Abstract
We present a novel “Top-down” strategy to design the long phosphorescent phosphors in the second biological transparency window via energy transfer. Inherence in this approach to material design involves an ingenious engineering for hybridizing the coordination networks of hosts, tailoring the topochemical configuration of dopants and bridging a cascaded tunnel for transferring the persistent energy from traps, to sensitizers and then to acceptors. Another significance of this endeavour is to highlight a rational scheme for functionally important hosts and dopants, Cr/Nd co-doped Zn1−xCaxGa2O4 solid solutions. Such solid-solution is employed as an optimized host to take advantage of its characteristic trap site level to establish an electron reservoir and network parameters for the precipitation of activators Nd3+ and Cr3+. The results reveal that the strategy employed here has the great potential, as well as opens new opportunities for future new-wavelength, NIR phosphorescent phosphors fabrication with many potential multifunctional bio-imaging applications.
Similar content being viewed by others
Introduction
There is an increasing interest in the use of long persistent phosphorescence in the biologically transparent window to drive the photonic bioprobe for tracing the cancer cells1. Long phosphorescent phosphors (LPPs) can help avoiding the challenging requirement of high-intensity illumination during the signal collection, which often leads to decreased signal-to-noise ratio and photon-induced deterioration of analytes2. This emerging research trend, which incorporates various fields of materials science, biology, chemistry, engineering, physics and pharmaceuticals, follows two main directions: operation waveband and persistent duration, with many relevant crossing points in between3,4. As we know, there are two biologically transparent windows: first one at 650–950 nm and second one at 1000–1350 nm5; Near-infrared (NIR) light in the first transparency window can penetrate biological tissues such as skin and blood more efficiently than visible light6, yet the second region has even lower absorption and scattering therefore offers more efficient tissue penetration7. However, the main researches about the operational waveband of NIR LPPs mainly focus on the short wavelength region, i.e. first NIR window.
In addition to altering the emission center and tailoring the crystal field surrounding the activator, another useful strategy to extend the operational waveband, is to transfer the persistent energy of sensitizers to acceptors8. In fact, although the afterglow properties are predominantly controlled by the active traps, more subtle effects, such as topochemical coordination-configuration of dopant ions, can also have a profound role to the spectroscopic features of LPPs, which has long been recognized as a significant issue lying at the heart of doping chemistry and photoluminescent theory9. Considering the advanced engineering of cascaded tunnel of energy transfer (traps → activator(A) → activator(B)) and going into the details of it, one has at one’s disposal several decades worth of well-established principles in the coincident matching of macroscopical and microscopic features in spectroscopy, coordination chemistry and network connectivity relating to activators and hosts10,11. Traditionally, materials scientists view such network-engineering design accessed via active impurities with a practical eye intent on describing integral architectures in terms of ion types, valency and radius, local coordination geometries, as well as their concomitant implications for electronegativity and chemical bonding12. However, due to the complex attribute of topological network, there are still remaining grand challenges: to gain better modulation for the local coordination configuration of dopants, to understand the principle linking the indispensable transfer channel of independent individual and to realize true predictability to the arrangements of traps and dopants (sensitizers, activators, or co-dopants) in coordinated network.
In this work we present a new “Top-down” approach to design and synthesize the long phosphorescent phosphors in the second biological transparent window. The material design approach employed here involves an ingenious engineering for hybridizing the coordination networks of hosts, tailoring the topochemical configuration of dopants and bridging cascaded channels for transferring the persistent energy from traps, to sensitizers, to acceptors. We present a closed energy transfer channel from Cr3+ to Nd3+ in ZnGa2O4 phosphor and invalid electronic reservoir in CaGa2O4 phosphor, respectively. Persistent energy-transfer could occur in Zn1–xCaxGa2O4 solid-solution because two dopants were successfully locked in a cage via the efficient crystal packing at an appropriate distance, in addition to the preservation of native electron traps. The hybrid network topologies and structural motifs, thus far will be outlined with particular emphasis on how specific route of energy transfer can be prepared via premeditatedly designing a material system. Such design strategy will notably open a vista of potential avenues for the design of new optical functional materials for the future.
Results and Discussion
Our strategy was inspired by the fundamental spectroscopic theory of energy transfer and local intercalation reaction in inorganic polycrystals (Fig. 1)13. In our view, a typical long phosphorescent phosphor (MSI, in Fig. 1a) features a prominent electron reservoir (C, in Fig. 1) with the distinct ability of storing and releasing the captured electrons, as well as a notable photon-emitter (A, in Fig. 1) with higher quantum efficiency under the condition of accurately matching lattice-coordination network and atomic radius14. A pre-established electronic transfer channel (AG, traps (C) → activator (A), in Fig. 1) ensures the long persistent phosphorescence. However, the topological network does not provide an opportunity for another activator (B, in Fig. 1) to embed itself into the suitable lattice site. Such structural constraint thus, closes the possible channel of energy transfer (ET, in Fig. 1) between (A) and (B), leading to the luminescent and phosphorescent quenching. Fortunately, the existing chemical and spectroscopic knowledge offer a far-sighted technique to select another material system (MSII, in Fig. 1b), which allows a synchronous precipitation of activator (A) and (B), as well as engineers a theoretically existent energy-transfer channel (activator (A) → activator (B)). But to our surprise, this scheme misses the necessary electron reservoir so as to completely decrease the probability of electrons trapping-detrapping (Fig. 1b).
Schematic illustration showing the influence of ions doping-pattern and network-structural motifs on energy transfer process between traps and dopants.
(a,b) represent the typical trapping and de-trapping process (AG) (traps (C) → activator (A)), as well as energy transfer process (ET) (activator (A) → activator (B)) in different material system (MSI and MSII), respectively. Hybrid Materials (MSIII) involves a solid-solution to offer the suitable coordination geometry for activators (A) and (B) and realize the cascaded energy transfer (traps (C) → activator (A) → activator (B)).
The use of solid-solution complexes to engineer predictable, multi-dimensional infinite networks has received ever-increasing attention in the area of chemistry and materials science15. Solid-solutions have already proven their superiorities in the areas of optical, optoelectronic, electrical and magnetic properties than the single component16. Pan et al. broke new ground in the field by using zinc gallogermanates solid-solution as the system, thereby achieving a super-long NIR afterglow emission time of 360 h3. Kobayashi et al. also demonstrated a state-of-the-art LixFePO4 solid-solution technology, opening the door for lithium ion batteries to take their place in large-scale applications17. In addition, a series of solid-solution, such as AgGa1−xAlxO2, Zn1−xCuxS, (SrTiO3)1−x(LaTiO2N)x, also have been developed and used as the advanced photocatalysts to enhance the photocatalytic activity of a given semiconductor photocatalyst18. Therefore, solid-solution highlights hybrid coordination network of host and is expected to open up a possibility in the visualization of the structural and functional binding process of traps and all activators into an independent system19. By rationally deploying an indirect intercalation complex comprised by polyhedron ligands of materials (MSI) and (MSII), hybrid coordination-network of novel solid-solution (MSIII, in Fig. 1c) is engineered to steady the activators (A) and (B), modulate the topochemical configuration of activators and realize the cascaded energy transfers, traps(C) → activator(A) → activator(B) (Fig. 1c). Such novel structural motif is anticipated to adopt a disturbance to native unit cell and bridging a predictable periodic coordination network.
To validate research idea, a typical NIR long phosphorescent phosphor ZnGa2O4: Cr was pursued as preferential material system, which has been proven capable of supporting high defect densities, thought to be associated primarily with Zn vacancies (VZn) and O vacancies (VO), as well as some antisite deficiencies (ZnGa)20. Making use of its defect capacity, ZnGa2O4: Cr has been demonstrated as a NIR photo-emitter with surprisingly long persistent phosphorescence in first NIR window (Supplementary Fig. S1). Here, we target the operating waveband in the second NIR window by transferring the persistent energy of Cr3+ to Nd3+ in Cr/Nd-codoped ZnGa2O4 LPPs. Nd3+ ion is chosen as the emission center in order to take advantage of the appropriate energy level characteristic, i.e. NIR-absorption (680, 750 and 800 nm) and NIR emission (1064 nm)21. The various sharp transitions of Nd3+ [4I9/2 → 4F5/2], [4I9/2 → 4F7/2], [4I9/2 → 4F9/2], just overlap the electron transition from metastable state (4T2) to ground state (4A2) of Cr3+, allowing the potential energy transfer from Cr to Nd22. However, no any NIR phosphorescence in the second NIR window can be observed in Cr/Nd-codoped ZnGa2O4 LPPs (Fig. 2a). In fact, the desired phosphorescence is still absent in Nd3+ singly doped ZnGa2O4 phosphor after ceasing the excitation (Supplementary Fig. S2). It is notable that the diffuse reflection spectrum consists of the characteristic transition bands centered at 530, 588, 688, 748 and 808 nm, respectively, corresponding to Nd3+ f-f transition, [4I9/2 → 2K13/2 + 4G7/2 + 4G9/2], [4I9/2 → 2G7/2 + 2G5/2], [4I9/2 → 4F9/2], [4I9/2 → 4F7/2], [4I9/2 → 4F5/2] in Nd3+ doped ZnGa2O4 phosphor (Supplementary Fig. S3)21; yet under the excitation at 748 nm, emission peak at 1064 nm, attributed to Nd3+ [4F3/2 → 4I11/2] transition is not identifiable (Supplementary Fig. S4). This attractive optical quenching-phenomenon of luminescence and phosphorescence may be not concerned with the trap distribution, but the microcosmic network architecture.
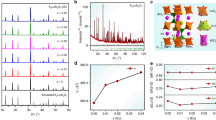
(a) Vis-NIR long persistent phosphorescence spectrum of ZnGa2O4: Cr/Nd phosphor. The inset shows a conjectural doping pattern of Cr and Nd. (b) XRD patterns for ZnGa2O4: xCr (x = 0.5% and 10%) and ZnGa2O4: xNd (x = 0.5% and 5%) phosphors. (c) Dependence of XRD peak ([PI] labeled in Fig. 2b) position and intensity as a function of Cr and Nd concentration in ZnGa2O4 phosphor. (d–e) 71Ga solid state NMR spectra of ZnGa2O4: xCr (x = 0.5% and 10%) and ZnGa2O4: xNd (x = 0.5% and 10%) samples. All spectra were recorded at a magnetic field of 11.7 T with a sample spinning frequency of 25 kHz.
In ZnGa2O4, a majority of [GaVI] cations occupy octahedral sites, whereas all of the [ZnIV] cations occupy tetrahedral sites23. As a preliminary conjecture, Cr3+ has proven its strong ability to substitute for Ga3+ in distorted octahedral coordination, whereas Nd3+ cannot be effectively introduced into this specific network configuration (inset of Fig. 2a). In order to identify this possibility of ion doping, we focus on the intricate topochemical coordination geometry of Cr and Nd ions in zinc gallate spinel. The elucidation is performed in detail by a combination of XRD data and 71Ga solid state nuclear magnetic resonance (NMR) studies. XRD patterns of ZnGa2O4: xCr (x = 0.5%, 5%, 10% and 20%) and ZnGa2O4: xNd (x = 0.5%, 5%, 10% and 20%) phosphors were measured and shown in Fig. 2b, Supplementary Fig. S5, S6. The peaks in XRD patterns of all Cr-doped samples are well indexed to pure ZnGa2O4 spinel structure (JCPDS 86-0848). In stark contrast, the higher doping content (up to 5%) of Nd ion gives rise to an impure phase, NdGaO3 (JCPDS, 70-3810) in Nd-doped samples. Another interesting phenomenon, i.e. XRD dominated peak (PI in Fig. 2b) shifting towards to higher 2θ value with the increment of Cr content, reveals a small linear variation in ZnGa2O4 unit cell lattice parameter with Cr3+ substitution, whereas no any shift of same peak is observed in Nd-doped samples, further ensuring the distinct phase splitting (Fig. 2c). In addition, a decline of the peak intensity in Fig. 2c also is present. Nevertheless, the causes of this decline may be different and rooted from either the substitution or the phase splitting.
NMR allows the observation of specific quantum mechanical and magnetic properties of atomic nucleus, as well as provides the detailed information about the structure, dynamics, reaction state and chemical environment of molecules24. Many scientific techniques exploit NMR phenomena to cover the interplay between the ligands and geometric centers, as well as study the topological network motif in crystals, microcrystalline powders, or anisotropic solutions, etc25. 71Ga solid-state NMR is famous for the permission of quantitative analyses to different Ga3+ central coordination state in inorganic solids26. Figure 2d,e shows the systematical physical investigations of Ga coordination geometry in Cr and Nd singly doped ZnGa2O4, respectively. For the undoped ZnGa2O4 samples, 71Ga NMR spectra exhibit two well-resolved resonances. The relative higher intensive signal at about 31 ppm is characteristic of sixfold coordinated Ga atoms and the other weaker one ~at 170 ppm corresponds to Ga atoms in the tetrahedral sites of the spinel structure26. It is necessary to mention that with increasing Cr content (from 0.5% to 10%), 71Ga NMR spectra present a significant broadening of spectral lines (Fig. 2d). In prominent contrast, scarcely any distinct influence on NMR spectral lines can be found by varying the Nd doping content in solid NMR spectra of ZnGa2O4: xNd (x = 0.5% and 10%) phosphors (Fig. 2e). The clear separation of NMR chemical shift at ~31 ppm between the two samples implies the precipitation of Cr3+ into the octahedral lattice site and the excludability of local configuration to Nd3+ ions. The NMR results are in accordance with XRD data, offering a powerful structural evidence to explain the interesting phenomena of phase splitting and luminescence quenching.
Actually, rare-earth elements generally form complexes which have high coordination numbers (CNs) and weak metal-ligand bonds, because of their large ionic radii and relatively low oxidation states27. Typically transition-metal and main-group elements have coordination numbers 2–6, while rare-earth metals have CNs > 628. The resulting coordination polyhedra include trigonal prisms (CN = 6) or its variation by stepwise capping of the prism face up to CN = 9, in addition to square antiprisms (CN = 8); Coordination number 3 is realized only under extreme conditions28. Therefore, to supply an ideal dwelling for Nd3+, a suitable material system should be proposed. Alkaline-earth metals have large ionic radii and various coordination-numbers 3–8 in different hosts, which ensure the selection of alkaline-earth gallates29. CaGa2O4 has a similar spinel crystal structure with ZnGa2O4. In CaGa2O4, [CaVI] cations occupy octahedral sites29. This configuration thus features a path of easy doping ion precipitation into the octahedral [CaVI] under the condition of matching geometrical lattice and atomic radius, which occurs with rare earth ion, Nd.
As expected, Fig. 3a exhibits the characteristic transitions of Nd3+ in Nd singly doped CaGa2O4 phosphor. However, the idealistic and aspirational long persistent phosphorescence is still absence in Cr singly, Nd singly and Cr/Nd doped CaGa2O4 phosphors, respectively (Supplementary Fig. S7). A possible cause of this problem is due to the lack of effective traps (Fig. 3b). In sharp contrast to Cr/Nd codoping ZnGa2O4, photoluminescence excitation (PLE) spectrum monitored at 1064 nm of Cr/Nd codoping CaGa2O4 sample consists of two specific excitation bands centered at ~410 and ~620 nm, in addition to Nd3+ characteristic f-f transitions (Fig. 3c), indicating an energy transfer from Cr3+ to Nd3+. Obviously, the strong one is attributed to the Cr3+ [4A2 → 4T1], while the weak one corresponds to Cr3+ [4A2 → 4T2]30. Further verification of energy transfer between Cr3+ and Nd3+ is supplied by emission spectrum and decay curve monitored at 1064 nm under the excitation wavelength at 410 nm (Fig. 3c and Supplementary Fig. S8). A possible channel of energy transfer from Cr3+ to Nd3+ is Cr3+ [4T2 → 4A2]: Nd3+ [4I9/2 → 4F5/2], [4I9/2 → 4F7/2], or [4I9/2 → 4F9/2], depending on the overlap between Cr3+ emission band and Nd3+ absorption band (Fig. 3d)31. As discussed above, due to the similar atomic radius and geometric configurations, Nd ions can easily precipitate on Ca lattice site in CaGa2O4, enabling the distinct photoluminescence (PL). To probe the lattice configuration and substitution progress in CaGa2O4, we performed XRD and solid state NMR experiments. X-ray diffraction pattern first confirms the crystallization of Nd-doped calcium gallate (Fig. 3e). In contrast to Nd-doped ZnGa2O4, all Nd-doped CaGa2O4 samples can be indexed as standard phase CaGa2O4 (JCPDS 16-0593). There is no any apparent observation of phase splitting from XRD data, even under a higher doping content of Nd3+, firmly supporting the rational inclusion of Nd3+ into an inert matrix, CaGa2O4. This result is also supported by 71Ga solid state NMR spectra. In contrast to ZnGa2O4 host, the undoped CaGa2O4 sample has a dominant chemical shift at 170 ppm (Fig. 3f). With increasing dopants content, CaGa2O4: Nd also has the same effect of NMR resonances’ line broadening and the linear increase of NMR resonances integrated intensity, strongly suggesting the successful substitution in substantial amounts of Nd into Ca lattice site.
(a) Static photoluminescence spectrum under excitation at 750 nm and the corresponding photoluminescence excitation spectrum monitored at 1064 nm of CGO-0.5Nd phosphor; (b) Thermoluminescence curves of 0.5%Cr-doped ZnGa2O4 and CaGa2O4 phosphors measured 30 s after irradiation ceased; (c) Photoluminescence spectrum under excitation at 410 nm and photoluminescence excitation spectrum monitored at 1064 nm of CGO-0.5Cr0.5Nd phosphor; (d) Schematic illustration showing the energy-level diagram of Cr3+ and Nd3+ in CaGa2O4 phosphors; (e) XRD patterns for CaGa2O4: xNd (x = 0.5%, 5% and 10%) phosphors; (f) 71Ga NMR spectra of CaGa2O4: xNd (x = 0, 0.5% and 5%) samples.
Seemingly, as the individual backbone, MGa2O4 (Zn and Ca) polymorph is chosen as the prototypical coordination network for its respective ability to engineer the functionally independent tunnel, traps(C) → activator(A), or activator(A) → activator(B), used to transfer the required energy. The only regret is the fundamentally missing connection of traps(C) → activator(A) → activator(B) in a separate material system. To address this issue, we anticipate a novel solid-solution Zn1-xCaxGa2O4 to bridge a new channel for transferring the persistent energy from traps to desired ions, based on the cautious consideration for crystal structure, ion valency and chemical bond relating to hosts and dopants. The desired NIR phosphorescence at 1064 nm is finally present in the afterglow spectra of Zn1-xCaxGa2O4 (x = 0.1, 0.3, 0.4 and 0.5) solid-solution (Fig. 4a). Significantly, we also observe a strong dependence (i.e. rising first followed by a decline) of phosphorescent peak intensity and decay dynamics on Ca concentration in Fig. 4a. We attribute this special spectral change of Nd3+ to the successful persistent energy transfer from Cr3+ to Nd3+, which is supported by the meticulous spectral studies of Nd3+ in an optimal Zn0.6Ca0.4Ga2O4: 0.5Cr/0.5Nd solid-solution: PLE band at 410 nm should be assigned to Cr3+ transition [4A2−4T2], while a distinct NIR PL peak at 1064 nm is observed under the excitation at 410 and 600 nm (Fig. 4a and Supplementary Fig. S9). The additional support for the formation of an unrestricted energy tunnel, traps → Cr3+ → Nd3+, is the analysis of kinetic processes in Z0.6C0.4GO: 0.5%Cr/xNd (x = 0, 0.5%, 1% and 2%) samples (Fig. 4b). PL decay dynamics study of Cr3+ shows a notable shortening in decay lifetime from 7.8 (Z0.6C0.4GO−0.5Cr), to 7.59 (Z0.6C0.4GO−0.5Cr0.5Nd), to 7.32 ms (Z0.6C0.4GO−0.5Cr2Nd), giving clear evidence of successfully simultaneous precipitation of two activators into the corresponding lattice along with the effective energy transfer from Cr3+ to Nd3+.
(a) persistence time monitored at 1064 nm as a function of Ca concentration (x = 0.1, 0.3, 0.4 and 0.5). The inset shows the long persistent phosphorescence spectra of Z1-xCxGO (x = 0.1, 0.3, 0.4 and 0.5) samples and photoluminescence excitation spectrum monitored at 1064 nm of sample Z0.6C0.4GO. (b) Normalized photoluminescent and phosphorescent decay curves of Z0.6C0.4GO: 0.5%Cr/xNd (x = 0, 0.5%, 1% and 2%) samples. The monitored transition is Cr3+ [4T2 → 4A2]. (c) Thermoluminescence curves of Z1-xCxGO (x = 0.1, 0.3, 0.4, 0.5 and 0.7) phosphors measured 30 s after irradiation ceased. The inset shows the dependence of TL peak position as a function of Ca concentration. (d) XRD patterns for Z1-xCxGO (x = 0.1, 0.4, 0.5 and 0.7) phosphors. (e) EDX mapping of sample Z0.6C0.4GO. (f) 71Ga NMR spectra of Z1-xCxGO (x = 0, 0.2, 0.4 and 0.7) phosphors. (g) Normalized Raman spectra of ZGO-0.5Nd, ZGO-5Nd, CGO-0.5Nd, CGO-5Nd and Z1-xCxGO (x = 0.1, 0.4 and 0.7) phosphors.
It should be noted that, to the best of our knowledge, this type of NIR long-persistence phosphorescence has not been previously reported to occur in hybrid coordination networks by engineering cascaded energy transfer channels. Such substantial progress is strongly influenced by two key attributes; one is trap distribution and another is network architecture. Apparently, the variation of trap distribution may be not a crucial factor in exploring the nature of transfer channel, because the indispensable electron reservoir is still steadily embedded in all the Zn1-xCaxGa2O4 solid-solutions (Fig. 4c). To probe the evolution of topological network-dependent topochemical coordination, the systematic characterization, such as, XRD, solid NMR, EDX mapping and Raman spectra should be conducted32. XRD peaks in Z1-xCxGO (x = 0.1, 0.4, 0.5 and 0.7) samples indicate their ZnGa2O4 spinel solid-solution nature, while the superimposed peaks in samples Z0.5C0.5GO and Z0.3C0.7GO can be well indexed by the diffraction peaks of ZnGa2O4 and CaGa2O4 (Fig. 4d and Supplementary Fig. S10). EDX mapping analysis reveals the solid-solutions have uniform distribution of Ca elements in all of the spinel solid-solutions (Supplementary Fig. S11, S12 and Fig. 4e). EDX experimental composition approximating the theoretical value supports the successful inclusion of Ca elements in spinel crystals (Supplementary Table S2).
71Ga NMR spectra have provided some insights into the coordination variation of Ga center in ZnGa2O4 and CaGa2O4 phosphors, due to the incorporation of Cr and Nd. it is also expected to manifest its ability in resolving the question of topochemical configuration’s evolution process, as the addition of Ca element. As shown in Fig. 4f, with increasing Ca content (0, 0.2, 0.4 and 0.7), two resonances at 170 and 31 ppm in 71Ga NMR spectra increasingly present the linear broadening. In these solid solutions, Zn-O and Ga-O tetrahedron could suppress the intrusion of Ca element due to the mismatch of coordination configuration. In fact, to steady Ca ion, parts of Ga-O octahedron must reorient to form the new polyhedron network Ca-O octahedron along with the transformation from Ga-O octahedron to Ga-O tetrahedron due to the decrease of Zn-O tetrahedron. In detail, for the samples Z1-xCxGO (x = 0, 0.2 ,0.4), the motion of local hybrid coordination-networks evolution include: (1) the precipitation of Ca on the lattice site of octahedron Ga, giving rise to the broadening of NMR resonance at 164 ppm; (2) the conversion from Ga-O octahedron to Ga-O tetrahedron, resulting in the enhancement of NMR resonance at 65 ppm. This interesting redeployment of network configuration thus permits the modification of topochemical state of dopants, as well as opens the possibility of bridging cascaded channels to transfer the persistent energy. To further validate the research idea aiming at the network configuration, Raman spectra of the fabricated samples also can be selected as the pertinent tool to further analyze the evolution of network architecture (Fig. 4g and Supplementary Fig. S13)33. In stark contrast to samples ZGO-0.5Nd and ZGO-5Nd, normalized Raman spectra of samples CGO-0.5Nd and CGO-5Nd do not exhibit the notable Raman peak shift and variation of Raman peak intensity, indicating a strong constraint of topological network to the migration of Nd ions in CaGa2O4. In fact, only two distinct Raman bands at ~1358 and 1434 cm−1 are present in the Raman spectrum of CGO-0.5Nd, while the Raman spectrum of ZGO-0.5Nd includes three identifiable Raman peaks at ~1341, 1389 and 1425 cm−1. Thus, Raman spectra of Z1-xCxGO (x = 0.1, 0.4 and 0.7) solid-solutions consequentially show a unit number decrease of Raman peaks with the increment of Ca content (inset of Fig. 4g). The variation of middle peak at 1401 cm−1 as a function of Ca doping content ensures the strong signature of the hybrid network structure, which is in accordance with the XRD and solid-state NMR data.
In summary, we report a principle of bridging cascaded energy transfer channels to activate long persistent phosphorescence in the second biological window and fabrication of novel near-infrared phosphorescent phosphor Cr/Nd codoped Zn1-xCaxGa2O4 solid-solutions. Structural studies offer the powerfully fundamental evidences to explain the closed energy transfer channel from Cr3+ to Nd3+ in ZnGa2O4 phosphor and invalidation of electronic reservoir in CaGa2O4 phosphor. We believe that the ingenious solid-solution technology featuring the superiority of engineering a hybrid coordination-network opens new paths for advanced dynamic management of activation energy and gives the inspiration to design future new-wavelength, NIR phosphorescent phosphors by energy transfer.
Methods
Materials
4N pure CaCO3, Ga2O3, ZnO, Nd2O3 and Cr2O3 were selected as the raw materials.
Preparation of ZnGa2O4: xCr/yNd
Phosphors with molar compositions of ZnGa2O4: xCr/yNd (x = 0, 0.5%, 5%, 10%, 20%; y = 0, 0.5%, 5%, 10%, 20%), (Supplementary Table S1) were prepared by the solid state reaction method. The reaction included a two-step thermal treatment (i.e., initial calcination at 800 °C for 5 h, secondary calcination at 1350 °C for 3 h).
Preparation of CaGa2O4: xCr/yNd
Phosphors with molar compositions of CaGa2O4: xCr/yNd (x = 0, 0.5%; y = 0, 0.5%, 5%, 10%), (Supplementary Table S1) were prepared by the solid state reaction method. The reaction included a two-step thermal treatment (i.e., initial calcination at 800 °C for 5 h, secondary calcination at 1200 °C for 3 h).
Preparation of Zn1-xCaxGa2O4: 0.5Cr/yNd
Phosphors with molar compositions of Zn1-xCaxGa2O4: 0.5Cr/yNd (y = 0, 0.5%, 1%, 2%; x = 0.1, 0.2, 0.3, 0.4, 0.5, 0.7), (Supplementary Table S1) were prepared by the solid state reaction method. The reaction included a two-step thermal treatment (i.e., initial calcination at 800 °C for 5 h, secondary calcination at 1350, 1350, 1300, 1300, 1270, 1250 °C for 3 h as a function of x, respectively).
Characterization
The prepared materials were analyzed by X-ray diffraction (Cu/Kα) to confirm the sole crystalline phase. Room-temperature photoluminescence (PL), photoluminescence excitation (PLE) spectra, afterglow spectra and decay curves were measured with a high-resolution spectrofluorometer (UK, Edinburgh Instruments, FLS920) equipped with a 500 W Xenon lamp as an excitation source, with a Hamamatsu R928P visible photomultiplier (PMT) (250–850 nm) and a liquid nitrogen-cooled Hamamatsu R5509-72 NIR PMT as the detectors. TL glow curves and TL excitation (TLE) spectra were measured with a FJ-427A TL meter (China, Beijing) to characterize defect properties. Unless otherwise mentioned, the samples were pre-annealed at 600 K before testing and some measurements were taken after pre-irradiating the samples for 10 min by using a xenon lamp. EDX images are characterized by a field emission scanning electron microscopy (FE-SEM), Nova NanoSEM 430. 71Ga Hahn echo NMR experiments were performed on Bruker Avance III spectrometers operating at magnetic fields of 111.4 T corresponding to 71Ga Larmor frequencies of 152.54 MHz) using Bruker 2.5 mm triple and double resonance probe heads. The 900 degree pulse length is 1.25 μm with a recycle delay of 8s. 71Ga chemical shifts were referenced relative to a 1.0 M aqueous solution of Ga(NO3)3. All 71Ga spectra were fitted using the Dmfit software. Raman spectra were collected with a Renishaw inVia Raman microscope irradiated by a visible laser at 532 nm.
Additional Information
How to cite this article: Qin, X. et al. Hybrid coordination-network-engineering for bridging cascaded channels to activate long persistent phosphorescence in the second biological window. Sci. Rep. 6, 20275; doi: 10.1038/srep20275 (2016).
References
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Abdukayum, A., Chen, J. T., Zhao, Q. & Yan, X. P. Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 135, 14125–14133 (2013).
Pan, Z. W., Lu, Y. Y. & Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 11, 58–63 (2012).
Maldiney, T. et al. Gadolinium-doped persistent nanophosphors as versatile tool for multimodal in vivo imaging. Adv. Funct. Mater. 25, 331–338 (2015).
Hong, G. S. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics. 8, 723–730 (2014).
Lv, R. C. et al. A yolk-like multifunctional platform for multimodal imaging and synergistic therapy triggered by a single near-infrared light. Acs Nano. 9, 1630–1647 (2015).
Hong, G. S. et al. In Vivo Fluorescence Imaging with Ag2S Quantum Dots in the Second Near-Infrared Region. Angew. Chem. Int. Edit. 51, 9818–9821 (2012).
Li, Y. et al. Tailoring of the trap distribution and crystal field in Cr3+-doped non-gallate phosphors with near-infrared long-persistence phosphorescence. NPG. Asia. Mater. 7, e180(1)-(11) (2015).
Horike, S., Umeyama, D., Inukai, M., Itakura, T. & Kitagawa, S. Coordination-network-based ionic plastic crystal for anhydrous proton conductivity. J. Am. Chem. Soc. 134, 7612–7615 (2012).
Oh, M. & Mirkin, C. A. Chemically tailorable colloidal particles from infinite coordination polymers. Nature. 438, 651–654 (2005).
Desiraju, G. R. Cryptic crystallography. Nat. Mater. 1, 77–79 (2002).
Zaworotko, M. J. Crystal engineering comes of age. Nat. Chem. 3, 653–653 (2011).
Miyakawa, T. & Dexter, D. Phonon sidebands, multiphonon relaxation of excited states and phonon-assisted energy transfer between ions in solids. Phys. Rev. B 1, 2961–2969 (1970).
Takasaki, H., Tanabe, S. & Hanada, T. Long-lasting afterglow characteristics of Eu, Dy codoped SrO-Al2O3 phosphor. J. Ceram. Soc. Jpn. 104, 322–326 (1996).
Zhou, S. F., Jiang, N., Wu, B. T., Hao, J. H. & Qiu, J. R. Ligand-driven wavelength-tunable and ultra-broadband infrared luminescence in single-ion-doped transparent hybrid materials. Adv. Funct. Mater. 19, 2081–2088 (2009).
Yan, S. et al. Zinc gallogermanate solid solution: A novel photocatalyst for efficiently converting CO2 into solar fuels. Adv. Funct. Mater. 23, 1839–1845 (2013).
Kobayashi, G. et al. Isolation of solid solution ohases in size-controlled LixFePO4 at room temperature. Adv. Funct. Mater. 19, 395–403 (2009).
Tsuji, I., Kato, H., Kobayashi, H. & Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1-x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 126, 13406–13413 (2004).
Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J. M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005).
Bessière, A. et al. Storage of visible light for long-lasting phosphorescence in chromium-doped zinc gallate. Chem. Mater. 26, 1365–1373 (2014).
Yraola, E., Molina, P., Plaza, J. L., Ramírez, M. O. & Bausá, L. E. Spontaneous emission and nonlinear response enhancement by silver nanoparticles in a Nd3+-doped periodically poled LiNbO3 laser crystal. Adv. Mater. 25, 910–915 (2013).
Li, Y. et al. A strategy for developing near infrared long-persistent phosphors: taking MAlO3: Mn4+, Ge4+ (M=La, Gd) as an example. J. Mater. Chem. C 2, 2019–2027 (2014).
Li, Y. et al. Long persistent and photo-stimulated luminescence in Cr3+-doped Zn–Ga–Sn–O phosphors for deep and reproducible tissue imaging. J. Mater. Chem. C 2, 2657–2663 (2014).
Ren, J. J. & Eckert, H. A Homonuclear Rotational Echo Double-Resonance Method for Measuring Site-Resolved Distance Distributions in I=1/2 Spin Pairs, Clusters and Multispin Systems. Angew. Chem. Int. Edit. 51, 12888–12891 (2012).
Barry, B. M. & Gillan, E. G. Low-temperature solvothermal synthesis of phosphorus-rich transition-metal phosphides. Chem. Mater. 20, 2618–2620 (2008).
Allix, M. et al. Considerable improvement of long-persistent luminescence in germanium and tin substituted ZnGa2O4 . Chem. Mater. 25, 1600–1606 (2013).
Trojan-Piegza, J., Niittykoski, J., Holsa, J. & Zych, E. Thermoluminescence and kinetics of persistent luminescence of vacuum-sintered Tb3+-doped and Tb3+, Ca2+-codoped Lu2O3 materials. Chem. Mater. 20, 2252–2261 (2008).
Dehnicke, K. & Greiner, A. Unusual complex chemistry of rare-earth elements: Large ionic radii-small coordination numbers. Angew. Chem. Int. Edit. 42, 1340–1354 (2003).
RepSaines, P. J., Elcombe, M. M. & Kennedy, B. J. Lanthanide distribution in some doped alkaline earth aluminates and gallates. J. Solid. State. Chem. 179, 613–622 (2006).
Zhuang, Y., Ueda, J. & Tanabe, S. Tunable trap depth in Zn(Ga1−xAlx)2O4: Cr, Bi red persistent phosphors: considerations of high-temperature persistent luminescence and photostimulated persistent luminescence. J. Mater. Chem. C 1, 7849–7855 (2013).
Li, Y. et al. Anti-stokes fluorescent probe with incoherent excitation. Sci. Rep. 4, 1–6 (2014).
Krumpel, A. H., Bos, A. J. J., Bessière, A., van der Kolk, E. & Dorenbos, P. Controlled electron and hole trapping in YPO4:Ce3+, Ln3+ and LuPO4:Ce3+, Ln3+ (Ln=Sm, Dy, Ho, Er, Tm). Phys. Rev. B 80, 085103 (1)-085103(10) (2009).
Van, den. Eeckhout., K., Poelmanm, D. & Smet, P. Persistent luminescence in Non-Eu2+-doped compounds. A Review. Materials. 6, 2789–2818 (2013).
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant Nos 51132004, 51072754, 51472091, 61475174), Guangdong Natural Science Foundation (Grant Nos S2011030001349, 2014A030310444), National Basic Research Program of China (Grant Nos 2011CB808100), China Postdoctoral Science Foundation (Grant Nos 2015M570707), 100 Talents Program of Chinese Academy of Sciences and Fundamental Research Funds for the Central Universities (Grant Nos 2013ZM0001, 2015ZM089). This work was also supported by the Open Fund of the State Key Laboratory of High Field Laser Physics (Shanghai Institute of Optics and Fine Mechanics.).
Author information
Authors and Affiliations
Contributions
J.R.Q. conceived and designed the experiments and was responsible for the project planning. X.X.Q. prepared the samples. X.X.Q., Y.L. and Y.L.W. investigated the spectroscopic properties. J.J.R. and R.L.Z. carried out the NMR study. Y.L. wrote the manuscript. J.R.Q., M.G., S.F.Z., Z.J.M., K.F. and G.P.D. checked the manuscript. All authors were involved in the discussion of the experimental results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Qin, X., Li, Y., Zhang, R. et al. Hybrid coordination-network-engineering for bridging cascaded channels to activate long persistent phosphorescence in the second biological window. Sci Rep 6, 20275 (2016). https://doi.org/10.1038/srep20275
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20275
This article is cited by
-
Afterglow, TL and OSL properties of Mn2+-doped ZnGa2O4 phosphor
Scientific Reports (2019)
-
A co-doping influence towards enhanced persistent duration of long persistent phosphors
Journal of Materials Science: Materials in Electronics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.