Abstract
The increasingly attractive stereotactic body radiotherapy (SBRT) treatment for stage I lung cancer is concomitant with a large amount of monitor units (MU), leading to excessive out-of-field dose and prolonged beam-on time. The study aims to reduce the MU number and shorten the beam-on time by optimizing the planning parameters. Clinically acceptable treatment plans from fourteen patients suffered from peripheral stage I non-small cell lung cancer (NSCLC) were created in the study. Priority for the upper objective of the target (PUOT), strength and Max MU setting in the MU objective function (MUOF) were adjusted respectively to investigate their effect on MU number, organs at risk (OARs) sparing and beam-on time. We found that the planning parameters influenced the MU number in a PUOT, strength and Max MU dependent manner. Combined with high priority for the UOT (HPUOT) and MUOF, the MU number was reduced from 443 ± 25 to 228 ± 22 MU/Gy without compromising the target coverage and OARs sparing. We also found beam-on time was proportional to MU number and it could be shortened from 7.9 ± 0.5 to 4.1 ± 0.4 minutes.
Similar content being viewed by others
Introduction
Retrospective studies have demonstrated that stereotactic body radiotherapy (SBRT) treatment was effective for medically inoperable early stage non-small cell lung cancer (NSCLC)1,2,3. It has been reported to achieve similar overall survival (OS), disease-free survival (DFS), local control (LC) and distant control (DC) as surgery while maintaining minimal toxicity4,5,6. The latest research in Lancet Oncology brings in the encouraging result that SBRT strategy achieves better outcome than surgery for operable stage I NSCLC7.
Single-fraction SBRT strategy has been widely used for lung cancer8,9,10 and several publications have demonstrated its safety, efficacy and minimal toxicity for NSCLC treatment11,12. As the dose regimen was usually larger than 25 Gy per fraction in single-fraction SBRT, the excessive monitor units (MU) and prolonged beam-on time has become an issue of concern. It was reported the required MU was in the range of 2000–10000 for a fraction dose in excess of 10 Gy13 and the average beam-on time ranged from 5 to 6 minutes in SBRT treatment with 25 Gy per fraction10,14. The MU number and beam-on time will be more than reported when larger dose regimens such as 30 Gy or 34 Gy was used. It has been estimated that doubled MU could translate into a potential increase in the risk of secondary cancers by a factor of 1.2–815,16 and the extended treatment time could increase the risk of tumor displacement during beam delivery17,18.
Eclipse treatment planning system (TPS) has incorporated the MU objective function (MUOF) to reduce MU number during treatment planning. To our knowledge, few researches have investigated the effect of planning parameters on MU number and beam-on time, particularly for SBRT treatment of lung cancer which always involves high dose fractionation.
Therefore, the study aims to reduce the MU number and beam-on time for SBRT treatment of lung cancer by optimizing the planning parameters in Eclipse.
Materials and Methods
Ethics statement
The protocol was approved by the Ethics Committee of Cancer Hospital of Shantou University Medical College. Since this is not a treatment-based study, our institutional review board waived the need for written informed consent from the participants. The methods in the study were performed in accordance with the approved guidelines and regulations.
CT scanning and contouring of organs at risk (OARs)
During April 2013 and May 2015, fourteen patients suffering from stage I lung cancer were enrolled in this study. Basic information of the patients was listed in Table 1. Patients were positioned supine on a vacuum bag (Medtec Medical, Inc, Buffalo Grove, IL) or a thermoplastic mask (Guangzhou Klarity Medical & Equipment Co., Ltd, Guangzhou, China). Patients were subjected to a four-dimensional computed tomography (4DCT) scans with Brilliance Big Bore CT (Philips, Inc, Netherlands) under uncoached free breathing. The CT images were then transferred to Eclipse TPS (V10, Varian Medical System, Inc., Palo Alto, CA) for target volume, OARs delineation and treatment planning. The gross target volume (GTV) accounting for ten breathing phases were contoured in the pulmonary windows by a radiation oncologist specialized in lung SBRT. Ten phases of the GTV were then used to form internal target volume (ITV). To account for setup inaccuracies and potential intra-fractional tumor shift, a 0.5 cm setup margin was added to the ITV to form the planning target volume (PTV). All plans were carried out on the averaged 4DCT. OARs, including aorta, esophagus, bronchial tree, heart, spinal cord, lung and chest wall (CW) were contoured according to the RTOG 0915 report19.
Treatment Planning
We prescribed 1 fraction of 25 Gy in all plans for small peripheral tumor according to previous publications10,14,20. Treatment planning was designed with dual partial arcs to exclude the entrance of the beam through the contralateral lung. All plans required a clockwise and a counterclockwise arc for each fraction. Collimator angles for all plans were set to 30° in one arc and 330° for another to minimize the contribution of the tongue-and-groove effect to the dose. The grouped fields were aligned to the center of the PTV. A dose-limiting structure (2 cm from PTV in any direction) was constructed to ensure a rapid dose fall-off outside the target. We used 6MV flattening filter free (FFF) beam on a TrueBeam Linac (Varian Medical Systems, Inc, Palo Alto, CA), selecting a maximum dose rate of 1400 MU/min and 114 control points to design the treatment plans. Optimization was performed with the progressive resolution optimizer (PRO) algorithm implemented in Eclipse 10.0. The objectives were adjusted to make sure the maximum dose was at least 120% of the prescription dose and centered in the GTV. Dose calculations were performed using the Anisotropic Analytic Algorithm (AAA) with a calculation grid size of 1 mm, taking into account heterogeneity correction. The plan calculated at the first time was used as a basedose plan for further optimization to compensate any underdose or “dose cloud” areas. The final dose calculation was normalized to guarantee that 95% of the PTV received the prescribed dose. All of the dose constraints, such as PTV, conformity of prescribed dose, intermediate dose spillage and critical organ dose-volume limits met the criterion of the RTOG 0915 protocol using single fraction19.
Planning parameters
Priority for the upper objective of the target (PUOT) and two MU constraints (strength and Max MU) in the MUOF were adjusted to investigate their effect on MU number, target coverage, OARs sparing and beam-on time. Upper objective of the target (UOT) is the objective in the optimizer to limit the maximum dose in the target and the priority for it determines the relative importance to achieve the objective. For example, if the UOT is set to 110% of the prescribed dose and given high priority, the optimizer will ensure the target receives no more than 110% of the prescribed dose taking the first priority. The MUOF tends to keep the MU number as low as possible in the optimization process, if clinically required. When using the MUOF, we need to define the strength and the Max MU values for the objective. The strength and Max MU values can be defined between 0–100 and 0–100000, respectively.
In the study, the priority for the lower objective of the target (PLOT) was set to 100 for all the treatment plans. Relatively, PUOT was set to 60 (low priority, LPUOT), 80 (medium priority, MPUOT) and 100 (high priority, HPUOT) to investigate their effects on MU number. Strength value in the MUOF was set to 25, 50, 75 and 100, respectively. Max MU setting was set to 100%, 85%, 75%, 50% and 25%, respectively. We must emphasize that 100%, 85%, 75%, 50% and 25% Max MU setting in the MUOF equals to 100%, 85%, 75%, 50% and 25% of total MU number calculated in the LPUOT, MPUOT and MPUOT plans without the MUOF. When changing the parameters investigated, we kept other optimization parameters unchanged during the RapidArc optimization to avoid their influence on the result of treatment plans.
Evaluation parameters
The evaluation parameters include the maximum, minimum and mean dose to the PTV. Conformity of prescribed dose (CI100%) was defined as the ratio of the volume of prescription isodose to the volume of PTV. Two parameters of D2cm and R50% were used to evaluate the intermediate dose spillage according the RTOG 0915 protocol19. D2cm was defined as the maximum dose 2 cm away from PTV in any direction. R50% was defined as the ratio of the volume of 50% prescription isodose to the volume of PTV. For the OARs, the analysis included the maximum dose to the aorta, esophagus, bronchial tree, heart and spinal cord. Meanwhile, the mean dose and a set of appropriate Vx values were used to assess the lung. Vx was the volume of the organ receiving a dose of x or more. For example, V40 was the volume of organ receiving a dose of 40 Gy or more. Although it was reported that V30 was a predictive parameter of radiation induced CW pain21, we have ignored the dosimetric change of CW due to the 1 × 25 Gy fraction scheme used in our study.
Statistical analysis
All data in this study were presented as the mean plus standard deviation (mean ± SD). The Statistical Package for Social Sciences (SPSS, version 17.0, Chicago, IL) was used for statistical analysis in the present study. We used the Friedman Test to determine the difference between groups. Comparisons of the sub-group data were compared using Wilcoxon signed-rank test. Differences were considered statistically significant when p < 0.05.
Results
Effect of PUOT on MU number
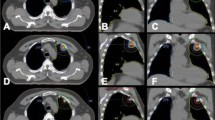
We found that the planning parameters in the optimizer influenced the MU number in a PUOT, strength and max MU dependent manner. MU number decreased with the PUOT increasing. MU number was 443 ± 25, 324 ± 55 and 260 ± 28 MU/Gy for the LPUOT, MPUOT and HPUOT groups, respectively. Effect of PUOT on the MU number was exhibited in Fig. 1a. The HPUOT group also spared the heart, spinal cord and lung while maintaining comparable target coverage than the other two groups. Dosimetric comparison of PTV and OARs for the LPUOT, MPUOT and HPUOT plans without the MUOF were shown in Table 2. Reduction of MU number was associated with less multileaf collimator (MLC) movement. MU related MLC movement in LPUOT, MPUOT and HPUOT groups without the MUOF were exhibited in Fig. 2a–c.
Effect of planning parameters on MU number.
(a) impact of PUOT without MUOF; (b,e,h) and (c,f,i) impact of strength and Max MU setting in LPUOT, MPUOT and HPUOT plans; (d,g,j) MU comparison with and without the MUOF in LPUOT, MPUOT and HPUOT groups, respectively; L = LPUOT, M = MPUOT, H = HPUOT, S = strength setting, Max MU = Max MU setting. S was set to 100 and Max MU was set to 50%. *indicates statistical significance (p < 0.05) using Wilcoxon signed-rank test.
Effect of MUOF on MU number
The strength and Max MU settings in the MUOF also influenced the MU number. MU number was continuously reduced with the strength increasing and Max MU setting decreasing, irrespective of LPUOT, MPUOT and HPUOT groups. With the maximum strength of 100, MU number reached the minimum while maintaining comparable dose to the OARs. We also found that the MU number continued to decrease at 100%, 85%, 75% and 50% Max MU setting, but no longer to decrease at 25%. Effect of the MUOF (strength and Max MU setting) on MU number in the LPUOT, MPUOT and HPUOT groups were also displayed in Fig. 1b–j. MLC movement with the MUOF in LPUOT, MPUOT and HPUOT groups were exhibited in Fig. 2d–i. Dosimetric comparison of PTV and OARs with and without the MUOF in the LPUOT, MPUOT and HPUOT plans were shown in Table 3, 4, 5, respectively.
Combined with HPUOT and MUOF, MU number could be reduced to as low as 228 ± 22 MU/Gy (Fig. 1j) without compromising the dose to the target and OARs (Table 5).
Effect of planning parameters (PUOT and MUOF) on beam-on time
We also found that beam-on time was proportional to MU number and the corresponding beam-on time for the LPUOT, MPUOT and HPUOT plans were 7.9 ± 0.5, 5.9 ± 1.0 and 4.7 ± 0.5 minutes (Fig. 3a). When incorporating the MUOF, mean beam-on time was furtherly reduced to 5.5 ± 1.0, 4.4 ± 0.4 and 4.1 ± 0.4 minutes for the LPUOT, MPUOT and HPUOT plans, respectively. Effect of the MUOF (strength and Max MU) on beam-on time in the LPUOT, MPUOT and HPUOT plans was exhibited in Fig. 3b–d.
Effect of planning parameters on beam-on time.
(a) impact of PUOT without MUOF; (b–d) impact of the MUOF in LPUOT, MPUOT and HPUOT groups; L = LPUOT, M = MPUOT, H = HPUOT, S = strength setting, Max MU = Max MU setting. S was set to 100 and Max MU was set to 50%. *indicates statistical significance (p < 0.05) using Wilcoxon signed-rank test.
Discussion
In this study, we have optimized the planning parameters to reduce the MU number and thus shorten the beam-on time. We found that the planning parameters influenced the MU number in a PUOT, strength and Max MU dependent manner. Combined with HPUOT, maximum strength of 100 and 50% Max MU setting in the MUOF, we reduce the MU number to as low as 228 ± 22 MU/Gy in SBRT treatment for lung cancer with 1 × 25 Gy fraction scheme. Meanwhile, the corresponding beam-on time was shortened to 4.1 ± 0.4 minutes. To the best of our knowledge, it was the first study to investigate the effect of PUOT and MUOF on the MU number for SBRT treatment of lung cancer involving high dose fraction scheme.
Clemente et al. had investigated the MU objective tool incorporated in the Eclipse TPS to reduce the MU number in prostate cancer patients using a conventional fractionation of 2 Gy per day22. They observed that the favorable combination of 100 strength and 50% Max MU in the MUOF could result in 28% reduction in the MU number whereas the deterioration in homogeneity index (HI) of the target was up to 23%. Moreover, they also found that the rectum and femoral heads dose were significantly higher in the MU-optimized group than that without incorporating the function. Our data that the combination of 100 strength and 50% Max MU setting achieved the lowest MU number was highly in accordance with their result. However, our finding that the function significantly reduced the total MU number (up to 15%) while maintaining comparable target coverage and improved OARs sparing differed from theirs. One explanation for the inconsistency is that the OARs are away from the target in peripheral lung patients but the OARs like bladder, rectum, small intestine and femoral heads are adjacent to the target in prostate cases. The anatomical characteristics in prostate cases might lead to the HI deterioration and more irradiation to the OARs when the MU number was enforced to be reduced. From the two independent researches, we speculate that the MUOF is more beneficial to cases where the target is away from the OARs, such as peripheral lung cancer patients and so on. When the target is adjacent to the OARs, MU number decreased at the cost of compromising HI of the target and OARs sparing. To our knowledge, this point has not been proposed and this study is the first to report and analyze it.
A waste of MU is worthy of attention in modern radiotherapy, particularly in intensity modulated radiotherapy (IMRT) treatment which can increase the MU number by a factor of 2 to 10 (typically 3–5) depending on the techniques and equipment used23. It is known and accepted that the scattered radiation administered to a patient’s body outside of the treatment volume is at first-order directly proportional to the applied MU in treatments with linear accelerators24. Excessive MU increases the leakage radiation and out-of-field dose and simultaneously enhances the risk of radiation induced second malignancies15,23,25. The issue of excessive MU and the radiation induced second malignancies needs to be taken into consideration due to the large fractional dose and good life expectancy achieved in SBRT treatment of lung cancer. The reported single-fraction SBRT used fractional dose up to 34 Gy9 and the total MU number will be larger compared with the 1 × 25 scheme in our study. Additionally, it was reported that 2-year OS and LC were 70% and 91% in 3201 patients with localized stage I NSCLC from a systematic review4. With treatment becoming more successful and survival rates rising, lowering the risk of radiation induced secondary cancer is of particular concern in lung SBRT treatment.
MU reduction also translates into beam-on time shortening. Many publications have reported the potential of volumetric modulated arc therapy (VMAT) combined FFF beams to improve the treatment efficiency compared with conventional IMRT13,26,27,28,29. However, in our study, we found the treatment efficiency could be further improved by optimizing the planning parameters. Shorter treatment time generally translated into substantially superior patient stability and treatment accuracy17, simultaneously reduced the likelihood of intrafractional baseline shifts in tumor position18. For high dose SBRT treatment of lung cancer, reducing the beam-on time is of clinical significance for two reasons: (1) Previous research regarding the target motion as a function of treatment time found the average time needed to maintain the target motion within 1 mm of translation or 1 degrees of rotational deviation was 5.9 min for thoracic tumors30. In our study, the beam-on time was shortened to less than 5.5 minutes on average with the MUOF, implying a stable target motion during the delivery. (2) For SBRT treatment with FFF beams which provides as high as 2400 MU/min dose rate, a maximum dose of 1 Gy can be delivered within the course of 2.5 seconds13. Therefore, it was important to shorten the beam-on time in SBRT treatment for lung patients, particularly when the large dose fraction schemes like 1 × 25 Gy, 1 × 30 Gy or 1 × 34 Gy were used.
Reducing the MU number also means less MLC modulation is required to achieve comparable dose distribution (Fig. 2). MLC interlock is a common linac accelerator (LA) failure and reduction of MLC movement probably helps to lower the MLC workload. Moreover, it also contributes to reduce the probability of dosimetric inaccuracy induced by MLC positioning error. It was reported the average changes in D95% caused by this errors was up to 8% in complex head and neck plans31.
Although the original goal of our research is to reduce the MU number in small peripheral NSCLC (≤3 cm) patients, we believe our experience is also useful for larger tumors (>3 cm) or other treatment sites because the tumor size doesn’t impact on the PUOT and MUOF setting. However, we need more experiments to confirm the speculation. It will be another interesting work for our future studies.
Although we have optimized the planning parameters to reduce the MU number, it does have some limitations. (1) The dose displayed in the study is physical dose. As SBRT treatment always involves large dose per fraction, it is necessary to convert the physical dose to biologically equivalent dose (BED) to account for the radiobiological effect. However, as the dose difference between the groups is so small that we have ignored the contribution of it. (2) Although the combination of HPUOT and MUOF is capable of reducing the MU number, it slightly prolongs the planning time by about 10–12 minutes because we need to know the total MU number calculated in HPUOT plan when using 50% Max MU setting. However, we found the planning parameters in study didn’t have any influence on the optimization time during each optimization.
Conclusions
The planning parameters in the optimizer influence the MU number in a PUOT, strength and Max MU dependent manner. Combined with HPUOT and MUOF, the MU number can be reduced to 228 ± 22 MU/Gy while maintaining comparable or improved OAR sparing in SBRT treatment for lung cancer.
Additional Information
How to cite this article: Huang, B.-T. et al. Monitor unit optimization in stereotactic body radiotherapy for small peripheral non-small cell lung cancer patients. Sci. Rep. 5, 18453; doi: 10.1038/srep18453 (2015).
References
Xia, T. et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 66, 117–125 (2006).
Baumann, P. et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 27, 3290–3296 (2009).
Timmerman, R. et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 303, 1070–1076 (2010).
Solda, F. et al. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol. 109, 1–7 (2013).
Zhang, B. et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol. 112, 250–255 (2014).
Zheng, X. et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 90, 603–611 (2014).
Chang, J. Y. et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 16, 630–637 (2015).
Wulf, J. et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys. 60, 186–196 (2004).
Hara, R. et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors Cancer. 106, 1347–1352 (2006).
Le, Q. T. et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 1, 802–809 (2006).
Fritz, P. et al. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol. 1, 30 (2006).
Videtic, G. M. et al. 30 Gy or 34 Gy? Comparing 2 single-fraction SBRT dose schedules for stage I medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 90, 203–208 (2014).
Ong, C. L. et al. Fast arc delivery for stereotactic body radiotherapy of vertebral and lung tumors. Int J Radiat Oncol Biol Phys. 83, e137–143 (2012).
Huang, B. T. et al. Comparison of Two RapidArc Delivery Strategies in Stereotactic Body Radiotherapy of Peripheral Lung Cancer with Flattening Filter Free Beams. PLoS One. 10, e0127501 (2015).
Hall, E. J. Intensity-modulated radiation therapy, protons and the risk of second cancers. Int J Radiat Oncol Biol Phys. 65, 1–7 (2006).
Kry, S. F. et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 62, 1195–1203 (2005).
Hoogeman, M. S., Nuyttens, J. J., Levendag, P. C. & Heijmen, B. J. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 70, 609–618 (2008).
Purdie, T. G. et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification and intrafraction tumor position. Int J Radiat Oncol Biol Phys. 68, 243–252 (2007).
RADIATION THERAPY ONCOLOGY GROUP. A Randomized Phase II Study Comparing 2 Stereotactic Body Radiation Therapy (SBRT) Schedules for Medically Inoperable Patients with Stage I Peripheral Non-Small Cell Lung Cancer. (2012) Available at: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0915. (Accessed: 24th July 2015).
Li, R. et al. Clinical implementation of intrafraction cone beam computed tomography imaging during lung tumor stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 87, 917–923 (2013).
Dunlap, N. E. et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 76, 796–801 (2010).
Clemente, S. et al. Monitor unit optimization in RapidArc plans for prostate cancer. J Appl Clin Med Phys. 14, 4114 (2013).
Cashmore, J., Ramtohul, M. & Ford, D. Lowering whole-body radiation doses in pediatric intensity-modulated radiotherapy through the use of unflattened photon beams. Int J Radiat Oncol Biol Phys. 80, 1220–1227 (2011).
Cozzi, L. et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 89, 180–191 (2008).
Palma, D. A., Verbakel, W. F., Otto, K. & Senan, S. New developments in arc radiation therapy: a review. Cancer Treat Rev. 36, 393–399 (2010).
Verbakel, W. F., Senan, S., Cuijpers, J. P., Slotman, B. J. & Lagerwaard, F. J. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol. 93, 122–124 (2009).
McGrath, S. D. et al. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol. 95, 153–157 (2010).
Zhang, G. G. et al. Volumetric modulated arc planning for lung stereotactic body radiotherapy using conventional and unflattened photon beams: a dosimetric comparison with 3D technique. Radiat Oncol. 6, 152 (2011).
Herbert, C. et al. Stereotactic Body Radiotherapy: Volumetric Modulated Arc Therapy Versus 3D Non-coplanar Conformal Radiotherapy for the Treatment of Early Stage Lung Cancer. Technol Cancer Res Treat. 12, 511–516 (2013).
Ma, L. et al. Nonrandom intrafraction target motions and general strategy for correction of spine stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 75, 1261–1265 (2009).
Mu, G., Ludlum, E. & Xia, P. Impact of MLC leaf position errors on simple and complex IMRT plans for head and neck cancer. Phys Med Biol. 53, 77–88 (2008).
Acknowledgements
This work was supported by Medical Scientific Research Foundation of Guangdong Province (A2015534), Shantou University Medical College Clinical Research Enhancement Initiative (201424) and Cooperative and Creative Center, Shantou University.
Author information
Authors and Affiliations
Contributions
B.T.H. conceived, designed the experiments and wrote the paper. Z.L. performed the experiments and collected the data. P.X.L. analyzed the data. J.Y.L. and C.Z.C. revised the paper. All authors have reviewed the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, BT., Lin, Z., Lin, PX. et al. Monitor unit optimization in stereotactic body radiotherapy for small peripheral non-small cell lung cancer patients. Sci Rep 5, 18453 (2016). https://doi.org/10.1038/srep18453
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18453
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.