Abstract
Inflammasomes are multiprotein complexes that include members of the NOD-like receptor family and caspase-1. Caspase-1 is required for the fusion of the Legionella vacuole with lysosomes. Caspase-11, independently of the inflammasome, also promotes phagolysosomal fusion. However, it is unclear how these proteases alter intracellular trafficking. Here, we show that caspase-11 and caspase-1 function in opposing manners to phosphorylate and dephosphorylate cofilin, respectively upon infection with Legionella. Caspase-11 targets cofilin via the RhoA GTPase, whereas caspase-1 engages the Slingshot phosphatase. The absence of either caspase-11 or caspase-1 maintains actin in the polymerized or depolymerized form, respectively and averts the fusion of pathogen-containing vacuoles with lysosomes. Therefore, caspase-11 and caspase-1 converge on the actin machinery with opposing effects to promote vesicular trafficking.
Similar content being viewed by others
Introduction
Actin filaments play a role in cell cycle, cellular motility and proper vesicle transport and are critical for providing structure and subcellular organization1. In addition, trafficking and fusion of the phagosome with the lysosome is a key host defense mechanism modulated by the actin machinery2. In order for fusion to occur, actin nucleates and polymerizes around the membranes of phagosomes providing a directional track to interact and fuse with lysosomes3. The dynamic process of actin polymerization and depolymerization is regulated by the phosphorylation status of cofilin4. Cofilin activity is inhibited by phosphorylation of the serine residue at position 3, which is mediated by the Rho small GTPase family: RhoA, Rac, or Cdc42. Activation of RhoA, Rac and Cdc42 in hematopoietic cells leads to the polymerization of actin into distinct structures. On the other hand, Slingshot family of protein phosphatases dephosphorylates and reactivates cofilin5,6. These modifications cycle cofilin from active to inactive states4. Whether these GTPase and phosphatases play a role during intracellular infections is unknown. In addition, it is also unclear if their activation is altered by the caspase-1 and caspase-11.
Legionella pneumophila (Legionella) is a facultative, intracellular pathogen that causes Legionnaire’s disease (LD). The majority of people exposed to Legionella remain asymptomatic or suffer only mild self-limiting infection. Cigarette smoking, chronic lung disease and immunosuppression have been consistently implicated as risk factors7,8. Legionella reaches the alveoli where it encounters and multiplies in human alveolar macrophages at the site of infection, which is crucial to the pathogenesis of LD9,10,11. Notably, human monocytes do not activate caspase-1 and -7 upon Legionella infection, allowing bacterial growth12,13,14,15. Primary human macrophages and the THP-1 monocytic cell line also do not activate caspase-1 in response to Legionella12,13,14.
In contrast, macrophages from C57BL/6 mice (designated wild-type (WT) in the text) restrict Legionella replication via activation of caspase-1, caspase-7 and caspase-11 resulting in fusion of the containing phagosome with the lysosome leading to bacterial degradation and growth restriction14. In WT mouse macrophages, bacterial flagellin leaks through the type-IV secretion system (T4SS) and is detected within the host cytosol by the NOD-like receptors (NLR) Nlrc4 and Naip5, leading to the activation of caspase-116,17,18,19,20,21. Independently of the inflammasome complex, caspase-11 is induced and activated upon Legionella infection contributing to bacterial clearance by promoting phagosome-lysosome fusion14. Accordingly, mouse macrophages lacking any of these factors (e.g. Nlrc4−/−, Naip5−/−, Casp1−/−, Casp-7−/−, or Casp11−/−) are permissive to Legionella infection17,22,23,24. However, the signaling pathway linking caspase-1 and caspase-11 to vesicular trafficking remains obscure.
Here we demonstrate that cofilin loses its basal phosphorylation status early upon Legionella infection in WT macrophages. Dephosphorylation of cofilin is accompanied by an increase in the filamentous actin (F-actin) and globular actin (G-actin) ratio and promotion of fusion of the Legionella-containing vacuole with the lysosome. We found that caspase-11 is required for the phosphorylation of RhoA and cofilin, whereas caspase-1 promotes the dephosphorylation of cofilin via Slingshot. In WT macrophages, where both caspases are active, the F/G ratio dynamically changes during Legionella infection, as it increases within 2 hours of infection and the pathogen-containing vacuole fuses with the lysosome. When caspase-11 or caspase-1 is lacking, such as in their corresponding single knock outs, cofilin remains phosphorylated or dephosphorylated, respectively. The F/G-actin ratio remains unchanged and the fusion of the pathogen-containing vacuole with the lysosome is halted and Legionella replicates. Notably, the enzymatic activity of caspase-11 is required for its ability to modulate actin polymerization. Interestingly, the absence of either caspase-11 or caspase-1 does not influence the trafficking of E. coli-containing vacuoles. Our results identify new key players downstream of caspase-11 and caspase-1 that modulate the trafficking of phagosomes harboring pathogenic intracellular microbes.
Results
The Legionella-containing vacuole fails to fuse with the lysosome in macrophages lacking caspase-1
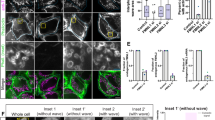
It has been previously shown that the Legionella-containing vacuole (LCV) fails to fuse with the lysosome in macrophages believed to only lack caspase-117,22. However, the caspase-1−/− macrophages used in previous publications also lacked caspase-1125,26,27,28,29. Thus, it is possible that caspase-11 alone is sufficient to promote phagosome-lysosome fusion, irrespective of caspase-1 expression. To test this hypothesis, we examined the role of caspase-1 using the single caspase-1 knock out (Casp-1−/−Casp-11Tg) during Legionella infection (a generous gift from Dr. Vishva Dixit, Genetech)30. We focused on mimicking early stages of physiological infection and we used low multiplicity of infection (MOI). First, we evaluated the trafficking of Legionella in Casp-1−/−Casp-11Tg bone marrow-derived macrophages (BMDMs). Fusion of the LCV with the lysosome was assessed by quantifying the number of GFP-expressing bacteria colocalized with LysoTracker™ red, a dye that stains acidic vacuoles. In WT macrophages, intracellular bacteria readily trafficked to lysosomes (~38%), while in Casp-1−/−Casp-11Tg cells, significantly less bacteria (~20%) did so (Fig. 1a,b). Conversely, Escherichia coli (E. coli) trafficked to the lysosome regardless of caspase-1 expression (~90%) (Fig. 1c,d).
Caspase-1 promotes fusion of the Legionella vacuole with lysosome.
Wild-type (WT) and Casp-1−/−Casp-11Tg macrophages were infected with (a) GFP-expressing Legionella (L.p.) or (c) Escherichia (E.) coli. Macrophages were stained with LysoTracker® red that accumulates in acidic compartments. Quantification of colocalization of LysoTracker® with (b) GFP-L.p. and (d) GFP-E.coli. Mean percent colocalization ± SEM from three biological replicates are represented. (b) significance, indicated by (*), was obtained by performing Student’s unpaired t-test. p = 0.0002 is ***, p = 0.01 is ** and p = 0.0113 is *.
To determine the independent role of caspase-1 in the pathogenesis of Legionnaire’s disease, replication of Legionella in macrophages (in vitro) and lungs (in vivo) of Casp-1−/−Casp-11Tg mice was assessed via colony forming units (CFUs). During in vitro infection, Casp-1−/−Casp-11Tg macrophages exhibited significantly more bacterial replication at 24, 48 and 72 hrs after infection (Fig. 2a). In vivo, bacterial loads in the lungs of the Casp-1−/−Casp-11Tg mice after 4 hrs of infection were similar, indicating equivalent initial infection doses. After 48 hrs of infection, significantly more Legionella CFUs were recovered from lungs of Casp-1−/−Casp-11Tg mice compared to that of WT counterparts (Fig. 2b). These results indicate that caspase-1 contributes to the efficient fusion of Legionella-containing vacuoles with the lysosome and restriction of the bacteria in vitro and in vivo.
Caspase-1 promotes the restriction of Legionella in vitro and in vivo.
Colony forming unit (CFU) assay of Legionella replication in WT and Casp-1−/−Casp-11Tg (a) in vitro after 1, 24, 48, 72 hrs post infection and (b) in vivo from lungs of WT and Casp-1−/−Casp-11Tg mice at 4 and 48 hrs after infection. (c) Live/Dead® immunofluorescence assay on WT, Casp-11−/−, Casp-1−/−Casp-11Tg BMDMs infected (2 hrs) or not (NT) with Legionella. (d) Quantification of Live/Dead® stain after 1, 2 and 4 hrs after infection and mean percent dead macrophages ± SEM from three biological replicates are represented. (a) significance, indicated by (*), was obtained by performing Student’s unpaired t-test. 24 hrs p = 0.000098 is ****, 48 hr p = 0.000087 is ****, 72 hr p = 0.0017 is **, respectively. (b) 48 hrs p = 0.021 is *. (d) significance of the Live/Dead assay was calculated by one-way ANOVA with Student’s t-test post-test. *** is p < 0.001 and ** is p < 0.01.
It has also been reported that caspases promote host cell death during infection in order to restrict bacterial replication31,32,33. Previous studies using the caspase-1/11 double knockout have shown significantly reduced levels of cell death during Legionella infection without reference to which caspase is a major determinant in the host cell death process34,35. To elucidate the singular role of these caspase in cell death, we performed a Live/Dead immunofluorescence assay where dead cells allow ethidium homodimer-1 (EthD-1) to reach their nuclei and stain them red. WT, Casp-11−/− and Casp-1−/−Casp-11Tg BMDMs were infected with Legionella and then stained with EthD-1. We found that after 1, 2 and 4 hrs infection, Casp-1−/−Casp-11Tg macrophages displayed significantly less cell death when compared to WT and Casp-11−/− BMDMs (Fig. 2c,d). There was no significant difference in percentage of cell death between WT and Casp-11−/− macrophages throughout the infection time course. These data indicate that during Legionella infection, caspase-1, not caspase-11, is a major determinant in promoting cell death that also contributes to the restriction of the bacterium.
The F/G actin ratio increases in macrophages upon Legionella infection
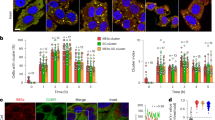
The host actin cytoskeleton dynamically changes during chemotaxis, phagocytosis and vesicle transport36. Late endosomes and phagosomes use filamentous (F) actin as a scaffold or track to move towards and efficiently fuse with other endocytic compartments and lysosomes37. To enhance our understanding of the role of caspases in vesicular trafficking during Legionella infection WT, Casp-11−/− and Casp-1−/− Casp-11Tg BMDMs were used to decipher the singular contribution of caspase-1 to actin modulation38. Macrophages were double stained with Alexa Fluor 568 phalloidin to label F-actin structures and Alexa Fluor 488 DNAse I to label unpolymerized G-actin and examined via confocal microscopy. Basally, WT macrophages expressed evenly distributed F-actin structures throughout the cell (Fig. 3a). Comparatively, Casp-11−/− and Casp-1−/−Casp-11Tg macrophages displayed F-actin that accumulates at the periphery of the cell, while there is more G-actin throughout the cell body (Fig. 3a). Upon Legionella infection of WT macrophages, F-actin structures localized in the vicinity of Legionella, whereas, in Casp-11−/− and Casp-1−/−Casp-11Tg macrophages, the distribution of F-actin remained unchanged (Fig. 3a). Notably, the distribution of F-actin during infection with non-pathogenic E. coli remained unchanged in the presence or absence of caspase-11 and caspase-1 (Fig. 3b). Since end point measurements of F-actin do not reflect actin dynamics, we assessed the changes in actin polymerization by measuring the ratio of polymerized (F) and unpolymerized (G) actin throughout 2 hours of infection. WT, Casp-11−/− and Casp-1−/−Casp-11Tg macrophages were infected with Legionella for 0.5, 1 and 2 hrs, lysed and soluble (G-actin) and insoluble (F-actin) cytoplasmic fractions were separated and analyzed by western blot. Restrictive WT macrophages exhibited dynamic reduction then increased ratios of F/G actin during Legionella infection. Comparatively, in permissive macrophages lacking caspases-11, the F/G ratio remained unchanged throughout infection (Fig. 4a,b). In the absence of caspase-1, the F/G actin ratio slightly decreased throughout the elapsed infection time (Fig. 4e,f). To determine if the modulation of the actin cytoskeleton is similar during pathogenic and non-pathogenic bacterial infection, Casp-11−/− and Casp-1−/−Casp-11Tg macrophages were infected with E. coli. Vacuoles harboring these non-pathogenic bacteria fuse with the lysosome in the absence or presence of caspase-1 and caspase-11 (Fig. 1c,d). The ratio of F/G actin was assessed throughout infection. WT, Casp-11−/− and Casp-1−/−Casp-11Tg cells exhibited steep reduction of the F/G actin ratios irrespective of caspase-1 and caspase-11 (Fig. 4c,d,g,h). Together, these data indicate that alteration of the F/G actin ratio is required for the trafficking of vacuoles harboring intracellular pathogens to lysosomes but not for those carrying non-pathogenic organisms. In addition, these results show that caspase-11 and caspase-1 are both necessary to increase the F/G actin ratio during Legionella infection but are dispensable for the trafficking of vacuoles containing non-pathogenic bacteria.
Caspase-11 and caspase-1 promote F-actin polymerization in the vicinity of the Legionella-containing vacuole.
Confocal analysis of WT, Casp-11−/− and Casp-1−/−Casp-11Tg macrophages double-labeled with Alexa Fluor 568 phalloidin (stains F-actin) and Alexa Fluor 488 DNAse I (stains G-actin) after 2 hrs infection with (a) Legionella (L.p.) or (b) Escherichia (E.) coli. F-actin structures in the vicinity of the Legionella vacuole in WT macrophages are indicated by white arrow, white tabs indicate their absence. Data are representative of three biological replicates performed independently.
F/G actin ratio is increased in WT macrophages, while Casp-11−/− and Casp-1−/−Casp-11Tg cells display less during Legionella (L.p.) infection.
G-actin/F-actin assay in WT and Casp-11−/− macrophages infected with (a) L.p. and (c) infected with E. coli for 0.5, 1 and 2 hrs. Dynamic changes in F/G actin ratios were quantified (b,d). G-actin/F-actin assay of WT and Casp-1−/−Casp-11Tg macrophages infected with (e) L.p. and (g) E. coli for 0.5, 1 and 2 hrs and changes in F/G ratios were quantified (f,h). Data are representative of three independent experiments.
Caspase-11 is required for the phosphorylation of cofilin, whereas caspase-1 promotes its dephosphorylation
Because caspase-11 and -1 are both required for the restriction of Legionella and are linked to the actin cytoskeleton during different cell processes14 (Figs 1, 2, 3, 4), we further investigated the signaling pathways that are shared or unique downstream of these caspases. Actin polymerization and depolymerization is regulated by cofilin phosphorylation status4,39. WT, Casp-11−/− and Casp-1−/−Casp-11Tg macrophages were infected with Legionella and the phosphorylation of cofilin was determined by western blot. Resting macrophages elicited different patterns of cofilin phosphorylation. While resting WT and Casp-1−/−Casp-11Tg macrophages demonstrated the phosphorylated form of cofilin, Casp-11−/− macrophages did not (Fig. 5a). Upon Legionella infection, WT macrophages lost the phosphorylation of cofilin within 1 hour infection while Casp-11−/− macrophages failed to alter the status of cofilin phosphorylation throughout infection. On the other hand, in Casp-1−/−Casp-11Tg cells, the phosphorylation of cofilin initially decreased, but then increased after 2 hrs of infection (Fig. 5a). Taken together, these data indicate that caspase-11 and caspase-1 play opposing roles in modulating cofilin phosphorylation status during infection, as caspase-11 is required for the phosphorylation of cofilin and caspase-1 is necessary for its dephosphorylation.
Caspase-11 and caspase-1 differentially regulate the phosphorylation of cofilin.
Immunoblot analysis of phospho-cofilin (P-cofilin) after infection with Legionella in (a) WT, Casp-11−/− and Casp-1−/−Casp-11Tg. (b) Nlrc4−/− and Naip5−/− macrophages. (c) P-cofilin immunoblots of WT cells infected with parental Legionella (L.p.), the T4SS (dotA−) mutant and flagellin (flaA−) mutant for 1, 2 and 4 hrs. (d) Lysates from WT macrophages infected with live and heat-killed Legionella analyzed for P-cofilin at 1 and 2 hrs after infection. Blots are representative of at least three independent experiments.
Nlrc4 and Naip5 are required for cofilin dephosphorylation upon Legionella infection
The NOD-like receptors (NLRs) Nlrc4 and Naip5 form a multiprotein inflammasome complex that is able to detect intracellular bacteria, triggering canonical pyroptosis and non-pyroptotic clearance of pathogens16,17,22,40. Because the inflammatory caspases are activated downstream of Nlrc4 and Naip5 and since caspase-11 and -1 have been shown to modulate the activity of cofilin, we investigated whether Nlrc4 and/or Naip5 are able to control the phosphorylation status of cofilin in response to Legionella. WT, Nlrc4−/− and Naip5−/− macrophages were infected and 1 and 2 hrs of infection, both Nlrc4−/− and Naip5−/− cells failed to dephosphorylate cofilin when compared to WT cells (Fig. 5b). Since Nlrc4 and Naip5 detect bacterial flagellin within the host cytosol, we determined if alterations in cofilin phosphorylation are dependent on flagellin. WT macrophages were infected with a T4SS (dotA−) and a flagellin (flaA−) mutant of Legionella. After 1, 2 and 4 hrs of infection, Western blot analysis showed that the dotA− and the flaA− mutants failed to modulate cofilin phosphorylation status when compared to the parental Legionella strain (Fig. 5c). To understand whether this process is due to an active process elicited by live bacteria, we infected WT cells with live or heat-killed Legionella. Live bacteria dephosphorylated cofilin, while the heat-killed bacteria failed to do so (Fig. 5d). Together, these data indicate that live, flagellated Legionella with a competent T4SS dephosphorylates and activates cofilin in a Nlrc4/Naip5/caspase-1 axis-dependent manner.
The activation of RhoA in response to Legionella requires caspase-11
The phosphorylation of cofilin is driven by the GTPases: RhoA, Rac, and/or Cdc42 through Limk in response to different stimuli1. Therefore, we assessed GTPase activity using specific G-LISA Activation Assays (Cytoskeleton). Lysates from WT and Casp-11−/− macrophages displayed similar levels of Rac activation in response to Legionella infection and this was confirmed by Western blot using phospho-Rac/Cdc42 antibodies (Fig. 6a and data not shown). Notably, Casp-11−/− macrophages exhibited significantly lower levels of RhoA activity during Legionella infection when compared to WT cells (Fig. 6b). These data indicate that upon Legionella infection, caspase-11 is required for RhoA GTPase activation, which is then accompanied by phosphorylation of cofilin.
Caspase-11-dependent phosphorylation of cofilin requires the RhoA GTPase activity.
Rac GTPase activity (a) and RhoA GTPase activity (b) from WT and Casp-11−/− macrophage lysates after infection with Legionella using specific G-LISA activation assay (Cytoskeleton) 0.25, 0.5, 1 and 2 hrs post infection. All data are representative of at least three independent experiments. G-LISA assays analyzed for significance by Student’s unpaired t-test. *** is p < 0.001, ** is p < 0.01 and * is p<0.05.
Caspase-1 is required for the activity of the phosphatase Slingshot during Legionella infection
Cofilin is dephosphorylated via the phosphatase Slingshot41. To determine if caspase-1 is required for the dephosphorylation of cofilin in response to Legionella infection by halting the activity of kinases or promoting the activity of phosphatases that converge on cofilin, we first assessed Rac1 and Cdc42 activation. WT and Casp-1−/−Casp-11Tg macrophages were infected with Legionella then analyzed by western blot using specific phospho-antibodies. Casp-1−/−Casp-11Tg macrophages displayed a similar trend of decreased levels of phospho-Rac1/Cdc42, compared to WT and Casp-11−/− counterparts indicating that modulation of Rac and/or Cdc42 is independent of caspase-11 and caspase-1 (Fig. 7a). Next, we assessed the activity of RhoA using a G-LISA assay specific for its active form (GTP bound). The activity of RhoA in WT and Casp-1−/−Casp-11Tg macrophages did not differ upon Legionella infection, indicating that the activity of RhoA was dependent on caspase-11 and not caspase-1 (Fig. 7b). We next determined if the activation of the phosphatase protein Slingshot is altered by the lack of caspase-1. The activation of the Slingshot protein is determined by its phosphorylation status as phosphorylation of Slingshot inhibits phosphatase activity while dephosphorylation activates Slingshot42. Using a specific antibody against the phosphorylated Ser-978, Fig. 7c shows that Slingshot is dephosphorylated during Legionella infection in WT and Casp-11−/− macrophages. Conversely, in Casp-1−/−Casp-11Tg macrophages, Slingshot remained phosphorylated (inactivated) throughout the infection (Fig. 7c). Together, this data demonstrate that the activation of Slingshot requires the presence of caspase-1 and not caspase-11.
Slingshot-mediated cofilin dephosphorylation in response to Legionella infection is dependent on caspase-1.
WT and Casp-1−/−Casp-11Tg macrophages assayed for activity of (a) Rac/Cdc42 by Western blot analysis using phospho-Rac/Cdc42 antibodies 1 and 2 hrs post infection, (b) RhoA GTPase using specific G-LISA activation 0.25, 0.5, 1 and 2 hrs after infection and (c) Slingshot by immunoblot using phospho-Slingshot specific antibody after 1 and 2 hrs infection with Legionella. Data are representative of at least three independent experiments. (b) Student’s unpaired t-test was performed to determine significance, time points exhibited no significance (NS).
The catalytic activity of caspase-11 is required for the modulation of cofilin phosphorylation status
Caspases, initially expressed as inactive zymogens, are activated by proteolytic processing in response to infection or insult stimuli43,44. After activation, caspases promote a myriad of downstream effects such as: production of inflammatory cytokines, restriction of intracellular bacteria, cell repair and survival33. To determine if the enzymatic activity of caspase-11 is required for the modulation of cofilin phosphorylation and hence actin polymerization, HEK293 cells were transduced with lentiviruses that contained either WT or a catalytically inactive (mut) caspase-11 tagged with a red fluorescent protein (RFP) (Fig. 8a). Cells stably expressing WT or mut-caspase-11 were infected with Legionella and the phosphorylation state of cofilin was assessed. HEK293 cells expressing caspase-11 or mut-caspase-11 exhibited disparate phospho-cofilin trends. Similar to WT macrophages, HEK293 cells expressing functional caspase-11 dephosphorylated cofilin upon Legionella infection. On the other hand, HEK293 cells with mut-caspase-11 failed to do so and instead the phosphorylation of cofilin was increased (Fig. 8b). Therefore, our data indicate that enzymatic activity of caspase-11 is essential for the dephosphorylation of cofilin during Legionella infection.
The dephosphorylation of cofilin is dependent on enzymatic activity of caspase-11.
Immunoblot analysis of lysates of HEK293 cells transduced with lentiviral construct (a) containing enzymatically active and inactive (mut) caspase-11 red fluorescent plasmid after 1 h infection with Legionella with phospho-cofilin (P-cofilin) antibody (b). Blots are representative of three independent experiments.
Discussion
Much of the recent work in innate immunity detailing the functions of caspases has focused on their role in cytokine production and cell death with less consideration on emerging ancillary functions44,45. Recently, studies implicating alternative functions for caspases have come to light, including: unconventional protein secretion, lipid metabolism, membrane repair, phagosome acidification and restriction of bacterial pathogens28,46,47,48. Caspases have been linked to the actin cytoskeleton in context of apoptosis5,49,50 and regulators and stabilizers of actin filaments have been found to be substrates of initiator and executioner caspases during apoptotic events51. However, whether caspases modulate the actin machinery independently of cell death is still unclear. To better understand the role of caspase-11 and caspase-1 in vesicular trafficking, we focused on physiological doses of Legionella infection and early trafficking events that mimic early infection stages. Hence, our data describe non-pyroptotic consequences of caspase activation.
The inducible caspase-11, like other caspases, executes apoptotic functions when strongly induced and activated and elicits non-apoptotic roles when moderately activated7,28,51,52,53. Reports have indicated that activation of caspase-11 leads to cell death in response to high doses of Legionella35,52,54,55. Conversely, using different bacterial strains and opsonization, Zamboni’s group nicely described how caspase-1 but not caspase-11 is required for cell death in response to Legionella infection33. The elegant work by Isberg group showed that low, physiological doses of Legionella actually upregulate anti-apoptotic genes, positively controlled by the transcriptional regulator nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) thus, extending host cell survival56. Similarly, work from Nunez group demonstrated that caspase-1 promotes the trafficking of Legionella vacuole and increases bacterial clearance irrespective of cell death17.
In our study, using physiological doses of infection, we found that the death of WT and Casp-11−/− macrophages is comparable, similar to that of Zamboni’s work33. Yet, Legionella survival in vivo and in vitro was significantly increased in Casp-11−/− mice and their derived macrophages14. Accordingly, we found that at low infection levels, in WT cells, caspase-11 promotes the fusion of the Legionella-containing vacuole (LCV) with lysosomes leading to bacterial degradation, whereas in macrophages lacking caspase-11, the trafficking of the LCV is stalled and the pathogen escapes degradation14. On the other hand, in Casp-1−/− macrophages, the LCV also escapes fusion with the lysosome while the host cell survives Fig. 1,2c,d. Therefore, according to our work and others in the field, it is clear that the intracellular survival of Legionella is not solely dictated by the death of the host cell (Table 1).
Here we report that resting WT macrophages exhibit uniform distribution of both F- and G-actin moieties, whereas Casp-11−/− and Casp-1−/−Casp-11Tg macrophages demonstrate F-actin accumulation at the cell periphery. Diverse distributions of F- and G-actin in uninfected WT, Casp-11−/− and Casp-1−/−Casp-11Tg did not affect bacterial uptake since macrophages harbored similar numbers of Legionella or E. coli at 1 hour post infection. Interestingly, caspase-11 is required for the phosphorylation of cofilin, while caspase-1 is essential for its dephosphorylation upon Legionella infection. Like Casp-1−/−Casp-11Tg macrophages, macrophages lacking Nlrc4 or Naip5 also failed to dephosphorylate cofilin, confirming the contribution of these NLRs to the actin machinery upstream of caspase-1.
Notably, WT cells displayed dynamic changes in the F/G ratio during infection. However, vacuoles containing non-pathogenic bacteria such as E. coli traffic to the lysosome independently of any changes in the F/G ratio and irrespective of caspase-1 and caspase-11. The mechanism by which caspases recognize vacuoles that contain pathogenic organisms is unknown. Various reports have indicated that caspase-11 itself is a sensor for the bacterial factor lipopolysaccharide (LPS), in models where LPS is delivered to the cytoplasm or in cases where pathogens escape the intracellular vacuole57,58,59. Notably, Legionella LPS mutants still dephosphorylate cofilin in WT cells (data not shown). Convergence of caspase-11 and caspase-1 on cofilin is dependent on bacterial flagellin, as WT macrophages infected with either a flagellin (flaA−) or T4SS, (dotA−) mutant failed to dephosphorylate cofilin. These results indicate that access of flagellin to the cytosol is essential for the activation of caspase-11 and caspase-1 to alter the activation of cofilin.
Regulation of cofilin phosphorylation is mediated by upstream kinases and phosphatases (Fig. 9). These players are differentially phosphorylated in order to be activated. The molecular GTPases; RhoA, Rac and Cdc42 promote downstream signaling to phosphorylate and activate Limk, whereas ATP, histamine, calcineurin and λ phosphatase dephosphorylate Slingshot to promote its activation5,6,41,60,61,62. Casp-11−/− exhibits defects in Rho-GTPase activation while Rho activity was similar in WT and Casp-1−/−Casp-11Tg cells. On the other hand, dephosphorylation (activation) of the Slingshot phosphatase does not occur in cells lacking caspase-1 but proceeds efficiently in WT and Casp-11−/− macrophages during Legionella infection. These data demonstrate that both caspases modulate cofilin phosphorylation in an opposing mode by differentially regulating upstream RhoA and Slingshot (Fig. 9). This intricate balance between phosphorylation and dephosphorylation of actin regulators is essential for vesicular trafficking to occur since it modulates the amount of polymerized and depolymerized actin.
Caspase-11 is required for the activation of RhoA GTPase, while caspase-1 is essential for Slingshot phosphorylation.
Accordingly, caspase-11 promotes the phosphorylation of cofilin and actin depolymerization, whereas caspase-1 promotes the dephosphorylation of cofilin and actin polymerization. The dynamics of F/G actin ratio facilitates the fusion of the Legionella vacuole with lysosomes.
Together our findings demonstrate for the first time the opposing effects of caspase-11and caspase-1 on the activation of an actin-associated factor. On the other hand, Yuan’s group has linked caspase-11 activity to cofilin modulation, via actin interacting protein 1 (Aip1), to promote lymphocyte migration63. More recently, it was found that Casp-11−/− T cells migrate less efficiently into lymphoid tissues64. Likewise, Nlrc4 has been implicated in mediating actin polymerization, promoting cell rigidity and preventing cell migration65,66. Whether this recent Nlrc4 function is mediated through caspase-1 or caspase-11 remains to be determined.
The importance of the cytoskeleton in host defense is supported by the fact that Legionella devotes several molecules secreted through its T4SS that alter the cytoskeletal network2,67,68,69,70. Purification and proteomic analysis of pathogen-containing vacuoles identified the members of the actin machinery on the vacuolar membrane71. The involvement of actin in bacterial pathogenesis has been previously reported without description of a clear mechanism. Studies have demonstrated that increased actin polymerization during S. typhimurium, L. donovani, or Mycobacteria spp. prevents the fusion of the lysosome37,65,72,73,74. Therefore, the strategy of specific bacteria to modulate the cytoskeleton is an efficient tactic used by professional intracellular organisms in order to subvert restrictive vesicle trafficking.
These data raise the intriguing possibility that host cells activate caspases to promote cellular immunity by engaging their alternative non-apoptotic functions to control intracellular replication of pathogenic microbes.
Methods
Bacterial Strains
Legionella pneumophila (Legionella) strain Lp02 is a streptomycin resistant (Smr) thymine-auxotroph derivative of Philadelphia-1. The flaA− and T4SS (dotA−) mutants have been previously described75. Lp02 and dotA− were complemented with a plasmid for green fluorescent protein (GFP). All in vitro infections were performed at an MOI of 0.5, unless otherwise stated and were performed in the absence of thymidine, ferric nitrate and L-cysteine to restrict extracellular growth. Non-fluorescent Escherichia coli (E. coli) strain BL21DE3 was grown overnight to Log phase as previously described14,22.
Mice
Wild-type (restrictive) C57BL/6 (B6) were purchased from The Jackson Laboratory (Bar Harbor ME). Casp-11−/− mice were generously donated by Dr. Yuan at Harvard Medical School76. Casp-1−/−/Casp-11Tg mice were a gift from Dr. Vishva Dixit at Genentech38. Naip5−/− mice were from Dr. Russell Vance at University of California Berkeley24. All mice were housed in a pathogen-free facility and experiments were performed with approval and in accordance with regulations and guidelines from the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University (Columbus OH).
Cell culture
Bone marrow-derived macrophages (BMDMs) were cultured as previously described77. Construction of red fluorescent caspase-11 and mutant caspase-11 using the pLenti6/V5 TOPO vector (Invitrogen Life Technologies) was previously described78. To generate HEK293 cells stably expressing red fluorescent caspase-11 and mutant caspase-11. Cells were transduced with lentivirus at an MOI of 1 in the presence of 6 μg/mL polybrene for 8–16 hrs. Afterwards, cell culture media was replaced and cells were selected with blasticidin (5 μg/mL) for 10 days resulting in a homogeneous yield of stably transduced cell line.
Immunoblotting
Cell lysates were prepared with an isotonic buffer (10 mM HEPES, 5 mM MgCl2, 1 mM EGTA, 142 mM KCl with NP-40), in the presence of complete, EDTA-free, protease inhibitor cocktail (Roche). Samples were clarified, denatured with SDS buffer and boiled. Proteins were separated by SDS-PAGE and then transferred to a polyvinylidene fluoride (PVDF) membrane (Biorad). Blots were probed with antibodies against caspase-11 (Sigma), caspase-1 (Genentech), phospho-cofilin, cofilin, Rac/Cdc42 and phospho-Rac (Cell Signaling), actin (Abcam), calreticulin (Stressgen). Detection was achieved using appropriate secondary antibodies conjugated with horseradish peroxidase (HRP), as previously described12,14,22,79.
Confocal Microscopy
F- and G-actin were visualized from Legionella-infected macrophages using Alexa Fluor® 568 phalloidin and Alexa Fluor® 488 DNAse I (Molecular Probes) and for studies examining colocalization of GFP-expressing bacteria (Legionella and E. coli) with the lysosome, Lysotracker™ red was used to stain acidic vesicles of infected BMDMs, as previously described14. To examine cell death, after infection macrophages were stained with 4 uM ethidium homodimer-1 (EthD-1) from the LIVE/DEAD® viability/cytotoxicity kit (Molecular Probes), according to manufacturer’s instructions. Images were captured using laser scanning confocal fluorescence microscope with a 60X objective (Olympus Fluoview FV10i).
F/G actin Assay in Live cells
Amounts of G- and F-actin were assessed from lysates of WT, Casp-11−/− and Casp-1−/−Casp-11Tg according to the manufacturer’s recommendations (BK037, Cytoskeleton). Briefly, macrophages were infected and lysed with F-actin stabilization buffer. Lysates were then ultracentrifuged and cytoplasmic fractions were separated and run out on an SDS-PAGE gel. Antibodies specific for actin were then used to visualize amounts of F- and G-actin.
RhoA and Rac GTPase Activity
Lysates from Legionella-infected macrophages were assayed for Rac1 and RhoA GTPase activity by G-LISA assay according to manufacturers recommendations (RhoA, BK124 and Rac, BK128; Cytoskeleton). Briefly, cell lysates were incubated on a Rac1 or RhoA affinity plate and then this colorimetric assay was developed with HRP detection reagent. Samples were read on a Spectra Max M2 plate reader (Molecular Devices) and results were expressed as absorbance at 490 nm.
Statistical Analysis
Data were analyzed using GraphPad Prism software. Data in figures are presented as mean averages of at least 3 independent experiments and error bars represent SEM. Comparisons of groups for statistical significance were analyzed with Student’s two-tailed t-test. p value ≤0.05 was considered significant.
Additional Information
How to cite this article: Caution, K. et al. Caspase-11 and caspase-1 differentially modulate actin polymerization via RhoA and Slingshot proteins to promote bacterial clearance. Sci. Rep. 5, 18479; doi: 10.1038/srep18479 (2015).
References
Pollard, T. D. & Cooper, J. A. Actin, a central player in cell shape and movement. Science 326, 1208–1212, doi: 10.1126/science.1175862 (2009).
Franco, I. S. & Shuman, H. A. A pathogen’s journey in the host cell: Bridges between actin and traffic. Bioarchitecture 2, 38–42 (2012).
Marion, S. et al. Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion. Traffic 12, 421–437, doi: 10.1111/j.1600-0854.2011.01158.x (2011).
Bamburg, J. R. & Bernstein, B. W. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep 2, 62, doi: 10.3410/B2-62 (2010).
Huang, T. Y., DerMardirossian, C. & Bokoch, G. M. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 18, 26–31, doi: 10.1016/j.ceb.2005.11.005 (2006).
Kurita, S., Gunji, E., Ohashi, K. & Mizuno, K. Actin filaments-stabilizing and -bundling activities of cofilin-phosphatase Slingshot-1. Genes Cells 12, 663–676, doi: 10.1111/j.1365-2443.2007.01078.x (2007).
Fernandez-Sabe, N. et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics and outcomes. Medicine (Baltimore) 82, 159–169, doi: 10.1097/01.md.0000076005.64510.87 (2003).
Carratala, J. et al. Risk factors for nosocomial Legionella pneumophila pneumonia. Am J Respir Crit Care Med 149, 625–629, doi: 10.1164/ajrccm.149.3.8118629 (1994).
Horwitz, M. A. & Silverstein, S. C. Legionnaires’ disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest 66, 441–450, doi: 10.1172/JCI109874 (1980).
Horwitz, M. A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med 158, 2108–2126 (1983).
Khweek, A. A. & Amer, A. Replication of Legionella pneumophila in Human Cells: Why are We Susceptible? Front Microbiol. PMCID:3109522 1, 133, doi: 10.3389/fmicb.2010.00133 (2010).
Abdelaziz, D. H. et al. Apoptosis-associated speck-like protein (ASC) controls Legionella pneumophila infection in human monocytes. J Biol Chem. PMCID:3030324 286, 3203–3208, doi: 10.1074/jbc.M110.197681 (2011).
Abu Khweek, A. et al. Biofilm-derived Legionella pneumophila evades the innate immune response in macrophages. Front Cell Infect Microbiol 3, 18, doi: 10.3389/fcimb.2013.00018 (2013).
Akhter, A. et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47, doi: 10.1016/j.immuni.2012.05.001 (2012).
Kortmann, J., Brubaker, S. W. & Monack, D. M. Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J Immunol 195, 815–819, doi: 10.4049/jimmunol.1403100 (2015).
Abdelaziz, D. H., Amr, K. & Amer, A. O. Nlrc4/Ipaf/CLAN/CARD12: more than a flagellin sensor. Int J Biochem Cell Biol. PMCID:2862870 42, 789–791, doi: 10.1016/j.biocel.2010.01.003 (2010).
Amer, A. et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281, 35217–35223, doi: 10.1074/jbc.M604933200 (2006).
Miao, E. A., Andersen-Nissen, E., Warren, S. E. & Aderem, A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 29, 275–288, doi: 10.1007/s00281-007-0078-z (2007).
Sutterwala, F. S. & Flavell, R. A. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin Immunol 130, 2–6, doi: 10.1016/j.clim.2008.08.011 (2009).
Pereira, M. S. et al. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J Immunol 187, 6447–6455, doi: 10.4049/jimmunol.1003784 (2011).
Zamboni, D. S. et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7, 318–325, doi: 10.1038/ni1305 (2006).
Akhter, A. et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5, e1000361, doi: 10.1371/journal.ppat.1000361 (2009).
Lamkanfi, M. et al. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol 178, 8022–8027, doi: (2007).
Lightfield, K. L. et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9, 1171–1178, doi: 10.1038/ni.1646 (2008).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7, 569–575, doi: 10.1038/ni1344 (2006).
Mariathasan, S.A.S.C., Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect 9, 664–671, doi: 10.1016/j.micinf.2007.01.017 (2007).
Suzuki, T. et al. Differential regulation of caspase-1 activation, pyroptosis and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog 3, e111, doi: 10.1371/journal.ppat.0030111 (2007).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831, doi: 10.1016/j.cell.2007.12.040 (2008).
Case, C. L., Shin, S. & Roy, C. R. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun 77, 1981–1991, doi: 10.1128/IAI.01382-08 (2009).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature, doi: 10.1038/nature15541 (2015).
Bitar, D. M., Molmeret, M. & Abu Kwaik, Y. Molecular and cell biology of Legionella pneumophila. Int J Med Microbiol 293, 519–527 (2004).
Santic, M., Asare, R., Doric, M. & Abu Kwaik, Y. Host-dependent trigger of caspases and apoptosis by Legionella pneumophila. Infect Immun 75, 2903–2913, doi: 10.1128/IAI.00147-07 (2007).
Cerqueira, D. M., Pereira, M. S., Silva, A. L., Cunha, L. D. & Zamboni, D. S. Caspase-1 but Not Caspase-11 Is Required for NLRC4-Mediated Pyroptosis and Restriction of Infection by Flagellated Legionella Species in Mouse Macrophages and In Vivo. J Immunol 195, 2303–2311, doi: 10.4049/jimmunol.1501223 (2015).
Asare, R. et al. Genetic susceptibility and caspase activation in mouse and human macrophages are distinct for Legionella longbeachae and L. pneumophila. Infect Immun 75, 1933–1945, doi: 10.1128/IAI.00025-07 (2007).
Casson, C. N. & Shin, S. Inflammasome-mediated cell death in response to bacterial pathogens that access the host cell cytosol: lessons from Legionella pneumophila. Front Cell Infect Microbiol 3, 111, doi: 10.3389/fcimb.2013.00111 (2013).
Bokoch, G. M. Regulation of innate immunity by Rho GTPases. Trends Cell Biol 15, 163–171, doi: 10.1016/j.tcb.2005.01.002 (2005).
Kjeken, R. et al. Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles. Mol Biol Cell 15, 345–358, doi: 10.1091/mbc.E03-05-0334 (2004).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121, doi: 10.1038/nature10558 (2011).
Arber, S. et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805–809, doi: 10.1038/31729 (1998).
Fortier, A., Diez, E. & Gros, P. Naip5/Birc1e and susceptibility to Legionella pneumophila. Trends Microbiol 13, 328–335, doi: 10.1016/j.tim.2005.05.007 (2005).
Kligys, K. et al. The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization and motility behavior of keratinocytes. J Biol Chem 282, 32520–32528, doi: 10.1074/jbc.M707041200 (2007).
Niwa, R., Nagata-Ohashi, K., Takeichi, M., Mizuno, K. & Uemura, T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 108, 233–246, doi: (2002).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–426, doi: (2002).
Salvesen, G. S. & Ashkenazi, A. Snapshot: caspases. Cell 147, 476–476 e471, doi: 10.1016/j.cell.2011.09.030 (2011).
Martinon, F. & Tschopp, J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14, 10–22, doi: 10.1038/sj.cdd.4402038 (2007).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145, doi: 10.1016/j.cell.2006.07.033 (2006).
Srivastava, S. S. & Krishnasastry, M. V. Cell membrane repair pathway involves sensing of dynamics of caveolae and caspase-1. Adv Exp Med Biol 749, 117–129, doi: 10.1007/978-1-4614-3381-1_9 (2012).
Sokolovska, A. et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol 14, 543–553, doi: 10.1038/ni.2595 (2013).
Banga, S. et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci USA 104, 5121–5126, doi: 10.1073/pnas.0611030104 (2007).
Lee, J. M. et al. The involvement of caspase-11 in TPEN-induced apoptosis. FEBS Lett 582, 1871–1876, doi: 10.1016/j.febslet.2008.04.056 (2008).
Mashima, T., Naito, M. & Tsuruo, T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene 18, 2423–2430, doi: 10.1038/sj.onc.1202558 (1999).
Aachoui, Y. et al. Caspase-11 Protects Against Bacteria That Escape the Vacuole. Science, doi: 10.1126/science.1230751 (2013).
Kang, S. J., Wang, S., Kuida, K. & Yuan, J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ 9, 1115–1125, doi: 10.1038/sj.cdd.4401087 (2002).
Case, C. L. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci USA 110, 1851–1856, doi: 10.1073/pnas.1211521110 (2013).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci USA 111, 6046–6051, doi: 10.1073/pnas.1321700111 (2014).
Losick, V. P. & Isberg, R. R. NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med 203, 2177–2189, doi: 10.1084/jem.20060766 (2006).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249, doi: 10.1126/science.1240248 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253, doi: 10.1126/science.1240988 (2013).
Meunier, E. & Broz, P. Interferon-induced guanylate-binding proteins promote cytosolic lipopolysaccharide detection by caspase-11. DNA Cell Biol 34, 1–5, doi: 10.1089/dna.2014.2701 (2015).
Raftopoulou, M. & Hall, A. Cell migration: Rho GTPases lead the way. Dev Biol 265, 23–32, doi: (2004).
Ridley, A. J. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev 5, 24–30 (1995).
Allen, W. E., Jones, G. E., Pollard, J. W. & Ridley, A. J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci 110 (Pt 6), 707–720 (1997).
Li, J. et al. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol 9, 276–286, doi: 10.1038/ncb1541 (2007).
Bergsbaken, T. & Bevan, M. J. Cutting Edge: Caspase-11 Limits the Response of CD8+ T Cells to Low-Abundance and Low-Affinity Antigens. J Immunol 195, 41–45, doi: 10.4049/jimmunol.1500812 (2015).
Man, S. M. et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc Natl Acad Sci USA 111, 17588–17593, doi: 10.1073/pnas.1419925111 (2014).
Meresse, S. et al. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol 3, 567–577, doi: (2001).
Franco, I. S., Shohdy, N. & Shuman, H. A. The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog 8, e1002546, doi: 10.1371/journal.ppat.1002546 (2012).
Bennett, T. L. et al. LegC3, an effector protein from Legionella pneumophila, inhibits homotypic yeast vacuole fusion in vivo and in vitro. PLoS One 8, e56798, doi: 10.1371/journal.pone.0056798 (2013).
O’Brien, K. M., Lindsay, E. L. & Starai, V. J. The Legionella pneumophila effector protein, LegC7, alters yeast endosomal trafficking. PLoS One 10, e0116824, doi: 10.1371/journal.pone.0116824 (2015).
Michard, C. et al. The Legionella Kinase LegK2 Targets the ARP2/3 Complex To Inhibit Actin Nucleation on Phagosomes and Allow Bacterial Evasion of the Late Endocytic Pathway. MBio 6, e00354–00315, doi: 10.1128/mBio.00354-15 (2015).
Herweg, J. A. et al. Purification and proteomics of pathogen-modified vacuoles and membranes. Front Cell Infect Microbiol 5, 48, doi: 10.3389/fcimb.2015.00048 (2015).
Holm, A., Tejle, K., Magnusson, K. E., Descoteaux, A. & Rasmusson, B. Leishmania donovani lipophosphoglycan causes periphagosomal actin accumulation: correlation with impaired translocation of PKCalpha and defective phagosome maturation. Cell Microbiol 3, 439–447, doi: (2001).
Guerin, I. & de Chastellier, C. Disruption of the actin filament network affects delivery of endocytic contents marker to phagosomes with early endosome characteristics: the case of phagosomes with pathogenic mycobacteria. Eur J Cell Biol 79, 735–749 (2000).
Guerin, I. & de Chastellier, C. Pathogenic mycobacteria disrupt the macrophage actin filament network. Infect Immun 68, 2655–2662 (2000).
Berger, K. H. & Isberg, R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7, 7–19 (1993).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509, doi: (1998).
Stanley, E. R. Murine bone marrow-derived macrophages. Methods Mol Biol 75, 301–304, doi: 10.1385/0-89603-441-0:301 (1997).
Gavrilin, M. A. et al. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol 188, 3469–3477, doi: 10.4049/jimmunol.1102272 (2012).
Abdulrahman, B. A. et al. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy 7, 1359–1370, doi: 10.4161/auto.7.11.17660 (2011).
Acknowledgements
We thank Drs. Yuan, Dixit and Vance for generous gift of Casp-11−/−, Casp-1−/−/Casp-11Tg and Naip5−/− mice, respectively. We thank Dr. Kopp for reading and editing the manuscript. Studies in the Amer laboratory are supported by Ohio State University (OSU) Bridge funds, Public Health Preparedness for Infectious Diseases (PHPID), Center for Clinical and Translational Science (CCTS), R21 AI113477 and R01 HL094586. MT is supported by American Association of Immunology (Careers in Immunology) fellowship. KK is supported by Deutsche Forschungsgemeinschaft (DFG - German Research Foundation).
Author information
Authors and Affiliations
Contributions
K.C. designed, performed, analyzed, interpreted data and contributed to the writing of the manuscript. M.A.G., M.T., D.L. and K.K. contributed to performance of experiments and editing of the manuscript. S.H. and A.K. contributed to the discussion and editing of the manuscript. A.O.A. designed the experiments, analyzed and interpreted the results and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Caution, K., Gavrilin, M., Tazi, M. et al. Caspase-11 and caspase-1 differentially modulate actin polymerization via RhoA and Slingshot proteins to promote bacterial clearance. Sci Rep 5, 18479 (2016). https://doi.org/10.1038/srep18479
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18479
This article is cited by
-
Gasdermin D restricts Burkholderia cenocepacia infection in vitro and in vivo
Scientific Reports (2021)
-
CASP4 gene silencing in epithelial cancer cells leads to impairment of cell migration, cell-matrix adhesion and tissue invasion
Scientific Reports (2018)
-
Actin polymerization is reduced in the anterior cingulate cortex of elderly patients with schizophrenia
Translational Psychiatry (2017)
-
Growth inhibition of cytosolic Salmonella by caspase-1 and caspase-11 precedes host cell death
Nature Communications (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.