Abstract
Retinitis pigmentosa (RP) is a rare heterogeneous genetic retinal dystrophy disease and despite years of research, known genetic mutations can explain only approximately 60% of RP cases. We sought to identify the underlying genetic mutations in a cohort of fourteen Indian autosomal recessive retinitis pigmentosa (arRP) families and 100 Indian sporadic RP cases. Whole-exome sequencing (WES) was performed on the probands of the arRP families and sporadic RP patients and direct Sanger sequencing was used to confirm the causal mutations identified by WES. We found that the mutations of EYS are likely pathogenic mutations in two arRP families and eight sporadic patients. Specifically, we found a novel pair of compound heterozygous mutations and a novel homozygous mutation in two separate arRP families and found two novel heterozygous mutations in two sporadic RP patients, whereas we found six novel homozygous mutations in six sporadic RP patients. Of these, one was a frameshift mutation, two were stop-gain mutations, one was a splicing mutation and the others were missense mutations. In conclusion, our findings expand the spectrum of EYS mutations in RP in the Indian population and provide further support for the role of EYS in the pathogenesis and clinical diagnosis of RP.
Similar content being viewed by others
Introduction
Retinitis pigmentosa (RP; OMIM 226800) is a highly heterogeneous genetic disease characterized by progressive visual loss caused by the impairment of retinal photoreceptors1. The worldwide prevalence of RP is approximately one in 3,500–5,0002 and it can be inherited as an autosomal recessive (50–60%), an autosomal dominant (30–40%) or an X-linked trait (5%–15%). Thus far, more than 70 genes and loci have been identified for RP (https://sph.uth.edu/RetNet/). However, these genes account for only approximately 60% of RP cases3. Therefore, unknown RP genes remain to be identified and novel RP genes would provide valuable information for the diagnosis, prevention and treatment of RP.
The EYS gene (OMIM 612424, NM_001142800) corresponds to the RP25 locus and was identified as the gene causing autosomal recessive retinitis pigmentosa (arRP) in 2008 and it is mainly expressed in the retina4. The human EYS gene encodes a homologue of the Drosophila eye spacemaker (SPAM) protein and is essential for the development and morphology of photoreceptors5. Thus far, 15 mutations have been reported in EYS for RP patients and the types of mutations include missense mutations, nonsense mutations, insertions, deletions and splice site mutations2,6,7,8,9,10.
However, there has been limited success when using traditional approaches to screen potential genes for RP because many techniques for positional cloning and gene identification are relatively time consuming, expensive and inefficient. Recently, whole-exome sequencing (WES) by next-generation sequencing (NGS) has become an efficient method for identifying genetic variants at the whole-genome level. In several studies, NGS has provided a promising alternative approach for the molecular diagnosis and genetic identification of RP11,12. Because of relatively high levels of consanguinity and large numbers of offspring per family, hereditary-disease gene analysis is highly effective in the Indian population. In this study, which is part of an international collaborative project, we used WES to identify disease-causing genes for RP in the Indian Population and the results indicated novel mutations in the EYS gene in two consanguineous Indian families and eight sporadic Indian RP patients. Our findings expand the mutation spectrum of EYS within the Indian population and demonstrate that WES by NGS is a powerful tool for the genetic diagnosis of RP.
Methods
Patient recruitment and ethics statement
All study protocols were approved by the Ethics Review Board of Aravind Medical Research Foundation of India and the Hospital of the University of Electronic Science and Technology of China and the Sichuan Provincial People’s Hospital and all experiments were performed in accordance with the approved protocols. Fourteen families with arRP, 100 patients with sporadic RP and 1000 control individuals with no history of retinal diseases were recruited in this study. Samples were collected from the Aravind Eye Hospital of India. Written informed consent was obtained from all individuals who participated in this study or from their legal guardians in the case of minors. Venous blood samples were obtained from all subjects in EDTA vacutainers.
DNA extraction
Total genomic DNA was isolated with DNA extraction kits according to the manufacturer’s instructions (TianGen, Beijing, China) and stored at −20 °C for later use. The integrity of the DNA was assessed through 1% agarose gel electrophoresis.
Whole-exome sequencing and data analysis
DNA samples from the probands of arRP families and 100 sporadic RP patients were subjected to WES at Axeq Technology Inc., Seoul, Korea. In brief, the workflow of WES was as follows. First, the genomic DNA samples were fragmented into 150–200 bp fragments and then ligated to paired-end adaptors. Exome enrichment was performed according to the manufacturer’s protocol with the Agilent SureSelect Human All Exon 50 MB kit V5 (Santa Clara, Californian, U.S.A.), which covers 20,965 genes and 334,378 exons in the Consensus Coding Sequence Region database. The captured libraries were subjected to a quality assessment with an Axeq 2100 Bioanalyzer and sequenced on an Illumina HiSeq 2000 sequencer. The raw image files were processed by Illumina base calling software v.1.7 for base calling with default parameters and the sequences of each individual were generated as 90-bp paired-end reads. High-quality sequencing reads of each sample were aligned to the reference human genome hg19 UCSC assembly (http://genome.ucsc.edu/) using the Burrows-Wheeler Aligner (BWA) program v.0.5.9-r16 (http://bio-bwa.sourceforge.net/)13. The SNPs and indels were detected by SAMTOOLS v.0.1.19 (http://samtools.sourceforge.net/) using the ‘mpileup’ command. The variants that had depths less than 10 and those located outside of the exome-capture regions were filtered out. For all of the samples, we used individual base-calling algorithms.
SnpEff v4.1 software was used for variants annotation (http://sourceforge.net/projects/snpeff/files/databases/v4_1/).
Gene filtration and annotation
The detected variants were filtered and annotated based on four databases: NCBI CCDS (http://www.ncbi.nlm.nih.gov/CCDS/ccdsBrowse.cgi), RefSeq (http://www.ncbi.nlm.nih.gov/RefSeq/), Ensembl (http://www.ensembl.org) and Encode (http://genome.ucsc.edu/ENCODE). Exclusion steps were performed to identify the candidate variants. Variants within intergenic, intronic and UTR regions and synonymous mutations were excluded and variants in dbSNP138 (http://www.ncbi.nlm.nih.gov/projects/SNP/), the 1000 Genome project (ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp), the YH database (http://yh.genomics.org.cn/), the HapMap Project (ftp://ftp.ncbi.nlm.nih.gov/hapmap) and our in-house database generated from 1600 samples sequenced by WES were also excluded. Moreover, potentially damaging effects of the variants on protein structure/function were predicted by SIFT (http://sift.bii.astar.edu.sg/) and Ployphen2 (http://genetics.bwh.havard.edu/pph2).
Sanger sequencing
Sanger sequencing was performed to confirm the variants identified by WES. Primers flanking the candidate loci were designed using Primer 5.0 and synthesized by Invitrogen, Shanghai, China. The primers used for Sanger sequencing are shown in supplementary Table S1. PCR amplification was performed and the products were purified using the PCR purification kit from Qiagen following the manufacturer’s instructions. The purified PCR products were then sequenced on an ABI3730 genetic analyser. The sequencing results were compared with the EYS gene reference sequence (NC_000006.12) to confirm the candidate nucleotide variants.
Results
Clinical features of Indian families with arRP and sporadic RP
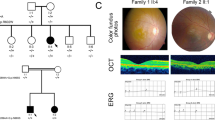
The detailed clinical data for two arRP families and eight sporadic RP patients are shown in Table 1. Ophthalmic examinations identified two affected members in family ARRP-206 and three affected members in ARRP-49. The sporadic patients and patients in the arRP families exhibited similar clinical features of RP, such as night blindness and low visual acuity. Representative fundus photographs and optical coherence tomography (OCT) are shown in Fig. 1 (ARRP-49), Fig. 2 (SP-14) and Fig. 3 (SP-48), which indicates an obvious waxen appearance of the discs, bone-spicule pigmentation in the midperiphery, attenuation of the retinal arteries (Fig. 1A) and atrophy of the foveal and retinal layer (Fig. 1B). The patients’ parents and other unaffected family members did not show any RP features. ARRP-49 and ARRP-206 showed an autosomal recessive inherited pattern (Figs 4A and 5A).
Fundus photographs and OCT pictures of the proband in family ARRP-49.
The fundus photographs (A) show typical changes (waxy-pale disc, arteriolar attenuation and bone-spicule pigment) of fundus in the left eye (OS LE) and right eye (OD RE); the OCT pictures of the left and right eyes (B) show atrophy of the foveal and retinal layers (macular thickness: macular cube 512 × 128).
Pedigree of the family ARRP-49 and mutations identified by Sanger sequencing analysis.
A shows the segregation of compound heterozygous changes c.8422G>A (MU1) and c.7868G>A (MU2). Genotypes are presented as follows: MU1/MU2 represents individuals with both mutations as compound heterozygous; MU1/+ and +/MU2 indicate heterozygous carriers; +/+ indicates individuals carrying two wild-type alleles. NA denotes DNA samples that were unavailable.
Pedigree of the family ARRP-206 and mutations identified by Sanger sequencing.
A shows the segregation of a homozygous mutation c.1871G>A (MU3). The proband is indicated by an arrow. MU3/MU3 represents a homozygous mutant, whereas MU3/+ indicates a heterozygous carrier. Black symbols indicate affected individuals; white symbols indicate unaffected individuals. NA denotes DNA samples that were unavailable.
Mutation analysis using whole-exome and Sanger sequencing
WES was conducted on the probands of 14 arRP families and 100 sporadic RP patients. The mean read depth of the target regions of each sample ranged from 52-66X and the average throughput depth of the target region in each sample ranged form 84-104.9X (See supplementary table S2 for detailed information.) To identify the underlying genetic mutations, we focused on the functional SNPs/indels with a homozygous or compound heterozygous status, including nonsynonymous variants, splice acceptor and donor site mutations and frameshift insertions or deletions. These variants were compared with 5 SNP databases (dbSNP138, the 1000 Genomes Project, the HapMap Project, the YH database and the in-house database). Finally, three compound heterozygous mutations and seven homozygous mutations in the EYS gene were identified in two arRP families and eight sporadic RP patients. The detailed WES results for these patients are illustrated in supplementary table S2 and NGS results for the SNP quality and depth of these mutations are listed in Table S3. In family ARRP-49, we used the recessive compound heterozygous inheritance model and found one pair of compound heterozygous mutations (c.8422G>A (p.A2808T) and c.7868G>A (p.G2623E)) (Table 2) in the EYS gene (NM_001142800). In family ARRP-206, we found a homozygous single-nucleotide mutation (c.1871G>A (p.S624L)) (Table 2) in ARRP-206. To confirm the accuracy of the mutations identified by exome sequencing, PCR-based Sanger sequencing was performed to validate these mutations in the other family members and the results demonstrated that the parents were unaffected carriers of c.7868G>A (p.G2623E, father) and c.8422G>A (p.A2808T, mother) in family ARRP-49 and c.1871G>A (p.S624L, father) in family ARRP-206. The inheritance pattern showed complete co-segregation of the mutations with the disease phenotype (Figs 2 and 3). The detailed genotype results of the family members in ARRP-49 and ARRP-206 are illustrated in supplementary Table S4.
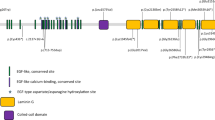
In 100 sporadic RP patients, we found two compound heterozygous mutations in two patients, RP:S-10 and RP:S-18 (Table 2, Fig. 6A,B) and six novel likely pathogenic mutations in six patients (Table 2, Fig. 7). These mutations included two stop-gain mutations, one splicing mutation, six missense mutations and one frameshift deletion (Table 2). These mutations either affect predicted functional regions of EYS, such as the Laminin G (LamG), EGF, EGF-like calcium-binding (EGF-CA) and EGF-like domains (Fig. 6A), or affect evolutionarily conserved amino acid residues (Fig. 6B).
Additional disease-causing mutations of other known RP genes or previously identified mutations of EYS were not found in any of these patients. According to the Exome Aggregation Consortium database (http://exac.broadinstitute.org/) and our 1000 control results, homozygous mutations or compound heterozygous mutations were not detected in the normal control population.
Mutation prediction analysis of the EYS gene
SIFT, PROVEAN14 and SMART were used to predict the effect of the identified amino acid substitutions on the EYS protein function. Most mutations are located at the protein functional domain LamG or EGF/EGF-like/EGF-CA, which may affect the function of the protein (Table 2, Fig. 8A).
Discussion
EYS spans 2.0 Mb of genomic DNA and encodes 3,165 amino acids and it is considered one of the largest genes expressed in the human eye. EYS is a multi-domain protein that starts with a signal peptide of 21 amino acids. It contains 28 epidermal growth factor (EGF) or EGF-like domains at the N-terminus and five LamG domains at the C-terminus and is highly expressed in retinal photoreceptors9. Previous studies have shown that a homologous protein of EYS in Drosophila named Spacemaker (spam) is involved in luminal space formation and plays an essential role in the formation of matrix-filled interrhabdomeral space in Drosophila15,16,17. However, its biological function in the human retina remains unknown. Further investigations for EYS function in the retina are essential for deep understanding of the pathological mechanism of retinal degeneration.
EYS is a major causative gene for RP. Thus far, the mutations identified in EYS include p.D904Qfs*17, p.S754Afs*6, p.T657Afs*5, p.W2640*, p.E1836* and others5,9,18. Most of these reported mutations result in a truncated EYS protein, which reveals that the C-terminus of EYS is essential for its function in the retina. In this study, we found a pair of compound heterozygous mutations, c.8422G>A (p.A2808T) and c.7868G>A (p.G2623E), in EYS in family ARRP-49. Both mutations affect a conserved amino acid residue. The mutation p.A2808T in exon 43 is a single nucleotide polymorphism (rs111991705) with an allele frequency of 0.009826. This mutation results in the replacement of a small hydrophobic amino acid (alanine) by a polar residue (threonine). Previously, Audo, I., et al. reported the same heterozygous mutation c.8422G>T in a French RP patient18. This French RP patient had another heterozygous mutation c.3329C>G18. The novel mutation p.G2623E in exon 40 identified in our study lies in a region of disulphide bond modification (http://www.uniprot.org/uniprot/Q5T1H1), which may affect the formation of disulphide bonds, thereby impairing protein function. These two mutations together may account for the incidence of RP in family ARRP-49. The novel homozygous mutation c.1871G>A (p.S624L) in EYS is likely the causative mutation for family ARRP-206. This missense mutation leads to the replacement of a small-sized polar residue, serine, by a hydrophobic lysine. However, this mutation is not highly evolutionarily conserved and further studies are warranted to ascertain the impact of this mutation on EYS functions.
The majority of RP cases show the sporadic form19,20 and the inheritance pattern of this form is difficult to ascertain. In our study, we found two compound heterozygous mutations in the sporadic RP patients RP:S-10 and RP:S-18. In patient RP:S-10, the compound heterozygous mutations c.C4060G (p.Q1536E) and c.A5038G (p.N1680D) were found and these mutations are located in exon 26 and the unknown region of the protein, respectively. In patient RP:S-18, the compound heterozygous mutations c.G1418T (p.G473V) and c.C2971T (p.L991F) were found and these mutations are located in the unknown region and the EGF domain of the protein, respectively. However, there were several limitations in this study. Because the blood samples of the intra-family members of the sporadic RP patients were difficult to collect, we could not assess the genotypes of these intra-family members, which included their parents, siblings or offspring. Therefore, we could not be certain whether these two variants originate from distinct alleles or the same allele in these two sporadic patients.
In sporadic patients RP:S-22 and RP:S-48, we found two novel homozygous stop-gain mutations, c.8388C>A (p.Y2796X) and c.3024C>A (p.C1008X), respectively and these mutations resulted in the generation of truncated proteins in the fourth LamG domain at the C-terminal and the fifth EGF-CA domain at the N-terminal, respectively. The truncated proteins likely impair the function of EYS. These nonsense mutations could cause the degradation of EYS mRNA via nonsense-mediated mRNA decay (NMD)21. We also found a novel frameshift deletion mutation in exon 43 and a splicing-site mutation in exon 15 in patients RP:S-2 and RP:S-40. Both mutations led to abnormal proteins. In sporadic patients RP:S-14 and RP:S-34, we found missense mutations in the LamG and EGF domains, respectively and the SIFT/PROVEAN predictions indicate that these mutations may be harmful to the protein. Iwanami et al., demonstrated that mutation types were related to the severity of the RP symptoms22. In our study, the correlation between genotype and phenotype of patients is not readily obvious due to the small number of subjects. More studies about the correlation between genotype and phenotype of RP would give the better understanding of the diagnosis and prediction of the incidence of RP.
In summary, we identified three novel compound heterozygous mutations in EYS and seven novel homozygous mutations for RP in the Indian population. Our study not only expands the spectrum of EYS mutations for arRP in the Indian population but also shows that WES can be an effective tool for identifying causative mutations in RP patients and diagnosing genetic diseases.
Additional Information
How to cite this article: Di, Y. et al. Whole-exome Sequencing Analysis Identifies Mutations in the EYS Gene in Retinitis Pigmentosa in the Indian Population. Sci. Rep. 6, 19432; doi: 10.1038/srep19432 (2016).
References
Hartong, D. T., Berson E. L. & Dryja T. P. Retinitis pigmentosa. Lancet 368, 1795–809 (2006).
Chen, X. et al. Targeted next-generation sequencing reveals novel EYS mutations in Chinese families with autosomal recessive retinitis pigmentosa. Sci. Rep. 5, 8927 (2015).
Hamel, C., Retinitis pigmentosa. Orphanet J Rare Dis 1, 40 (2006).
Collin, R. W. et al. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am. J. Hum. Genet. 83, 594–603 (2008).
Abd, E. M. et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat. Genet. 40, 1285–7 (2008).
Barragan, I. et al. Mutation spectrum of EYS in Spanish patients with autosomal recessive retinitis pigmentosa. Hum. Mutat. 31, E1772–800 (2010).
Bandah-Rozenfeld, D. et al. Novel null mutations in the EYS gene are a frequent cause of autosomal recessive retinitis pigmentosa in the Israeli population. Invest. Ophthalmol. Vis. Sci. 51, 4387–94 (2010).
Hosono, K. et al. Two novel mutations in the EYS gene are possible major causes of autosomal recessive retinitis pigmentosa in the Japanese population. PLoS One 7, e31036 (2012).
Huang, Y. et al. Identification of a novel homozygous nonsense mutation in EYS in a Chinese family with autosomal recessive retinitis pigmentosa. BMC Med. Genet. 11, 121 (2010).
Abd, E. M. et al. Identification of novel mutations in the ortholog of Drosophila eyes shut gene (EYS) causing autosomal recessive retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 51, 4266–72 (2010).
Avila-Fernandez, A. et al. Whole-exome sequencing reveals ZNF408 as a new gene associated with autosomal recessive retinitis pigmentosa with vitreal alterations. Hum. Mol. Genet. 24, 4037–48 (2015).
Katagiri, S. et al. Whole exome analysis identifies frequent CNGA1 mutations in Japanese population with autosomal recessive retinitis pigmentosa. PLoS One 9, e108721 (2014).
Li, H. & Durbin, Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–60 (2009).
Choi, Y. et al. Predicting the functional effect of amino acid substitutions and indels. PLoS One 7, e46688 (2012).
Zelhof, A. C. et al. Transforming the architecture of compound eyes. Nature 443, 696–9 (2006).
Husain, N. et al. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev. Cell 11, 483–93 (2006).
Gurudev, N., Yuan M. & Knust E., chaoptin, prominin, eyes shut and crumbs form a genetic network controlling the apical compartment of Drosophila photoreceptor cells. Biol. Open 3: 332–41 (2014).
Audo, I. et al. EYS is a major gene for rod-cone dystrophies in France. Hum. Mutat. 31: e1406–35 (2010).
Jay, M., Figures and fantasies: the frequencies of the different genetic forms of retinitis pigmentosa. Birth Defects Orig. Artic Ser 18: 167–73 (1982).
Kaplan, J. et al. Clinical and genetic heterogeneity in retinitis pigmentosa. Hum Genet 85: 635–42 (1990).
Schweingruber, C. et al. Nonsense-mediated mRNA decay - mechanisms of substrate mRNA recognition and degradation in mammalian cells. Biochim Biophys Acta 1829, 612–23 (2013).
Iwanami, M. et al. High prevalence of mutations in the EYS gene in Japanese patients with autosomal recessive retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 53, 1033–40 (2012).
Acknowledgements
The authors thank all of the patients and family members for their participation. This study was supported by grants from the National Natural Science Foundation of China (81170883 and 81430008 to Z.Y., 81271007 and 81470668 to X. Zhu 81100693 and 81570848 to Q.C., 81300802 to L.H. 8140043 to Y.Z., 81271049 and 81470667 to D. Zhang), the Department of Science and Technology of Sichuan Province (2012SZ0219, 2014SZ0169, 2015SZ0052 to Z.Y., 2015SZ0060 to Y.L., 2014JQ0023, 2014FZ0122, 16CXTD0066 to X.Zhu 2015JZ0004 to C.Q.) and the Department of Health of Sichuan Province (130145 to X.Zhu).
Author information
Authors and Affiliations
Contributions
Y.D., L.H., P.S., R.K., B.B., S.L., C.Q., X.Z., Y.Z., Z.J. and L.Z. conducted the experiments. Y.D., L.H., Y.L., D.Z., Y.L., H.Z. and Y.Y. analysed the data. Y.D., F.L., X.Z. and Z.L.Y. wrote the manuscript. X.Z. and Z.L.Y. designed and oversaw the overall experiment. All of the authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Di, Y., Huang, L., Sundaresan, P. et al. Whole-exome Sequencing Analysis Identifies Mutations in the EYS Gene in Retinitis Pigmentosa in the Indian Population. Sci Rep 6, 19432 (2016). https://doi.org/10.1038/srep19432
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19432
This article is cited by
-
The medaka mutant deficient in eyes shut homolog exhibits opsin transport defects and enhanced autophagy in retinal photoreceptors
Cell and Tissue Research (2023)
-
Dissecting the role of EYS in retinal degeneration: clinical and molecular aspects and its implications for future therapy
Orphanet Journal of Rare Diseases (2021)
-
“Genetic and clinical findings in an ethnically diverse retinitis pigmentosa cohort associated with pathogenic variants in EYS”
Eye (2021)
-
Whole genome sequencing and in vitro splice assays reveal genetic causes for inherited retinal diseases
npj Genomic Medicine (2021)
-
Ablation of EYS in zebrafish causes mislocalisation of outer segment proteins, F-actin disruption and cone-rod dystrophy
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.