Abstract
The mitogen- and stress-activated kinase MSK1/2 plays a decisive role in apoptosis. In analogy to apoptosis of nucleated cells, suicidal erythrocyte death called eryptosis is characterized by cell shrinkage and cell membrane scrambling leading to phosphatidylserine (PS) externalization. Here, we explored whether MSK1/2 participates in the regulation of eryptosis. To this end, erythrocytes were isolated from mice lacking functional MSK1/2 (msk−/−) and corresponding wild-type mice (msk+/+). Blood count, hematocrit, hemoglobin concentration and mean erythrocyte volume were similar in both msk−/− and msk+/+ mice, but reticulocyte count was significantly increased in msk−/− mice. Cell membrane PS exposure was similar in untreated msk−/− and msk+/+ erythrocytes, but was enhanced by pathophysiological cell stressors ex vivo such as hyperosmotic shock or energy depletion to significantly higher levels in msk−/− erythrocytes than in msk+/+ erythrocytes. Cell shrinkage following hyperosmotic shock and energy depletion, as well as hemolysis following decrease of extracellular osmolarity was more pronounced in msk−/− erythrocytes. The in vivo clearance of autologously-infused CFSE-labeled erythrocytes from circulating blood was faster in msk−/− mice. The spleens from msk−/− mice contained a significantly greater number of PS-exposing erythrocytes than spleens from msk+/+ mice. The present observations point to accelerated eryptosis and subsequent clearance of erythrocytes leading to enhanced erythrocyte turnover in MSK1/2-deficient mice.
Similar content being viewed by others
Introduction
The closely related mitogen- and stress-activated kinases MSK1 and MSK2 are involved in signal transduction that governs survival and apoptosis of nucleated cells1,2,3,4,5,6,7. Stimulators of MSK1 include the Ras-mitogen-activated protein kinase (MAPK)/p38 MAPK signal transduction pathway1,8,9,10. MSK1 participates in a wide array of cellular functions, including regulation of immediate-early gene expression9,11, an effect attributed to its ability to phosphorylate histone H1 and H3 and thus fostering the modification of chromatin structure3,6,9,12. Moreover, MSK1 contributes to the regulation of NF-κB activation2,13, of cAMP-response element11,14,15, of caspase activity16 and of Bad phosphorylation17. Furthermore, MSK1/2 deficiency enhances the formation of PGE218.
Similar to nucleated cells, erythrocytes may undergo suicidal death or eryptosis, which is characterized by cell shrinkage and cell membrane scrambling19. Triggers of eryptosis include activation of Ca2+-permeable cation channels20,21,22,23,24,25,26, which are activated by PGE227. The activation of the channels leads to Ca2+ entry, activation of Ca2+-sensitive K+ channels, exit of KCl with osmotically obliged water and, thus, to cell shrinkage28. Cytosolic Ca2+ further stimulates scrambling of the erythrocyte membrane with exposure of phosphatidylserine at the cell surface26,29,30,31,32. The Ca2+ sensitivity of cell membrane scrambling is increased by ceramide33. Phosphatidylserine exposing erythrocytes are rapidly phagocytosed and thus cleared from circulating blood34,35,36,37. Accordingly, accelerated eryptosis enhances the turnover of erythrocytes, which may lead to anemia, if the accelerated loss of erythrocytes is not compensated by a similar increase of erythrocyte formation, which is evident from reticulocytosis19.
In the present study, we explored whether MSK1/2 influences the survival of erythrocytes in response to pathophysiological cell stressors such as hyperosmotic shock and energy depletion. To this end, the eryptotic phenotype was characterized in mice lacking functional MSK1/2 (msk−/−) and their corresponding wild type mice (msk+/+).
Results
Absence of overt anemia but increased reticulocytosis in msk−/− mice
The present study addressed the impact of MSK1/2 on eryptosis in mice. To this end, experiments were performed in mice lacking functional MSK1/2 (msk−/−) and corresponding wild type mice (msk+/+). As a first approach, a blood count was performed. As shown in Table 1, erythrocyte count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were not significantly different between msk−/− than in msk+/+ mice. Mean corpuscular hemoglobin (MCH) was, however, slightly but significantly increased in msk−/− as compared to msk+/+ mice (Table 1). Reticulocyte count was significantly higher in msk−/− than in msk+/+ mice, pointing to enhanced erythrocyte formation in msk−/− mice (Table 1).
Expression of MSK1 and MSK2 in human and murine erythrocytes
Immunoblotting was employed to test whether MSK1 and/or MSK2 are expressed in erythrocytes. To this end, erythrocytes from humans or from mice were isolated and purified. Equal amounts of protein lysates were made and immunoblotting was performed. GAPDH served as a loading control. Expression of MSK1 and MSK2 was determined in lysates from murine whole blood and from purified murine erythrocytes. As illustrated in Fig. 1, the incubation with MSK1 and MSK2 specific antibodies both yielded a band of 90 (MSK1) and 86 (MSK2) kDa in murine and human erythrocytes, respectively.
MSK1 and MSK2 expression in murine and human erythrocytes.
(A) Original Western blots of MSK1 (~90 kDa) and GAPDH (~37 kDa) in murine whole blood (lane 1), 1:6.4 diluted whole blood (lane 2) and purified erythrocyte (RBC) preparation (lane 3) and human erythrocytes (lane 4). (B) Original Western blots of MSK2 (~86 kDa) and GAPDH (~37 kDa) in murine whole blood (lane 1), 1:3.7 diluted whole blood (lane 2) and purified erythrocyte (RBC) preparation (lane 3) and human erythrocytes (lane 4).
Increased susceptibility of msk−/− erythrocytes to osmosensitive eryptosis and hemolysis
Further experiments then addressed the susceptibility of MSK1/2-deficient erythrocytes to osmotic shock, a known stimulator of eryptosis, i.e. increase of phosphatidylserine exposure and decrease of cell volume26. Prior to osmotic shock, annexin V-binding reflecting phosphatidylserine exposure at the erythrocyte surface was similar in both msk−/− and msk+/+ erythrocytes (Fig. 2). Following exposure of erythrocytes for 1 h to hyperosmotic Ringer (addition of 550 mM sucrose), however, the annexin V-binding was significantly higher in msk−/− than in msk+/+ erythrocytes (Fig. 2). To depict cell shrinkage, forward scatter of msk−/− and msk+/+ erythrocytes was determined in flow cytometer analysis. As shown in Fig. 3, forward scatter was significantly reduced by hyperosmotic shock in erythrocytes from both msk−/− and msk+/+ mice. The effect, however, tended to be more pronounced in msk−/− erythrocytes than in msk+/+ erythrocytes. Further experiments explored the resistance of erythrocytes to a decline of extracellular osmolarity. As illustrated in Fig. 4, the resistance of erythrocytes to decreases of osmolarity was significantly lower in msk−/− than in msk+/+ mice. Thus, MSK1/2 deficiency enhances the sensitivity of erythrocytes to both hyper- and hypoosmotic shock.
Effect of hyperosmolarity on phosphatidylserine abundance at the surface of erythrocytes from msk−/− and msk+/+ mice.
(A) Histogram overlay and (B) Means ± SEM (n = 7) of annexin V-binding erythrocytes in isosmotic (black line) or hyperosmotic (red line, +550 mM sucrose) Ringer. #,###(p < 0.05; p < 0.001) from isosmotic, ***(p < 0.001) from msk+/+.
Increased vulnerability of msk−/− erythrocytes to energy-sensitive eryptosis
Additional experiments were performed in the presence and absence of glucose, as energy depletion is known to foster eryptosis38. As shown in Fig. 5, annexin V-binding reflecting phosphatidylserine exposure at the erythrocyte surface was significantly increased by 12 h glucose depletion, an effect significantly higher in msk−/− than in msk+/+ erythrocytes. Furthermore, as shown in Fig. 6, forward scatter was significantly reduced by energy depletion in erythrocytes from both msk−/− and msk+/+ mice. This effect tended to be larger in msk−/− than in msk+/+ erythrocytes, an effect, however, not reaching statistical significance (Fig. 6).
Effect of energy depletion on phosphatidylserine abundance at the surface of erythrocytes from msk−/− and msk+/+ mice.
(A) Histogram overlay and (B) Means ± SEM (n = 3−4) of annexin V-binding erythrocytes in glucose-containing (black line, +Glucose) or glucose-depleted (red line, −Glucose) Ringer. ###(p < 0.001) from +Glucose. ***(p < 0.001) from msk+/+.
Enhanced in vivo clearance and entrapment of eryptotic erythrocytes in the spleens of msk−/− mice
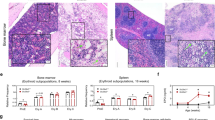
Eryptotic erythrocytes are rapidly cleared from circulating blood36. Thus, additional experiments were performed to disclose a possible effect of MSK1/2 deficiency on erythrocyte clearance. To determine the life span of circulating erythrocytes, blood was drawn from msk−/− and msk+/+ mice and erythrocytes were labelled with CFSE and injected autologously in the mice of the respective genotype. As shown in Fig. 7A, within 4 and 5 days CFSE-labeled msk−/− erythrocytes disappeared from circulating blood of msk−/− mice more rapidly than CFSE-labeled msk+/+ erythrocytes from circulating blood of msk+/+ mice. Thus, the life span of msk−/− erythrocytes in msk−/− mice was significantly shorter than the life span of msk+/+ erythrocytes in msk+/+ mice. The labelled erythrocytes were mainly trapped in the spleen. The ratio of spleen weight to body weight was slightly but significantly larger in msk−/− mice as compared to msk+/+ mice (Fig. 7B). The number of fluorescent annexin V-binding and thus phosphatidylserine-exposing erythrocytes as visualized by fluorescence confocal microscopy was again higher in the spleens from msk−/− mice than in the spleens from msk+/+ mice reflecting enhanced trapping of eryptotic erythrocytes in msk−/− mice (Fig. 7C,D).
Enhanced clearance and splenic entrapment of eryptotic erythrocytes in msk−/− mice.
(A) Means ± SEM (n = 3−4) of the percentages of autologously-injected circulating CFSE-labeled erythrocytes plotted against time. (B) Means ± SEM of the spleen/body weight ratio (mg/gram) of msk−/− (n = 21) and msk+/+ (n = 33) mice. (C) Confocal images of CFSE-dependent (left panels), annexin V-dependent (middle panels) and merged fluorescence (right panels) and (D) Means ± SEM (n = 3−4) of number of CFSE and annexin V positive splenic erythrocytes from msk−/− and msk+/+ mice. *(p < 0.05) from msk+/+.
Discussion
According to the present observations, a lack of MSK1/2 enhances the susceptibility of erythrocytes to undergo suicidal erythrocyte death or eryptosis following pathophysiological cell stressors such as hyperosmotic shock and energy depletion. The MSK1/2-deficient (msk−/−) mice did not exhibit overt anemia but showed marked increase in erythrocyte turnover that contributes to a mild increase in splenic mass. Moreover, the erythrocytes from msk−/− mice are more sensitive than erythrocytes from msk+/+ mice to triggers of eryptosis, including hyperosmotic shock and energy depletion. On the other hand, MSK1/2 deficiency decreases the resistance against hemolysis following decrease of extracellular osmolarity. Apparently, MSK1/2 deficiency increases the sensitivity of erythrocytes to both cell shrinkage and cell swelling.
Hyperosmotic shock and energy deletion trigger eryptosis only in a subset of the erythrocyte population, indicating that the circulating erythrocytes are not uniformly sensitive to those triggers of eryptosis. As a matter of fact, the susceptibility of circulating erythrocytes towards triggers of eryptosis increases with erythrocyte age39,40. On the other hand, evidence has been reported that newly formed erythrocytes are highly susceptible to suicidal death, a phenomenon called neocytolysis41,42,43. Along those lines, considerable diversity of lysophosphatidic acid (LPA) induced Ca2+ influx and phospatidylserine translocation was observed in seemingly morphologically homogeneous erythrocyte populations44. The Ca2+ response to LPA was virtually lacking in reticulocytes and still highly variable in old erythrocytes44.
Collectively, the present observations highlight the significance of MSK1/2 for erythrocyte survival. Phosphatidylserine-exposing cells are bound to macrophages45, engulfed and degraded46 and thus rapidly cleared from circulating blood36,37. Along those lines, msk−/− erythrocytes are cleared more rapidly from the circulation. The accelerated erythrocyte death and clearance from circulating blood is outweighed by compensatory increase of erythropoiesis in msk−/− mice, which is reflected by increased numbers of circulating reticulocytes in those mice.
Mechanistically, exposure of erythrocytes to hypertonic extracellular environment in vitro simulates the osmotic conditions encountered in the kidney medulla. Under pathological conditions such as acute renal failure, erythrocytes are trapped in the kidney medulla, thus predisposing erythrocytes to eryptosis33. It is, therefore, tempting to speculate that MSK1/2 influences erythrocyte survival and its ramifications in systemic conditions such as renal failure. The MSK1/2 upstream molecule p38 MAPK orchestrates adaptation to hypertonicity in mammalian cells47,48. In nucleated cells, hypertonic shock modulates cAMP response element-binding protein via activation of MSK1-dependent signaling49. In erythrocytes, a similar parallel can be drawn as hyperosmotic shock elicits phosphorylation of p38 MAPK that regulates the eryptosis machinery50. The msk−/− erythrocytes have further an enhanced sensitivity to the eryptotic effect of cellular energy deprivation, another powerful stimulator of eryptosis38. Signaling involved in the regulation of eryptosis following cellular energy depletion includes protein kinase C, AMP activate kinase and Janus kinase 319.
According to the present data MSK1/2 contributes to both osmo- and energy-sensitive regulation of erythrocyte survival. Without stimulation of eryptosis, the percentage of eryptotic cells is similar in msk−/− mice and in msk+/+ mice. The susceptibility of the erythrocytes from msk−/− mice to eryptosis is, however, apparent following osmotic shock and energy depletion. Eryptosis is enhanced by erythrocyte age, a wide variety of anemia-causing xenobiotics and endogenous substances19 and several clinical disorders, including iron deficiency, phosphate depletion, hepatic failure, dehydration, fever, Hemolytic Uremic Syndrome, end stage renal disease, sepsis, malaria, malignancy and Wilson’s disease19,51. Eryptosis may further influence erythrocyte storage for transfusion52. MSK1 deficiency may enhance the susceptibility to the eryptotic effect of those xenobiotics, endogenous substances and clinical disorders. In view of the accelerated clearance of erythrocytes and a mild splenomegaly in msk−/− mice, triggers of eryptosis are apparently operative in the blood of those mice.
Phosphatidylserine-exposing erythrocytes adhere to the vascular wall53,54,55,56,57 and to other erythrocytes58; they further stimulate blood clotting53,59,60. Thus, excessive eryptosis may compromise microcirculation. Along those lines, enhanced eryptosis has been suggested to participate in the vascular injury of metabolic syndrome61.
In conclusion, lack of MSK1/2 leads to enhanced susceptibility to suicidal erythrocyte death or eryptosis following osmotic shock and energy depletion leading to accelerated splenic trapping of circulating erythrocytes.
Materials and Methods
Human erythrocytes
Highly purified erythrocyte concentrates were provided by the blood bank of the University of Tübingen. The erythrocyte concentrates were virtually free of white blood cells and contained less than 1% platelets. The Committee approving the experiments, in name, is the ethics committee of the University of Tübingen, given report number: 184/2003V. Informed consent was obtained from all subjects.
Mice
Experiments were performed in 9- to 16-wk-old MSK1/2-deficient mice (msk−/−) as well as sex-and age matched wild-type mice (msk+/+) which were fed a control diet (C1314; Altromin, Heidenau, Germany) and had access to drinking water ad libitum. The msk−/− mice have been described previously15,18. The animals were maintained under specific pathogen-free conditions and all experiments described in the methods were carried out in accordance with the approved guidelines (American Physiological Society as well as the German law and the EU Animals Scientific Procedures Act for the welfare of animals) and were approved by local authorities of the state of Baden-Württemberg.
Blood count and isolation of murine erythrocytes
For all experiments except for the blood count, heparin blood was retrieved from the retrobulbar plexus of mice62. For the blood count, EDTA blood was analyzed using an electronic hematology particle counter (type MDM 905 from Medical Diagnostics Marx; Butzbach, Germany) equipped with a photometric unit for haemoglobin determination. To obtain pure erythrocytes, murine erythrocytes were separated utilizing Ficoll (Biochrom AG, Germany) and washed twice with Ringer solution containing (in mM): 125 NaCl, 5 KCl, 1 MgSO4 and 32 HEPES/NaOH (pH 7.4), 5 glucose and 1 CaCl2.
Reticulocyte count
For determination of the reticulocyte count EDTA-whole blood (5 μl) was added to 1 ml Retic-COUNT (Thiazole orange) reagent from Becton Dickinson. Samples were stained for 30 min at room temperature and flow cytometry was performed according to the manufacturer’s instructions. Forward scatter (FSC), side scatter (SSC) and Thiazole orange-fluorescence intensity (in FL-1) of the blood cells were determined. The number of Retic-COUNT positive reticulocytes was expressed as the percentage of the total gated erythrocyte populations. Gating of erythrocytes was achieved by analysis of FSC vs. SSC dot plots using CellQuest software.
Determination of the osmotic resistance
For measurement of osmotic resistance 2 μl erythrocyte pellets were exposed in a 96 well plate for 2 min to phosphate-buffered saline (PBS) solutions (in mM: 1.05 KH2PO4, 2.97 Na2HPO4, 155.2 NaCl) of decreasing osmolarity as prepared by mixing a PBS solution with a defined volume of distilled water. After centrifugation (500 g for 5 min), the Hb concentration of the supernatants was determined photometrically (at 405 nm).
Incubations and solutions
For in vitro analysis of eryptosis, erythrocytes were isolated by washing two times and subsequent incubation in vitro at a hematocrit of 0.4% in Ringer solution at 37 °C for the indicated time periods. Where indicated, glucose was removed or sucrose (550 mM) added to the Ringer solution.
Phosphatidylserine exposure and forward scatter
After incubation, erythrocytes were washed once in Ringer solution containing 5 mM CaCl2. The cells were then stained with annexin V-FITC (1:250 dilution; Immunotools, Friesoythe, Germany) at a 1:500 dilution. After 15 min, samples were measured by flow cytometric analysis (FACS-Calibur; BD). Cells were analyzed by forward scatter and annexin V-fluorescence intensity was measured with an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACS calibur (BD, Heidelberg, Germany).
Measurement of the in vivo clearance of fluorescence-labeled erythrocytes
The in vivo clearance of fluorescence-labeled erythrocytes was determined as described previously63. Briefly, erythrocytes (obtained from 200 μl blood) were fluorescence-labeled by staining the cells with 5 μM carboxyfluorescein-diacetate-succinimidyl-ester (CFSE) (Molecular Probes, Leiden, Netherlands) in PBS and incubated for 30 min at 37 °C. After washing twice in PBS containing 10% FCS the pellet was resuspended in Ringer solution (37 °C) and 100 μl of the CFSE-labelled erythrocytes (50% hematocrit) were injected into the tail vein of the recipient mouse. As indicated, blood was retrieved from the tail veins of the mice and CFSE-dependent fluorescence intensity of the erythrocytes was measured as described above. The percentage of CFSE-positive erythrocytes was calculated in % of the total labelled fraction determined 10 min after injection.
Confocal microscopy
For the detection of annexin V-binding and CFSE-dependent fluorescence of erythrocytes in the spleen, the spleens of msk−/− and msk+/+ mice were homogenized mechanically in 1 ml cold PBS. The suspension was then centrifuged at 500 g for 10 min at 4 °C. The cell pellet was resuspended in 200 μl cold PBS. Five μl of Annexin V-APC (BD, Heidelberg, Germany) were added and incubation was carried out for 20 min at 37 °C protected from light. Then, the suspension was transferred onto a glass slide and mounted with Prolong® Gold antifade reagent (Invitrogen). Images were taken on a Zeiss LSM 5 EXCITER Confocal Laser Scanning Microscope (Carl Zeiss MicroImaging GmbH, Germany) with a water immersion Plan-Neofluar 63/1.3 NA DIC.
Immunoblotting
To remove the haemoglobin, 200 μl erythrocyte pellet (1 × 109 cells) were haemolysed in 50 ml of 20 mM HEPES/NaOH (pH 7.4) containing 1 complete protease inhibitor cocktail (Roche). Ghost membranes were pelleted (20,000 g for 20 min at 4 °C) and lysed in 200 μl lysis buffer (125 mM NaCl, 25 mM HEPES/NaOH (pH 7.4), 10 mM Na2-EDTA, 10 mM NaF, 10 mM Na-pyrophosphate tetrabasic decahydrate, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid, 1% Triton X-100, 0.4% β-mercaptoethanol and 1 complete protease inhibitor cocktail. Lysed ghost membranes were solubilized in Laemmli sample buffer at 95 °C for 5 min and stored at −20 °C. The murine erythrocytes were washed after isolation from full blood by a single purification step with Ficoll and then lysed in the same lysis buffer as above.
For each lane, equal amounts of protein were loaded and resolved by 8–10% SDS-PAGE precast gel (Invitrogen). For immunoblotting, proteins were electrotransferred onto a PVDF membrane and blocked with 5% non-fat milk in TBS-0.1% Tween 20 (TBS-T) at room temperature for 1 h. The membrane was incubated with rabbit anti-MSK1 (C27B2; #3489) antibody (1:500; 90 kDa) (Cell signaling, USA) or rabbit anti-MSK2 (NBP2-30079) antibody (1:1000; 86 kDa, Novus Biological, USA) or 1:1000 anti-GAPDH antibody (1:1000; 37 kDa, Cell Signaling) at 4 °C overnight in 5% BSA. After washing with TBS-T the blots were incubated with secondary anti-rabbit antibody (1:2000; Cell Signaling) for 1 h at room temperature. After washing, antibody binding was detected with the ECL detection reagent (Life technologies, Germany).
Statistics
Data are expressed as arithmetic means ± SEM and statistical analysis was made using ANOVA or t-test, as appropriate. n denotes the number of different erythrocyte specimens studied.
Additional Information
How to cite this article: Lang, E. et al. Accelerated apoptotic death and in vivo turnover of erythrocytes in mice lacking functional mitogen- and stress-activated kinase MSK1/2. Sci. Rep. 5, 17316; doi: 10.1038/srep17316 (2015).
References
Dumka, D. et al. Activation of the p38 Map kinase pathway is essential for the antileukemic effects of dasatinib. Leuk. Lymphoma 50, 2017–2029 (2009).
Joo, J. H. & Jetten, A. M. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 287, 123–135 (2010).
Kannan-Thulasiraman, P., Katsoulidis, E., Tallman, M. S., Arthur, J. S. & Platanias, L. C. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J. Biol. Chem. 281, 22446–22452 (2006).
Mu, M. M. et al. A role of mitogen and stress-activated protein kinase 1/2 in survival of lipopolysaccharide-stimulated RAW 264.7 macrophages. FEMS Immunol. Med. Microbiol. 43, 277–286 (2005).
Odgerel, T. et al. MSK1 activation in acute myeloid leukemia cells with FLT3 mutations. Leukemia 24, 1087–1090 (2010).
Healy, S., Khan, P., He, S. & Davie, J. R. Histone H3 phosphorylation, immediate-early gene expression and the nucleosomal response: a historical perspective. Biochem. Cell Biol. 90, 39–54 (2012).
Moens, U. & Kostenko, S. Structure and function of MK5/PRAK: the loner among the mitogen-activated protein kinase-activated protein kinases. Biol. Chem. 394, 1115–1132 (2013).
Aggeli, I. K., Beis, I. & Gaitanaki, C. Oxidative stress and calpain inhibition induce alpha B-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell Signal. 20, 1292–1302 (2008).
Dunn, K. L., Espino, P. S., Drobic, B., He, S. & Davie, J. R. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell Biol. 83, 1–14 (2005).
Saldeen, J., Lee, J. C. & Welsh, N. Role of p38 mitogen-activated protein kinase (p38 MAPK) in cytokine-induced rat islet cell apoptosis. Biochem. Pharmacol. 61, 1561–1569 (2001).
Wiggin, G. R. et al. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 22, 2871–2881 (2002).
Kim, Y. H., Lee, D. H., Jeong, J. H., Guo, Z. S. & Lee, Y. J. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 75, 1946–1958 (2008).
Koh, H. S. et al. CD7 expression and galectin-1-induced apoptosis of immature thymocytes are directly regulated by NF-kappaB upon T-cell activation. Biochem. Biophys. Res. Commun. 370, 149–153 (2008).
Staples, C. J., Owens, D. M., Maier, J. V., Cato, A. C. & Keyse, S. M. Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J. Biol. Chem. 285, 25928–25940 (2010).
Ananieva, O. et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 9, 1028–1036 (2008).
El Mchichi, B., Hadji, A., Vazquez, A. & Leca, G. p38 MAPK and MSK1 mediate caspase-8 activation in manganese-induced mitochondria-dependent cell death. Cell Death Differ. 14, 1826–1836 (2007).
She, Q. B., Ma, W. Y., Zhong, S. & Dong, Z. Activation of JNK1, RSK2 and MSK1 is involved in serine 112 phosphorylation of Bad by ultraviolet B radiation. J. Biol. Chem. 277, 24039–24048 (2002).
MacKenzie, K. F. et al. MSK1 and MSK2 inhibit lipopolysaccharide-induced prostaglandin production via an interleukin-10 feedback loop. Mol. Cell. Biol. 33, 1456–1467 (2013).
Lang, F., Abed, M., Lang, E. & Foller, M. Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 21, 138–153 (2014).
Bernhardt, I., Weiss, E., Robinson, H. C., Wilkins, R. & Bennekou, P. Differential effect of HOE642 on two separate monovalent cation transporters in the human red cell membrane. Cell. Physiol. Biochem. 20, 601–606 (2007).
Duranton, C., Huber, S. M. & Lang, F. Oxidation induces a Cl(-)-dependent cation conductance in human red blood cells. J. Physiol. 539, 847–855 (2002).
Duranton, C. et al. Electrophysiological properties of the Plasmodium Falciparum-induced cation conductance of human erythrocytes. Cell. Physiol. Biochem. 13, 189–198 (2003).
Huber, S. M., Gamper, N. & Lang, F. Chloride conductance and volume-regulatory nonselective cation conductance in human red blood cell ghosts. Pflugers Arch. 441, 551–558 (2001).
Kaestner, L., Christophersen, P., Bernhardt, I. & Bennekou, P. The non-selective voltage-activated cation channel in the human red blood cell membrane: reconciliation between two conflicting reports and further characterisation. Bioelectrochemistry 52, 117–125 (2000).
Kaestner, L. & Bernhardt, I. Ion channels in the human red blood cell membrane: their further investigation and physiological relevance. Bioelectrochemistry 55, 71–74 (2002).
Lang, K. S. et al. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 10(2), 249–256 (2003).
Lang, P. A. et al. PGE(2) in the regulation of programmed erythrocyte death. Cell Death Differ. 12, 415–428 (2005).
Lang, P. A. et al. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell Physiol. 285, C1553–C1560 (2003).
Berg, C. P. et al. Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ. 8, 1197–1206 (2001).
Brand, V. B. et al. Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell. Physiol. Biochem. 13, 347–356 (2003).
Bratosin, D. et al. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 8, 1143–1156 (2001).
Daugas, E., Cande, C. & Kroemer, G. Erythrocytes: death of a mummy. Cell Death Differ. 8, 1131–1133 (2001).
Lang, K. S. et al. Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ. 11, 231–243 (2004).
Foller, M. et al. Anemia and splenomegaly in cGKI-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 105, 6771–6776 (2008).
Foller, M. et al. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 23, 1072–1080 (2009).
Kempe, D. S. et al. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 20, 368–370 (2006).
Dinkla, S. et al. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis. 3, e410 (2012).
Klarl, B. A. et al. Protein kinase C mediates erythrocyte “programmed cell death” following glucose depletion. Am. J. Physiol. Cell Physiol. 290, C244–C253 (2006).
Ghashghaeinia, M. et al. The impact of erythrocyte age on eryptosis. Br. J. Haematol. 157, 606–614 (2012).
Ghashghaeinia, M. et al. Age Sensitivity of NFkappaB Abundance and Programmed Cell Death in Erythrocytes Induced by NFkappaB Inhibitors. Cell. Physiol. Biochem. 32, 801–813 (2013).
Kaestner, L. & Bogdanova, A. Regulation of red cell life-span, erythropoiesis, senescence and clearance. Front. Physiol. 5, 269 (2014).
Rice, L. & Alfrey, C. P. Modulation of red cell mass by neocytolysis in space and on Earth. Pflugers Arch. 441, R91–94 (2000).
Risso, A., Ciana, A., Achilli, C. & Minetti, G. Survival and senescence of human young red cells in vitro. Cell. Physiol. Biochem. 34, 1038–1049 (2014).
Wang, J. et al. Morphologically homogeneous red blood cells present a heterogeneous response to hormonal stimulation. PLoS One 8, e67697 (2013).
Fadok, V. A. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 (2000).
Boas, F. E., Forman, L. & Beutler, E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc. Natl. Acad. Sci. USA. 95, 3077–3081 (1998).
Sheikh-Hamad, D. & Gustin, M. C. MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am. J. Physiol. Renal Physiol. 287, F1102–1110 (2004).
Arsenijevic, T. et al. Hyperosmotic stress induces cell cycle arrest in retinal pigmented epithelial cells. Cell Death Dis. 4, e662 (2013).
Gorbatenko, A. et al. Hyperosmotic stress strongly potentiates serum response factor (SRF)-dependent transcriptional activity in Ehrlich Lettre Ascites cells through a mechanism involving p38 mitogen-activated protein kinase. J. Cell. Physiol. 226, 2857–2868 (2011).
Gatidis, S. et al. p38 MAPK activation and function following osmotic shock of erythrocytes. Cell. Physiol. Biochem. 28, 1279–1286 (2011).
Lang, E. et al. Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 61, 275–284 (2015).
Kriebardis, A. G. et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J. Cell. Mol. Med. 11, 148–155 (2007).
Andrews, D. A. & Low, P. S. Role of red blood cells in thrombosis. Curr. Opin. Hematol. 6, 76–82 (1999).
Closse, C., Dachary-Prigent, J. & Boisseau, M. R. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br. J. Haematol. 107, 300–302 (1999).
Gallagher, P. G. et al. Altered erythrocyte endothelial adherence and membrane phospholipid asymmetry in hereditary hydrocytosis. Blood 101, 4625–4627 (2003).
Pandolfi, A. et al. Mechanisms of uremic erythrocyte-induced adhesion of human monocytes to cultured endothelial cells. J. Cell. Physiol. 213, 699–709 (2007).
Wood, B. L., Gibson, D. F. & Tait, J. F. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood 88, 1873–1880 (1996).
Steffen, P. et al. Stimulation of human red blood cells leads to Ca2+-mediated intercellular adhesion. Cell Calcium 50, 54–61 (2011).
Chung, S. M. et al. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler. Thromb. Vasc. Biol. 27, 414–421 (2007).
Zwaal, R. F., Comfurius, P. & Bevers, E. M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 62, 971–988 (2005).
Zappulla, D. Environmental stress, erythrocyte dysfunctions, inflammation and the metabolic syndrome: adaptations to CO2 increases? J. Cardiometab. Syndr. 3, 30–34 (2008).
Feger, M., Mia, S., Pakladok, T., Nicolay, J. P., Alesutan, I., Schneider, S. W., Voelkl, J. & Lang, F. Down-regulation of renal klotho expression by Shiga toxin 2. Kidney Blood Press Res 39(5), 441–449 (2014).
Lang, P. A. et al. Accelerated clearance of Plasmodium-infected erythrocytes in sickle cell trait and annexin-A7 deficiency. Cell. Physiol. Biochem. 24, 415–428 (2009).
Acknowledgements
The authors are grateful to Tanja Loch and Sari Rübe for the meticulous preparation of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (Nr. La 315/13-3).
Author information
Authors and Affiliations
Contributions
F.L. and S.M.Q. designed the project and wrote the main manuscript text, E.L., R.B., A.F., M.S.S., Y.S., C.Z., M.G., S.G., A.L., K.J., K.M.R., T.F.A., M.F., E.S., W.P.S., J.S.C.A. and S.M.Q. performed the acquisition, analysis and/or interpretation of data. R.B., A.F., M.S.S., Y.S., C.Z. and S.M.Q. prepared the figures and all authors read and reviewed the manuscript and approved the final version.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lang, E., Bissinger, R., Fajol, A. et al. Accelerated apoptotic death and in vivo turnover of erythrocytes in mice lacking functional mitogen- and stress-activated kinase MSK1/2. Sci Rep 5, 17316 (2015). https://doi.org/10.1038/srep17316
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17316
This article is cited by
-
Association between vitamin D status and eryptosis–results from the German National Cohort Study
Annals of Hematology (2023)
-
Casein kinase 1α mediates eryptosis: a review
Apoptosis (2023)
-
Inhibition of MSK1 Promotes Inflammation and Apoptosis and Inhibits Functional Recovery After Spinal Cord Injury
Journal of Molecular Neuroscience (2019)
-
Genetic deficiency of the tumor suppressor protein p53 influences erythrocyte survival
Apoptosis (2018)
-
Blunted apoptosis of erythrocytes in mice deficient in the heterotrimeric G-protein subunit Gαi2
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.