequent generations following classic Mendelian law18,19,20,23,24,28,35. Although CRIPR/Cas9 has become the choice of technology for targeted genome mutagenesis in plants, its use has not been reported in ornamental plants. Because most ornamental plants are highly heterozygous and the economic importance of individual ornamental species is relatively small compared with other horticultural crops, genome-related research in ornamental plants has been retarded36. Affordable CRISPR/Cas9 technology is therefore poised to facilitate gene function research and genetic modification in ornamentals. Petunia is one of the most popular bedding plant species and a model system for comparative research37. It is a good species in which to demonstrate the application of Cas9 technology in ornamentals.
In nearly all of the studies producing stable plant mutants, the Cas9 and sgRNA expression cassettes have been combined into a single binary vector. The relatively large Cas9 expression cassette (usually larger than 5 kb) creates inconvenience in the manipulation of destination vectors. The sequential transformation strategy—transferring Cas9 and sgRNA expression cassettes sequentially into plants—should therefore make the conduction of CRISPR/Cas9-mediated mutations easier. Here, we show that the CRISPR/Cas9 system is highly efficient in petunia for targeted mutagenesis. Homozygous chromosomal fragment deletions between two paired sgRNA targeted sites are readily detectable in T0 transgenic plants. The sequential transformation is an alternative strategy for Cas9-mediated genome modification in those plants amenable to Agrobacterium-mediated leaf-disc transformation.
Results
High frequency of targeted mutagenesis induced by constructs combining pcoCas9 and an sgRNA
Phytoene desaturase (PDS) is a key enzyme in carotenoid biosynthesis. It is required for the biosynthesis of chlorophyll. Disruption of PDS will cause an albino phenotype that can be easily recognized38. To design sgRNAs to target the petunia PDS gene, a fragment of PhPDS (GenBank ID: KP677483) genomic DNA was amplified with the primers PDS-F1 and PDS-R1 (Fig. 1A and see Supplementary Table S1 online) and the sequence was determined by Sanger sequencing. Subsequently, two sgRNAs, sgR1 and sgR2, were designed to contain guide sequences complementary to different DNA strands of PhPDS in the exon regions, flanking an intron of 745 bp in length (Fig. 1A).
Schematic of expression cassettes used in this study.
(A) Schematic of the petunia PDS gene fragment between primers PDS-F1 and PDS-R1, indicating the sgRNA target sites and sequences, the location of PCR primers.  indicates exon. ▬ indicates intron. (B) Binary plasmids for targeted genome mutagenesis. NPTII, Neomycin phosphotransferase II; PAM, proto-spacer adjacent motif; PAT, phosphinothricin N-acetyltransferase.
indicates exon. ▬ indicates intron. (B) Binary plasmids for targeted genome mutagenesis. NPTII, Neomycin phosphotransferase II; PAM, proto-spacer adjacent motif; PAT, phosphinothricin N-acetyltransferase.
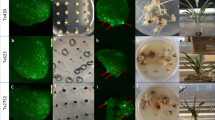
Albino calli began to appear at approximately four weeks after the transformation of leaf discs with the CRISPR/Cas9 constructs containing single sgRNA: pGGE1c and pGGE2c (Fig. 1B). All of the green and albino callus islets were excised from the leaf segments and sub-cultured to promote shoot formation. Plantlets regenerated from the same callus islet were regarded as an independent transgenic line. PCR was performed on more than 30 randomly selected Basta-resistant plants to detect the integration of transfer DNA (T-DNA) and all of them displayed the transgene. We therefore regarded all the Basta-resistant plants as transgenic plants. Three months after transformation, a total of 214 transgenic plant lines, including albino, mosaic and green lines (Fig. 2A), were produced from approximately 300 leaf discs (200 for pGGE1c and 100 for pGGE2c) transformed with pGGE1c or pGGE2c. The proportion of transgenic lines with albino phenotype was 87.5% and 58.1% for pGGE1c and pGGE2c respectively (Table 1). Because the Petunia hybrida inbred line Mitchell Diploid (MD) is a diploid with two copies of each gene, the albino phenotype indicated that both copies of the target PDS gene had been destroyed in these transgenic seedlings.
Targeted mutagenesis in petunia using the CRISPR/Cas9 system.
(A) Bleaching phenotype of PDS gene mutated petunia shoots. Left, albino shoots. Middle, mosaic shoots. Right, wild type. (B) Targeted mutagenesis of PDS in pGGE1c transgenic shoots. (C) Targeted mutagenesis of petunia PDS in pGGE2 transgenic shoots using a sequential transformation approach. Deletions are denoted by red dashes. Insertions and a replacement are indicated by red letters. Figures in brackets denote the no. of detected clones with the mutation type indicated for that row. For chromatograms, see Supplementary Figures S1 and 2 online.
To evaluate the types of mutation in the PDS locus of transgenic plants, PCR amplification was performed on three randomly selected albino plant lines transformed with pGGE1c, using the primers PDS-F1 and PDS-R2 (Fig. 1A and see Supplementary Table S1 online). PCR products were cloned and the mutation types were determined by Sanger sequencing. Plant lines were regarded as homozygous if the mutations of all of the sequenced clones (at least five clones) were the same, bi-allelic if different mutations occurred on the two copies of the target gene, or chimeric if more than three different mutations occurred on the same transgenic plant. For the three selected pGGE1c lines, one plant line was homozygous; with a 1 bp insertion located 4 bp upstream of the PAM sites (Fig. 2B, line 1c-7). One plant line (Fig. 2B, line 1c-3) was bi-allelic; one allele had a 1 bp insertion and the other allele carried a 1 bp replacement combined with a 1 bp insertion. The other transgenic line (Fig. 2B, line 1c-1) was chimeric, comprising three alleles with different deletions ranging from 1 bp to 63 bp.
Efficient gene mutation by sequential transformation of Cas9 and sgRNA expression cassettes
To determine whether targeted mutagenesis could be achieved by sequential transformation of Cas9 and sgRNA(s), the pK7WGF2::hCas94 (Addgene ID, 46965) was first transferred to MD using Agrobacterium-mediated transformation. After selecting with 10mg/L G418, 16 independent G418 resistant plant lines were regenerated. Ten of them were subjected to PCR amplification using hCas9-specific primers (see supplementary Table S1 online) and eight plant lines showed the presence of the hCas9 gene. At least 20 leaf segments from each of the eight hCas9 transgenic lines were used as recipients for transformation with plasmid pGGE2. Thirty-six shoot lines with albino phenotype were regenerated from 50 discs of two hCas9 plant lines (HC-40 and HC-27) and accounted for 55.6% of the total transgenic lines regenerated from these two hCas9 plants (Table 1). This ratio was similar to that obtained using single transformation with pGGE2c containing the same sgR2 (58.1%, Table 1).
PCR amplification was performed on four randomly selected albino plant lines transformed with pGGE2, using primers PDS-F2 and PDS-R1 (Fig. 1A). Sequencing results of PCR products showed that all four plant lines were bi-allelic. In one line (line 2–2), one allele had a single adenine insertion and the other allele carried a 5 bp deletion (Fig. 2C). In a second line (line 2–23), one allele had an adenine insertion and the other allele carried an 8 bp deletion (Fig. 2C). The other two lines displayed a third type of mutation: each genome had an allele with a single adenine insertion and another with a single thymine insertion (Fig. 2C, lines 2–7 and 2–12). The results indicated that targeted mutagenesis in petunia can be achieved by sequential transformation of Cas9 and sgRNA expression cassettes.
In addition, the hCas9 transgenic plants were grown into mature plants and allowed to flower and set seed. None of these plants showed any morphological or developmental variation compared with the non-transgenic plants; this indicated that the expression of hCas9 had no obvious detrimental effects on plant development in petunia.
Chromosomal fragment deletion induced by CRISPR/Cas9
In order to test the capacity of the CRISPR/Cas9 system to induce chromosomal fragment deletion between two Cas9 cut sites in petunia, pGGE3c (Fig. 1B) was transferred into MD leaf segments. Sixteen albino lines were selected for PCR detection using primers PDS-F1 and PDS-R1 (Fig. 1A). Three of these lines showed a fragment close to or smaller than 1000 bp (Fig. 3A), suggesting a deletion between sgR1/Cas9 and sgR2/Cas9 cut sites. Sequencing results for the three truncated PCR products showed that they represented two classes of deletion. Fragment 1 (F1) represented an 870 bp deletion that included the 867 bp fragment between two expected Cas9 cut sites, 1 bp beyond the sgR1/Cas9 cut site and 2 bp beyond the sgR2/Cas9 cut site (Fig. 3C,D). Fragments 2 and 3 represented a 1570 bp deletion combined with a 6 bp filler DNA (TGGTGG, Fig. 3C,D), which was too short to permit a sound conclusion about its origin. Surprisingly, besides the inclusion of the fragment between the two Cas9 cut sites, the 1570 bp deletion also contained a 554 bp-long fragment extending beyond the sgR1/Cas9 cut site and a 149 bp fragment extending beyond the sgR2/Cas9 cut site; these results indicated that resection of the DSBs occurred before the breaks were re-joined. An electrophoretogram of amplicons and sequencing results demonstrated that line 3c-5 carried a homozygous deletion (Fig. 3A,D), whereas lines 3c-1 and 3c-6 contained mono-allelic deletions (Fig.3A,D).
Generation of chromosomal fragment deletion by targeting two sites in the PDS locus of petunia.
(A,B) Detection of deletion mutations in pGGE3c (A) and pGGE3 (B) transgenic shoots respectively. The agarose gel images indicate PCR bands amplified using primers PDS-F1 and PDS-R1. F1-5 indicates the PCR fragments with desired deletions. (C) Sanger sequencing chromatograms of the deletions from F1, F2 and F4 in A, B and D. (D) Deletion types in five shoot lines represented by PCR fragments F1-5. (E,F) Other mutation types found in sgR1 (E) and sgR2 (F) target sites. Five clones per PCR product were sequenced. For chromatograms, see Supplementary Figures S3–5 online.
The sequential transformation method was also tested for its ability to produce chromosomal fragment deletion in the petunia PDS locus. The vector pGGE3 (Fig. 1B) was transformed into HC-40 (carrying hCas9) leaf discs. Ten independent plant lines showing a bleaching phenotype were selected for PCR amplification using primers PDS-F1 and PDS-R1 (Fig. 1A). Two of these plant lines showed truncated PCR fragments of approximately 1000 bp in length (Fig. 3B, F4 and 5). Sequencing results showed that F4 and 5 were identical and represented a deletion between the two expected Cas9 cut sites, without additional indels (Fig. 3C,D). Taken together, the electrophoretogram and sequencing results suggested that lines 3–2 and 3–5 carried a homozygous deletion of 867 bp in the PDS locus. As a control experiment, when the vector pGGE3 was transformed into MD plants, no albino phenotype was observed (Table 1). The sequential transformation method can therefor be used in creating chromosomal fragment deletion in petunia.
To further identify the mutation types in pGGE3 and pGGE3c transgenic plants, eight PCR fragments from albino plants without the desired deletions were cloned and sequenced. The results confirmed that all of them carried targeted mutations. The mutations were mainly short indels, except that line 3c-9 contained a 366 bp deletion at the sgR1 targeted site (Fig. 3E,F). Mutations occurring at sgR1 and sgR2 targeted sites accounted for 77.5% and 82.5% of the total sequenced clones respectively, suggesting that both sgR1 and sgR2 possessed high efficiency.
Discussion
In this work, for the first time, the CRISPR/Cas9 system was used to generate targeted mutagenesis in the genome of petunia plants. When constructs targeting single PDS sites were transformed into petunia, the proportion of bleaching phenotype occurring in T0 Basta-resistant plant lines was between 55.6% and 87.5%. These mutation frequencies were similar to those previously reported in other plants, including Arabidopsis(71.2%)20, tobacco (81.8–87.5%)22, tomato (48–75%)28, poplar (51.7%)33 and rice (85.4%)39. DSBs are mainly repaired by NHEJ in somatic cells17. It has been postulated that species-specific differences in NHEJ contribute significantly to the evolution of genome size17. Nevertheless, present data, including our results, suggest that genome size does not have a significant influence on the efficiency of targeted genome mutagenesis mediated by the CRISPR/Cas9 system. It is anticipated that Cas9 technology will promote gene function analysis and genetic improvement of highly heterozygous ornamental plants.
An advantage of the CRISPR/Cas9 system is the capacity to create targeted deletions between two Cas9 cut sites. This capacity was first verified using transient systems3,21,22,25,40. It was also demonstrated using transient systems that the fragment deletion efficiency was negatively correlated with the distance between two paired gRNA/Cas9 cut sites41. Stable transgenic plants with fragment deletions have also been obtained, but most of them involved short deletions (<250 bp)20,28,33,39,42. The creation of only short deletions will limit the applicability of the technique in some applications, such as the deletion of marker genes. Xie et al. obtained a mutant with monoallelic 727 bp deletion of MPK5 and a mutant with a homozygous 357 bp deletion of MPK141 and Zhou et al. obtained four plants with monoallelic large chromosomal fragment (~245 kb) deletions24. Both studies used rice as plant material. In this work, five transgenic plant lines with deletions close to 1 kb were identified from 26 albino plant lines; three of them were homozygous deletions and two were monoallelic. Because albino plants accounted for more than 45% of the single sgRNA transgenic plants in this study and targeting one gene with two sgRNAs greatly increases mutation frequency41, we deduced that the proportion of plant lines with the desired deletions should be more than 8.7% of the transgenic plants; furthermore, the proportion showing homozygous deletion should be more than 5.2%. These results indicate that homozygous large deletions can be readily identified in the first generation.
In additional to the length of deletion, the efficiency of deletion may be influenced by other factors such as cell type and phase of the cell-division cycle, efficiencies of different sgRNAs, direction and base composition of the two paired sgRNAs, location and context of the target. These factors need to be addressed in future.
The homozygous deletions reached 60% (3/5) of the total deletions in this work. A previous report showed that homozygous mutations with 47–90 bp deletions were identified from stably transgenic tomato plants and they occupied 50% (9/18) of the total deletion mutants28. The proportion of the homozygous deletion mutations in tomato (50%)28 and petunia (60%) was higher than the expected if all the deletions on the two alleles of each genome were generated independently. Ma et al. found in rice that the actual homozygous mutation frequency was much higher than would be expected if all the mutations were produced by the NHEJ mechanism39. They reasoned that the HR-based repair mechanism may be involved in the formation of homozygous mutations. However, it has been proven that HR is only a minor DSB repair pathway in somatic cells1. Another surprising result was that the deletions were identical between lines 3c-5 and 3c-6 and also between lines 3–2 and 3–5. A possible explanation for the identical deletions between different lines and the high proportion of homozygous deletions is that NHEJ is influenced by cell state and chromosome structure. However, the total number of reported fragment deletion events between two Cas9 cut sites in stable transformants was nevertheless very low. More data are needed to permit a valid interpretation of the deletion patterns.
Our results showed that the sequential transformation strategy worked well in petunia, with respect to both targeted mutagenesis and deletion. Although novel CRISPR/Cas9 tool-kits using Golden Gate ligation or Gibson Assembly have made the assembly of multiple sgRNA expression cassettes simple39,40,41,42 and have removed the advantage of the sequential transformation strategy in DNA fragment assembly, sequential transformation still has some other advantages, including the improvement of transformation efficiency. Because transformation efficiency declines linearly with increasing plasmid size43 and the expression cassette of Cas9 is usually larger than 5 kb, small sgRNA plasmids unloading Cas9 will make cloning manipulation easier. Secondly, the employment of a Cas9 transgenic plant line in genome editing experiments can provide an identical expression level of Cas9 for genome modification. This will facilitate the comparison of different guide RNA constructs.
In addition, if the cargo capacity of a vector is limited, an sgRNA construct unloading Cas9 will also facilitate the combination of sgRNA with other elements such as a long HR repair template, transcriptional regulation elements and transgene expression cassettes.
There are also some disadvantages for the sequential transformation strategy. First, the resistance gene used to select Cas9 transgenic plants will preclude the use of the same selectable marker gene in sgRNA transformation. However, if necessary, the resistance gene in Cas9 plants can be destroyed or deleted using the method presented here. Another disadvantage is the difficulty of generating plants with the intended modification but carrying no foreign DNA. Because no toxic effect of Cas9 on plants has been observed, the presence of Cas9 in a plant genome may not be an acute problem in basic scientific research. Moreover, although difficult, foreign DNA can still be segregated out by outcrossing. In addition, the sequential transformation may only be applicable in certain situations. Recently, Mikami et al. introduced Cas9 and sgRNA expression cassettes sequentially into rice calli to evaluate the frequency of mutagenesis of different constructs44. They found that the sequential transformation is ‘laborious’ in rice. The difference between their results and ours may originate from the fact that the transformation protocol for rice is distinct from that for petunia. In contrast, virus-mediated genome editing in plants using the CRISPR/Cas9 system has been achieved by using a sequential transformation strategy45,46. Taken together, the sequential transformation is a good alternative strategy for Cas9-mediated genome editing in those plants amenable to leaf-disc transformation.
Material and Methods
Plant Materials
The Petunia hybrida inbred line Mitchell Diploid (MD) was used in all experiments. Surface sterilized seeds were sown on ½MS semi-solid medium and grown under long-day conditions (16h light/8h dark) at 25 °C. After two weeks, seedlings were cut within the hypocotyl region and shoots were re-rooted in MS semi-solid medium. Four weeks later, young leaves were collected for Agrobacterium-mediated transformation.
Vector Construction
Conventional molecular cloning procedures were used for vector construction. SgRNA expression constructs were synthesized using overlapping PCR with pUC119-AtU6::gRNA plasmid DNA (a gift from Prof. Jeen Sheen, Massachusetts General Hospital, Boston, Massachusetts, USA)3 as a template. PCR was carried out as described by Li et al.3. The PCR primers used in this study are listed in Supplementary Table S1 online. PCR products were cut with KpnI and EcoRI to release the sgRNA expression boxes and then inserted into the multiple cloning sites of pGreenII022947 to produce pGGE1 and pGGE2 (Fig. 1B). To combine the sgRNA expression cassettes with the plant codon optimized Cas9 (pcoCas9) expression cassette in a single vector, the pHBT-pcoCas93 plasmid DNA (a gift from Prof. Jeen Sheen) was cut with EcoRI and XhoI and then inserted into pGGE1 and pGGE2 separately to produce pGGE1c and pGGE2c (Fig. 1B). To combine sgR1 and sgR2 expression cassettes into a single plasmid, we amplified the sgR2 expression construct with PCR primers sgrF and sgrR (see Supplementary Table S1 online) to add XbaI and EcoRI to the 5′ and 3′ end of the expression cassette. After sequence verification, the sgR2 expression cassette was released by XbaI and EcoRI and inserted into pGGE1 to produce pGGE3 (Fig. 1B). The sgR2 expression construct was also released by XbaI and EcoRI and inserted into pGGE1c to produce pGGE3c (Fig. 1B). All these vectors were verified by restriction enzyme cutting and sequencing.
Agrobacterium-mediated transformation
The petunia transformation procedure was modified from the methods of Napoli et al.48 and Conner et al.49. In the single transformation protocol, vectors containing both Cas9 and sgRNA(s) (pGGE1c, pGGE2c or pGGE3c, Fig. 1B) were used. Glufosinate-ammonium (Basta) at 4 mg/L was used to select the transgenic plants.
In the sequential transformation protocol, the plasmid pK7WGF2::hCas94, obtained from Addgene (ID 46965), was initially transformed into MD plants. Subsequently, the hCas9 transgenic plants were used as recipients of sgRNA constructs pGGE2 and pGGE3 (Fig. 1B). For details of plant transformation, see Supplementary Methods online.
Detection of mutation
One albino shoot of each line was collected and genomic DNA was extracted using a standard CTAB method. Albino shoot DNAs of pGGE1c transgenic lines were amplified using primers designed to flank the target of sgR1 (PDS-F1 and PDS-R2, Fig. 1A). Genomic DNAs of albino pGGE2 transgenic plants were amplified using primers designed to flank the target of sgR2 (PDS-F2 and PDS-R1, Fig. 1A). The PCR products were purified using an AxyPrep PCR Clean-up kit (Axygen, Union City, CA, USA) and cloned using a pMD19-T cloning kit (Takara, Dalian, China) for sequencing.
Albino shoots of pGGE3 and pGGE3c transgenic lines were genotyped for deletion using the primers PDS-F1 and PDS-R1 (Fig.1A), which flank the targets of sgR1 and sgR2. PCR fragments close to or smaller than 1000 bp were selected, purified and sequenced. A further eight PCR fragments without distinct deletions were also cloned and sequenced.
A minimum of five clones per PCR product were sequenced using the M13F or M13R primers on an ABI3730 DNA analyser.
Additional Information
How to cite this article: Zhang, B. et al. Exploiting the CRISPR/Cas9 System for Targeted Genome Mutagenesis in Petunia. Sci. Rep. 6, 20315; doi: 10.1038/srep20315 (2016).
References
Puchta, H. & Fauser, F. Synthetic nucleases for genome engineering in plants: prospects for a bright future. Plant J. 78, 727–741, 10.1111/tpj.12338 (2014).
Bortesi, L. & Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 33, 41–52, 10.1016/j.biotechadv.2014.12.006 (2015).
Li, J. et al. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nature Biotechnol. 31, 688–691, 10.1038/nbt.2654 (2013).
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J. D. & Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nature Biotechnol. 31, 691–693, 10.1038/nbt.2655 (2013).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nature Biotechnol. 31, 686–688, 10.1038/nbt.2650 (2013).
Feng, Z. et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23, 1229–1232, 10.1038/cr.2013.114 (2013).
Jiang, W. et al. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 41, e188, 10.1093/nar/gkt780 (2013).
Mao, Y. et al. Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol. Plant 6, 2008–2011, 10.1093/mp/sst121 (2013).
Miao, J. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 23, 1233–1236, 10.1038/cr.2013.123 (2013).
Xie, K. & Yang, Y. RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant 6, 1975–1983, 10.1093/mp/sst119 (2013).
Voytas, D. F. Plant genome engineering with sequence-specific nucleases. Annu. Rev. Plant Biol. 64, 327–350 (2013).
Chen, K. & Gao, C. Targeted genome modification technologies and their applications in crop improvements. Plant Cell Rep. 33, 575–583, 10.1007/s00299-013-1539-6 (2014).
Belhaj, K., Chaparro-Garcia, A., Kamoun, S., Patron, N. J. & Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 32, 76–84, 10.1016/j.copbio.2014.11.007 (2015).
Kumar, V. & Jain, M. The CRISPR-Cas system for plant genome editing: advances and opportunities. J. Exp. Bot. 66, 47–57, 10.1093/jxb/eru429 (2015).
Jain, M. Function genomics of abiotic stress tolerance in plants: a CRISPR approach. Frontiers in Plant Sci. 6, 10.3389/fpls.2015.00375 (2015).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821, 10.1126/science.1225829 (2012).
Puchta, H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J. Exp. Bot. 56, 1–14, 10.1093/jxb/eri025 (2005).
Jiang, W., Yang, B. & Weeks, D. P. Efficient CRISPR/Cas9-mediated gene editing in Arabidopsis thaliana and inheritance of modified genes in the T2 and T3 generations. Plos One 9, 99225, 10.1371/journal.pone.0099225.g001 (2014).
Fauser, F., Schiml, S. & Puchta, H. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J. 79, 348–359, 10.1111/tpj.12554 (2014).
Feng, Z. et al. Multigeneration analysis reveals the inheritance, specificity and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 4632–4637, 10.1073/pnas.1400822111 (2014).
Belhaj, K., Chaparro-Garcia, A., Kamoun, S. & Nekrasov, V. Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 9, 10.1186/1746-4811-9-39 (2013).
Gao, J. et al. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 87, 99–110, 10.1007/s11103-014-0263-0 (2015).
Zhang, H. et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 12, 797–807, 10.1111/pbi.12200 (2014).
Zhou, H., Liu, B., Weeks, D. P., Spalding, M. H. & Yang, B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 42, 10903–10914, 10.1093/nar/gku806 (2014).
Upadhyay, S. K., Kumar, J., Alok, A. & Tuli, R. RNA-guided genome editing for target gene mutations in wheat. G3: Genes, Genomes, Genetics 3, 2233–2238, 10.1534/g3.113.008847/-/DC1 (2013).
Wang, Y. et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nature Biotechnol. 32, 947–951, 10.1038/nbt.2969 (2014).
Liang, Z., Zhang, K., Chen, K. & Gao, C. Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J. Genet. Genomics 41, 63–68, 10.1016/j.jgg.2013.12.001 (2014).
Brooks, C., Nekrasov, V., Lippman, Z. B. & Van Eck, J. Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiol. 166, 1292–1297, 10.1104/pp.114.247577 (2014).
Ito, Y., Nishizawa-Yokoi, A., Endo, M., Mikami, M. & Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 467, 76–82, 10.1016/j.bbrc.2015.09.117 (2015).
Ron, M. et al. Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Phsiol. 166, 455–469, 10.1104/pp.114.239392 (2014).
Wang, S. et al. Efficient targeted mutagenesis in potato by the CRISPR/Cas9 system. Plant Cell Rep. 34, 1473–1476, 10.1007/s00299-015-1816-7 (2015).
Jia, H. & Wang, N. Targeted genome editing of sweet orange using Cas9/sgRNA. Plos One 9, 10.1371/journal.pone.0093806 (2014).
Fan, D. et al. Efficient CRISPR/Cas9-mediated targeted mutagenesis in populus in the first generation. Sci. Rep. 5, 12217, 10.1038/srep12217 (2015).
Sugano, S. S. et al. CRISPR/Cas9-mediated targeted mutagenesis in the liverwort Marchantia polymorpha L. Plant Cell Physiol. 55, 475–481, 10.1093/pcp/pcu014 (2014).
Schiml, S., Fauser, F. & Puchta, H. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 80, 1139–1150, 10.1111/tpj.12704 (2014).
Yagi, M. Recent progress in genomic analysis of ornamental plants, with a focus on carnation. Horticulture J. 84, 3–13, 10.2503/hortj.MI-IRO1 (2015).
Gerats, T. & Vandenbussche, M. A model system for comparative research: Petunia. Trends Plant Sci. 10, 251–256, 10.1016/j.tplants.2005 (2005).
Qin, G. et al. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid and gibberellin biosynthesis. Cell Res. 17, 471–482, 10.1038/cr.2007.40 (2007).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284, 10.1016/j.molp.2015.04.007 (2015).
Lowder, L. G. et al. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant physiology 169, 971–985, 10.1104/pp.15.00636 (2015).
Xie, K., Minkenberg, B. & Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. U.S.A. 112, 3570–3575, 10.1073/pnas.1420294112 (2015).
Xing, H.-L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 10.1186/s12870-014-0327-y (2014).
Hanahan, D., Jessee, J. & Bloom, F. R. Plasmid transformation of escherichia-coli and other bacteria. Methods Enzymol. 204, 63–113 (1991).
Mikami, M., Toki, S. & Endo, M. Comparison of CRISPR/Cas9 expression constructs for efficient targeted mutagenesis in rice. Plant Mol. Biol. 88, 561–572, 10.1007/s11103-015-0342-x (2015).
Ali, Z. et al. Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol. Plant 8, 1288–1291, 10.1016/j.molp.2015.02.011 (2015).
Yin, K. et al. A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci. Rep. 5, 14926, 10.1038/srep14926 (2015).
Hellens, R. P., Edwards, E. A., Leyland, N. R., Bean, S. & Mullineaux, P. M. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832, 10.1023/a:1006496308160 (2000).
Napoli, C., Lemieux, C. & Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2, 279–289, 10.1105/tpc.2.4.279 (1990).
Conner, A. J., Albert, N. W. & Deroles, S. C. In Petunia: Evolutionary, Developmental and Physiological Genetics (eds Tom Gerats & Judith Strommer ) Ch. 19, 395–410 (Springer, 2009).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31272199) and the Program of Introducing Talents of Discipline to Universities (B12006).
Author information
Authors and Affiliations
Contributions
Y.G. designed the research. B.Z., X.Y., C.Y. and Y.G. performed vector construction, plant transformation and detection of mutations. Y.G. and M.L. contributed reagents and materials. Y.G. wrote the manuscript. All authors reviewed the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, B., Yang, X., Yang, C. et al. Exploiting the CRISPR/Cas9 System for Targeted Genome Mutagenesis in Petunia. Sci Rep 6, 20315 (2016). https://doi.org/10.1038/srep20315
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20315
This article is cited by
-
Applications of CRISPR/Cas9 Technology in Ornamental Plants
Plant Molecular Biology Reporter (2023)
-
CRISPR/Cas9 mediated editing of phytoene desaturase gene in squash
Journal of Plant Biochemistry and Biotechnology (2023)
-
CRISPR/Cas9-mediated editing of PhMLO1 confers powdery mildew resistance in petunia
Plant Biotechnology Reports (2023)
-
Dynamic evolution of small signalling peptide compensation in plant stem cell control
Nature Plants (2022)
-
An assessment on CRISPR Cas as a novel asset in mitigating drought stress
Genetic Resources and Crop Evolution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.