Abstract
VEGF-A, an angiogenic factor, is increased in the peritoneal fluid of women with endometriosis. The cytokine TGF-β1 is thought to play a role in the establishment of endometriosis lesions. Inhibitor of DNA binding (ID) proteins are transcriptional targets of TGF-β1 and ID1 has been implicated in VEGF-A regulation during tumor angiogenesis. Herein, we determined whether peritoneal expression of VEGF-A is regulated by TGF-β1 through the ID1 pathway in women with endometriosis. VEGF-A was measured in peritoneal fluid by ELISA (n = 16). VEGF-A and ID1 expression was examined in peritoneal biopsies (n = 13) and primary peritoneal and immortalized mesothelial cells (MeT5A) by immunohistochemistry, qRT-PCR and ELISA. VEGF-A was increased in peritoneal fluid from women with endometriosis and levels correlated with TGF-β1 concentrations (P < 0.05). VEGF-A was immunolocalized to peritoneal mesothelium and TGF-β1 increased VEGFA mRNA (P < 0.05) and protein (P < 0.05) in mesothelial cells. ID1 was increased in peritoneum from women with endometriosis and TGF-β1 increased concentrations of ID1 mRNA (P < 0.05) in mesothelial cells. VEGF-A regulation through ID1 was confirmed by siRNA in MeT5A cells (P < 0.05). Our data supports role for ID1 in the pathophysiology of endometriosis, as an effector of TGFβ1 dependent upregulation of VEGF-A and highlights a novel potential therapeutic target.
Similar content being viewed by others
Introduction
Endometriosis is a hormone-dependent benign disorder characterized by the presence of ectopic endometrial tissue commonly found on the pelvic peritoneum1,2. It is estimated to effect between 2–10% of women of reproductive age and it is associated with chronic pelvic pain and infertility1. Endometriosis is currently managed surgically or medically, however lesions reoccur in up to 75% of surgical cases and medical treatments have undesirable side effects3. The etiology of endometriosis is unclear. To date, the majority of research has centred on changes within the eutopic and ectopic endometrium of women with endometriosis, but there is now increasing evidence that the peritoneal mesothelial cells may contribute to the development and maintenance of endometriosis lesions4.
Angiogenesis is a crucial step in the development of endometriosis lesions. At a macroscopic level, lesions have been shown to be highly vascularized with new vessels developing from the surrounding peritoneum5. Vascular endothelial growth factor-A (VEGF-A), a potent angiogenic factor, is known to be increased in the peritoneal fluid of women with endometriosis compared to women without disease6,7. Levels correlate significantly with the stage of disease and appear to be hormonally regulated8. Reported sources of VEGF-A include ectopic endometrium and peritoneal macrophages9,10.
The largest cell population within the peritoneum, however, is peritoneal mesothelial cells and these cells intimately interact with the ectopic endometrium during the establishment of endometriotic lesions4. TGF-β1 is an established regulator of VEGF-A expression in several cell types and this pathway has been implicated in neoangiogenesis of several cancers11. Aberrant TGF-β1 signaling plays a critical role in the development of endometriosis lesions, which shares several parallels with tumorigenesis. Several studies have shown that TGF-β1 is increased in the peritoneal fluid, peritoneum and ectopic endometrium of women with endometriosis12,13,14,15, suggesting that the same over production of TGF-β1 that is seen in tumours and the surrounding stroma is also true for endometriosis lesions and the surrounding peritoneum. Furthermore, the importance of local TGF-β1 action is highlighted by changes in the expression of TGF-β signaling targets in the peritoneum adjacent to endometriosis lesions12,13,14,15.
One TGF-β-signaling target linked to the transcriptional regulation of angiogenesis is inhibitor of DNA binding protein 1 (ID1). ID1 is overexpressed in over 20 types of human cancers16 and we have recently shown that it is expressed in the peritoneum of women with endometriosis and regulated by TGF-β112. ID1 has recently been described as an oncogene and much of this evidence is based on ID1 regulation of VEGF-A, with an overexpression of ID1 leading to increases in VEGFA gene transcription and hence angiogenesis17,18. The role of ID1 in angiogenesis is further backed up with evidence that tumours failed to grow and/or metastasise in ID1 +/−; ID3 −/− mice due to poor vascularisation19. We hypothesized that the peritoneal mesothelium is a source of VEGF-A in endometriosis and that TGF-β1 regulates the expression of VEGF-A in the peritoneal mesothelial cell through the ID1 pathway, supporting lesion vascularization. Herein, we investigate the expression of VEGF-A in the peritoneal mesothelium and determine if it is regulated by TGF-β1 through an ID1 pathway in women with endometriosis.
Results
Increased concentrations of VEGF-A in the peritoneal fluid of women with endometriosis correlate with TGF-β1 concentrations
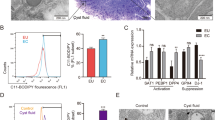
VEGF-A concentrations are increased in the peritoneal fluid of women with endometriosis compared to women without endometriosis (P < 0.05; Fig. 1A). We have reported in our previous studies that TGF-β1 concentration was significantly increased in the peritoneal fluid of women with endometriosis compared to women without endometriosis13. There was a significant positive correlation between the concentrations of VEGF-A and those of TGF-β1 in the peritoneal fluid from women with and without endometriosis (R = 0.39, P < 0.05; Fig. 1B). Immunohistochemistry shows VEGF-A to be localised to the peritoneal mesothelial cells of women with and without endometriosis (Fig. 1C).
VEGF-A protein concentrations are increased in peritoneal fluid from women with endometriosis compared to women without (A) and levels of VEGF-A positively correlate with TGF-β1 concentrations (B) (*p < 0.05 unpaired t-test, n = 8 each group). Immunohistochemistry of paraffin-embedded sections shows presence and localization of VEGF-A in peritoneal mesothelial cells of women with and without endometriosis, arrows indicate the peritoneal mesothelial cells (C). Endometrial tissue was used as positive control and no staining was observed in the isotype match control (n = 3 each group).
TGF-β1 regulates VEGF-A expression in peritoneal mesothelial cells
To address the question of whether TGF-β1 regulates VEGF-A expression in peritoneal mesothelial cells, we exposed HPMC and MeT-5A cells to physiological concentrations of TGF-β1 (2 ng/ml). TGF-β1 increased VEGFA mRNA expression (P < 0.05; Fig. 2A) and extracellular VEGF-A protein concentrations in HPMC at 12 hours (P < 0.05; Fig. 2B). In addition we confirmed and extended data from the HPMC by demonstrating TGF-β1 regulates VEGFA mRNA expression and VEGF-A protein secretion in the MeT-5A cell line (P < 0.05; Fig. 2C,D).
Effect of TGF-β1 on VEGF-A mRNA and protein expression in HPMC and MeT-5A cells.
TGF-β1 increased VEGFA mRNA (A) and VEGF-A protein expression (B) in the HPMC obtained from women with endometriosis at 12 hours (*p < 0.05 paired t-test, n = 6). TGF-β1 increased VEGFA mRNA at 12 and 24 hours (C) and significantly increased VEGF-A secretion into the extracellular environment in MeT-5A cells at 24 hours (D) (*p < 0.05 unpaired t-test, **p < 0.01 unpaired t-test, n = 3 each group).
TGF-β1 target ID1 has a peritoneal localization and its expression is increased in the peritoneum of women with endometriosis
The transcriptional regulatory protein ID1 is a known target of TGF-β1 and we found ID1 protein to be localized to the mesothelial, stromal and endothelial cells of the peritoneum (Fig. 3A). To investigate if ID1 is differentially expressed in the peritoneum of women with endometriosis, ID1 expression was quantified by RT-PCR in peritoneal biopsies from women with and without endometriosis. ID1 expression was increased in the peritoneum of women with endometriosis (P < 0.05; Fig. 3B).
Immunohistochemistry shows presence and localization of ID1 in mesothelial and endothelial cells of the peritoneum of women with and without endometriosis (A). Endometrial tissue was used as positive control and no staining was observed in the isotype match control (n = 3 each group). Peritoneum from women with endometriosis expressed significantly higher levels of ID1 mRNA when compared to women without endometriosis (B) (**p < 0.01 unpaired t-test, n = 8 no endometriosis, n = 3 endometriosis).
TGF-β1 increases ID1 expression in peritoneal cells and regulates VEGF-A expression through ID1
We next assessed the effects of TGF-β1 on ID1 expression in HPMC and MeT-5A cells. Exposure of HPMC to physiological levels of TGF-β1 for 12 hours increased ID1 expression (P < 0.05; Fig. 4A). Similarly, exposure of MeT-5A cells to TGF-β1 caused a rapid and sustained increase in ID1 mRNA expression (P < 0.05-P < 0.01; Fig. 4B).
Effect of TGF-β1 treatment on ID mRNA expression in HPMC obtained from women with endometriosis and MeT-5A cells.
Cells were treated with 2ng/ml TGF-β1 for between 3 and 24 hours. TGF-β1 up-regulated ID1 mRNA expression in HPMC at 12 hours (A) (*p < 0.05 paired t-test, n = 6 each group). TGF-β1 also increased ID1 mRNA expression in the MET-5A at all time points studied (B) (*p < 0.05 unpaired t-test, **p < 0.01 unpaired t-test, n = 3 each group).
To determine if the molecular regulation of VEGF-A by TGF-β1 is mediated via the ID1 pathway, siRNA was used to knock down ID1 expression in MeT-5A cells. ID1 siRNA significantly decreased TGF-β1-induced VEGFA mRNA expression (P < 0.001) and VEGF-A secretion (P < 0.01) in MeT-5A cells (Fig. 5A,B). Moreover, TGF-beta1 could not up-regulate ID1 after siRNA knockdown of ID1 as compared to the scrambled siRNA treated controls (P < 0.001; Fig. 5C), which supports successful siRNA knockdown of ID1. These data suggest that the regulation of VEGF-A by physiological concentrations of TGF-β1 is ID1-dependent.
Knockdown of ID1 in MeT-5A cells using siRNA in the presence and absence of TGF-β1.
ID1 siRNA significantly reduced VEGFA mRNA expression (A) and reduced VEGF-A protein secretion (B) in TGF-β1 treated MeT-5A cells after ID1 knockdown. TGF-beta1 could not up regulate ID1 (C). (*p < 0.05 unpaired t-test, ***p < 0.001 unpaired t-test, n = 3 each group).
Discussion
Herein, we demonstrate that human peritoneal mesothelial cells are a source of the increased VEGF-A known to be found within the peritoneal fluid of women with endometriosis. We also show that concentrations of VEGF-A positively correlate with levels of TGF-β1 within the peritoneal fluid and that physiological concentrations of TGF-β1 significantly increase the expression of VEGFA mRNA and VEGF-A protein from peritoneal mesothelial cells. In addition, we show that ID1 mRNA expression is increased in peritoneal biopsies from women with endometriosis compared to women without disease and that ID1 expression is increased in peritoneal mesothelial cells on exposure to physiological concentrations of TGF-β1. Knockdown of ID1 confirms that it is an intermediary molecule involved in TGF-β1 regulation of VEGFA expression and VEGF-A secretion.
Our observation that peritoneal fluid concentrations of VEGF-A are significantly increased in women with endometriosis, compared to women without endometriosis, is in agreement with previous reports7,9. In this study, we have extended these findings to show that VEGF-A concentrations positively correlate with levels of TGF-β1 in the peritoneal fluid, suggesting that TGF-β1 may regulate VEGF-A expression in the peritoneum. TGF-β is a known regulator of VEGF-A expression during tumorigenesis and we and others have previously shown TGF-β1 to be significantly increased in the peritoneal fluid of women with endometriosis13.
We have shown that the peritoneal mesothelium is a source of VEGF-A protein. As the peritoneal mesothelial cells are the largest cell fraction within the peritoneum20, it is likely that these cells contribute to the increasing concentrations of VEGF-A within the peritoneal fluid of women with endometriosis described above. Peritoneal mesothelial cells are known to secrete VEGF-A into the extracellular environment in trans differentiation and tumorigenesis where overexpression has been attributed to increased peritoneal fluid concentrations of TGF-β121. Furthermore, macroscopic examination of peritoneal endometriosis lesions, has shown that lesions are highly vascularized and that blood vessels are derived from the surrounding peritoneal tissue, suggesting that expression of VEGF-A in the peritoneum adjacent to endometriosis lesions may play a direct role in neoangiogenesis of endometriosis lesions22. HPMC and MeT-5A cells exposed to physiological concentrations of TGF-β1 expressed significantly higher levels of VEGFA mRNA transcripts and secreted significantly higher levels of VEGF-A protein, confirming that that peritoneal mesothelium may be a potential a source of increased VEGF-A levels in the peritoneal fluid of women with endometriosis. We believe this may in part explain the induction of neoangiogensis that is observed in the peritoneal tissue surrounding endometriosis lesions22.
The IDs are basic helix-loop-helix transcription factors that are transcriptional targets of the TGF-β signaling pathway involved in the regulation of cell differentiation, proliferation and angiogenesis23. Overexpression of TGF-β during tumorigenesis has been implicated in the dysregulation of IDs that leads to aberrant cell proliferation, epithelial-mesenchymal transition and neoangiogenesis24. In epithelial cells, TGF-β signaling through the Smad 2/3 pathway classically inhibits expression of ID genes by activating transcriptional repressor ATF3 which in turns binds to the ATF/CREB site within the ID promoter suppressing transcription25. However, TGF-β induced over expression of ID1 has been reported in at least one epithelial cell line and in several cancers26. Although the mechanisms for this largely remain elusive, one study has shown Smad3 but not Smad2 may be responsible for TGF-β induced ID1 overexpression27.
We have previously found ID1 to be increased in the peritoneum of women with endometriosis using a TGF-β signaling targets gene array12. Increased concentrations of TGF-β1 in the peritoneal fluid and peritoneum of women with endometriosis may be responsible for the increased ID1 expression in the peritoneum of women with endometriosis12,13. We demonstrated that physiological levels of TGF-β1 significantly increase ID1 expression in the HPMC and MeT-5A cells. This increase is consistent with a cancerous phenotype as ID1 is reported to be overexpressed in over 20 types of human cancers and ID1 overexpression is associated with poor clinical outcomes in patients with breast, cervical and endometrial carcinomas24. As the pathophysiology of endometriosis shares several parallels with tumor onset and progression, TGF-β1 dysregulation of IDs may play an important role in the development of endometriosis lesions. However, further work is needed to confirm that this is the dominant pathway in-vivo explaining elevated levels of VEGF-A in women with endometriosis because VEGF-A expression has been shown to be regulated through several different mechanisms in cancer biology5,9,10.
Importantly, we have demonstrated that TGF-β1 increases VEGFA expression and VEGF-A secretion through the ID1 pathway in a similar mechanism to that reported in several cancers28. IDs are known regulators of VEGF-A expression and a loss of ID function has been shown to lead to a decrease in VEGF-A expression28. ID1+/− ID3−/− mice fail to grow tumors due to little or no vascularisation of tumors and blood vessels in these mice fail to undergo neoangiogenesis19. As endometriosis lesions result from ectopic tissue implanting and proliferating in a similar fashion to cancer metastasis, the IDs may also play a crucial role in the development of endometriosis lesions.
Endometriosis is associated with chronic inflammation and there is accumulating evidence that key inflammatory factors play an important role in the pathophysiology of this disease4. Several of these factors may also play a role in this TGF-β1-ID1-VEGF-A pathway described in this paper. Hypoxia Inducible Factor 1-α (HIF-1α) is a transcription factor known to regulate VEGF expression and several studies have shown ID regulation of VEGF-A to be through HIF-1α29. We have previously shown HIF-1α to be increased in endometriosis lesions and the surrounding peritoneum13 and therefor it is possible that HIF-1α also plays a key role in TGF-β1 regulated VEGF-A expression. Other inflammatory mediators such as IL-1β, IL-6 and I-CAM1 have been reported to be overexpressed in the peritoneum and are associated with increased VEGF expression and hence neovascularisation in endometriosis4. Understanding the role of these and other inflammatory mediators in this pathway may provide a greater understanding of the pathophysiology of this disease.
In conclusion, this study demonstrates a functional role for ID1 in the peritoneum of women with endometriosis through the overexpression of VEGF-A to potentially increase neoangiogenesis at sites of endometriosis lesions. Blocking the expression of ID1 has been shown to decrease VEGF-A expression and hence angiogenesis during tumorigenesis and ID inhibitors are being explored as novel therapies for cancers. Thus, ID inhibitors may also be beneficial in the treatment of endometriosis24.
Methods
Subjects
Ethical approval for this study was obtained from the Lothian Research Ethics Committee (LREC 11/AL/0376). Informed written consent obtained from all patients and all of the methods were carried out in accordance with the approved guidelines. All women included in this study had regular 21–35 day menstrual cycles and none were taking hormonal medication at the time of surgery. All samples used within this study were from the luteal phase of the menstrual cycle which was confirmed by staining the endometrial biopsies with hematoxylin and eosin. Noyes’ criteria was used to determine the cycle phase. In addition, serum levels of progesterone and estradiol further confirmed the cycle phase. All women underwent laparoscopic surgery for the investigation of chronic pelvic pain and peritoneal fluid, primary human peritoneal mesothelial cells (HPMC), peritoneal biopsies, endometrial biopsies were collected at the start of surgery. There were no fundamental differences in the demographics, including; age, BMI, smoking status and presence of other pathologies of the women included within this study.
The women with endometriosis had macroscopic evidence of disease at laparoscopy and this was later confirmed by histology. The women without endometriosis displayed no evidence of endometriosis at laparoscopy and there was no evidence of other underlying pelvic pathology to explain their painful symptoms (e.g. adhesions). Peritoneal fluid (5–10 ml) was collected from women with (n = 8) and without (n = 8) endometriosis and stored in cryovials at −80 °C for later analysis. Primary human peritoneal mesothelial cells (HPMC) were isolated at the time of surgery by gentle brushing the pelvic mesothelium with a TaoTM brush followed by vigorously agitating in 15 ml of serum-containing culture media to dislodge cells, as previously described30.
In women with endometriosis, we collected peritoneal biopsies from peritoneum adjacent to endometriosis lesions (2–3 cm from lesion) (n = 3). In women without endometriosis, we collected peritoneal biopsies (0.5 cm diameter) from the Pouch of Douglas (n = 8). After collection, biopsies were divided into two portions with half stored in RNAlater at 4 °C for 24hrs before storage at −80 °C and half fixed in 4% neutral-buffered formalin (NBF) for 24hrs at 4 °C before storing in 70% ethanol prior to embedding in paraffin wax. All peritoneal biopsies collected were studied histologically to confirm the absence of endometriosis. All tissues were collected according to the Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) guidelines31.
Establishment of cell culture
Brushings of HPMC were collected from the pelvic brim in women with and without endometriosis at the beginning of surgery as previously described29, by gentle scraping of the pelvic mesothelium (away from the endometriosis lesion in the women with disease) with a TaoTM brush at the pelvic brim (QC Sciences, Virginia, USA). Brushes were vigorously agitated in 15 ml of serum-containing HOSE1 culture media to dislodge cells before transferring to a 75 cm2 culture flask and incubated at 37 °C under 5% CO2 in air (QC Sciences, Virginia, USA). HPMC were cultured as previously described in HOSE1 media containing; 40% media 199, 40% MCDB 105 and supplemented with 15% FBS, 0.5% penicillin/streptomycin and 1% L-glutamine, at 37 °C under 5% CO2 in air (Life Technologies Inc., Paisley UK and Sigma Chemical Co., Poole UK)30.
The mesothelial cell line, MeT-5A (CRL-9444, ATCC, Middlesex UK), was originally established by transfecting normal human mesothelial cells from the pleural cavity with a plasmid containing Simian virus (SV40) early region DNA and they express SV40 large T antigen (ECACC, Cambridge, UK). These cells are increasingly used in peritoneal mesothelial cell research and data obtained with MeT-5A cells are thought to be analogous to data obtained with HPMC20. The MeT-5A cells were cultured in Iscove’s Modified Dulbecco’s Media (IMDM) ((Life Technologies Inc.) supplemented with 10% FBS and 1% L-glutamine at 37 °C under 5% CO2 in air.
Experimental treatments of HPMC and MeT-5A cells
HPMC cells were plated at 1.5 × 105 cells/ml, in a 12 well plate with a minimum of five technical replicates per experimental protocol. MeT-5A cells were plated at 2 × 105 cells/ml, in a 12 well plate, with a minimum of three technical replicates per experimental protocol. Cells were left to adhere for 12 hours before being serum starved for 24 hours. Cells were exposed to physiological levels of recombinant human TGF-β1 (2 ng/ml). As HPMC are known to produce TGF-β ligands, control cell cultures were exposed to a TGF-β neutralising antibody (0.5 μg/ml) for between 3hr and 48hr.
Experimental treatments of MeT-5A cells with siRNA
MeT-5A cells were plated at 3 × 105 cells/well in a six well culture plate with ID1 siRNA or scrambled siRNA (Table 1) using the neofection transfection method. Two different siRNA sequences were used for optimal knockdown of selected genes of interest (Table 1). Cells were incubated for a total of 48 hours. Physiological concentrations of recombinant human TGF-β1 (2 ng/ml) or TGF-β receptor I small molecule inhibitor (10 μg/ml), SB 431542, were added to cultures 24 hours before the end of the siRNA incubation period. Knockdown of ID1 was performed both in the absence and presence of TGF-β1 (2 ng/ml). Successful transfection conditions were developed using positive control GAPDH siRNA where reduced gene expression was confirmed at the mRNA level by qRT-PCR, at the protein level by Western blotting and cytotoxicity was confirmed to be less than 15% using a lactate dehydrogenase assay (Source Bioscience, Nottingham, UK). Successful ID1 knockdown was confirmed at the mRNA level by qRT-PCR.
VEGF-A ELISA
VEGF-A ELISA was performed using the Human VEGF-A (DY293B) ELISA Duo set according to manufacturers instructions (R&D systems, Abingdon UK). ELISA plates were read using Lab Systems Multiscan EX Microplate reader at 450 nm with wavelength correction at 540 nm. Samples were quantified using standard curve analysis within the linear range of 16 pg/ml to 2000 pg/ml. Intra-assay CV was 2.5% and the between batch CV was 8.3% for cell culture supernatants and intra-assay CV was 1.9% and the between batch CV is 9.3% for peritoneal fluid.
TGF-β1 ELISA
TGF-β1 ELISA was performed using the Human TGF-b1 Quantikine kit (DB100B) according to manufacturers instructions (R&D systems, Abingdon UK). Peritoneal fluid and cell culture supernatant samples were assayed for active and total TGF-β1. For complete levels, samples were activated to the immunoreactive form by addition of 1 M HCL for 10 mins before neutralising with 1.2 M NaHO/0.5 M HEPES buffer. All peritoneal fluid complete samples were further diluted 1:2 in calibrator dilutant before addition to the pre-coated ELISA plate, all cell culture and active peritoneal fluid samples were added neat. Standards were prepared and added to ELISA plates before incubated for 2 hours at room temperature with shaking. Plates were washed 4 times in wash buffer and TGF-β1 conjugate antibody added and plates incubated for 2 hours at room temperature with shaking. Plates were washed 4 times in wash buffer before addition of the streptavidin-HRP and incubation for 30 minutes at room temperature with shaking and protection from light. Stop solution was added and ELISA plates were read using Lab Systems Multiscan EX Microplate reader at 450 nm with wavelength correction at 540 nm. Samples were quantified using standard curve analysis within the linear range of 2000 pg/ml to 16 pg/ml. Intra-assay CV is 2.5% and the between batch CV is 8.3% for cell culture supernatants and intra-assay CV is 1.9% and the between batch CV is 9.3% for peritoneal fluid (based upon serum).
Transcript analysis
RNA was extracted using the RNeasy Mini kit with on-column DNaseI digestion according to the manufacturer’s instructions (Qiagen, West Sussex, UK). First-strand cDNA synthesis was performed using Superscript VILO Master Mix according to the manufacturer’s instructions (Life Technologies). Quantitative (q)RT-PCR reactions were performed on an ABI Prism 7900 Fast system using brilliant III ultra-fast SYBR green QPCR master mix with standard running conditions. Pre-validated primers were used throughout this study and melt curves were analysed to confirm specific products (Primerdesign, Southampton, UK). Messenger RNA transcripts were quantified relative to the appropriate housekeeping gene GAPDH as determined by geNorm assay (Primerdesign) and using the 2−ΔCt or the 2−ΔΔCt method.
Immunostaining
Sections of paraffin embedded tissue were mounted onto microscope slides and dewaxed and rehydrated before antigen retrieval in 10 mM Tris 1 mM EDTA pH 9 with 5 min of pressure-cooking. Slides were washed before incubation with 3% hydrogen peroxide for 30 min followed by blocking in normal horse serum diluted 1:12 in Tris buffered saline with 0.5% Tween 20 (TBST20) for 30 min. Slides were incubated with primary antibody overnight at 4 °C (ID1 Santa Cruz sc-488 diluted 1:1000, VEGF-A Santa Cruz sc-507 diluted 1:100 or isotype match control Rabbit IgG Dako X0903) and then washed in TBST20 before incubation with species specific impress kit for 30 min at room temperature (Vector Laboratories, Peterborough, UK). After washing and incubation with 3, 3’-diaminobenzidine for 5 min slides were counterstained with hematoxylin, dehydrated and visualized by light microscopy, using an Olympus Provis microscope equipped with a Kodak DCS330 camera (Olympus Optical Co., London, UK and Kodak Ltd., Herts, UK). Due to the limited supply of peritoneal tissue, both positive and negative controls were performed on endometrial tissue.
Statistical analysis
All results are expressed as mean ± standard error of the mean of a minimum of 3 independent experiments. Quantitative RT-PCR and ELISA were analysed using paired and unpaired students’ t tests, as appropriate. All statistical results were generated using GraphPad PRISM version 5 statistical software and a P value of <0.05 was considered significant.
Additional Information
How to cite this article: Young, V. J. et al. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci. Rep. 5, 16859; doi: 10.1038/srep16859 (2015).
References
Giudice, L. C. Clinical practice. Endometriosis. The New England journal of medicine 362, 2389–2398, doi: 10.1056/NEJMcp1000274 (2010).
Mahmood, T. A. & Templeton, A. Prevalence and genesis of endometriosis. Human reproduction 6, 544–549 (1991).
Hickey, M., Ballard, K. & Farquhar, C. Endometriosis. Bmj 348, g1752, doi: 10.1136/bmj.g1752 (2014).
Young, V. J., Brown, J. K., Saunders, P. T. & Horne, A. W. The role of the peritoneum in the pathogenesis of endometriosis. Human reproduction update 19, 558–569, doi: 10.1093/humupd/dmt024 (2013).
Donnez, J., Smoes, P., Gillerot, S., Casanas-Roux, F. & Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod 13, 1686–1690 (1998).
McLaren, J., Prentice, A., Charnock-Jones, D. S. & Smith, S. K. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod 11, 220–223 (1996).
Kupker, W., Schultze-Mosgau, A. & Diedrich, K. Paracrine changes in the peritoneal environment of women with endometriosis. Human reproduction update 4, 719–723 (1998).
Shifren, J. L. et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. The Journal of clinical endocrinology and metabolism 81, 3112–3118, doi: 10.1210/jcem.81.8.8768883 (1996).
McLaren, J. et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. The Journal of clinical investigation 98, 482–489, doi: 10.1172/JCI118815 (1996).
McLaren, J. Vascular endothelial growth factor and endometriotic angiogenesis. Human reproduction update 6, 45–55 (2000).
Kaminska, B., Wesolowska, A. & Danilkiewicz, M. TGF beta signalling and its role in tumour pathogenesis. Acta biochimica Polonica 52, 329–337 (2005).
Young, V. J., Brown, J. K., Saunders, P. T., Duncan, W. C. & Horne, A. W. The peritoneum is both a source and target of TGF-beta in women with endometriosis. PloS one 9, e106773, doi: 10.1371/journal.pone.0106773 (2014).
Young, V. J. et al. Transforming growth factor-beta induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. The Journal of clinical endocrinology and metabolism 99, 3450–3459, doi: 10.1210/jc.2014-1026 (2014).
Pizzo, A. et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecologic and obstetric investigation 54, 82–87, doi: 67717 (2002).
Chegini, N., Gold, L. I. & Williams, R. S. Localization of transforming growth factor beta isoforms TGF-beta 1, TGF-beta 2 and TGF-beta 3 in surgically induced endometriosis in the rat. Obstetrics and gynecology 83, 455–461 (1994).
Wong, Y. C., Wang, X. & Ling, M. T. Id-1 expression and cell survival. Apoptosis: an international journal on programmed cell death 9, 279–289 (2004).
Sun, R., Chen, W., Zhao, X., Li, T. & Song, Q. Acheron regulates vascular endothelial proliferation and angiogenesis together with Id1 during wound healing. Cell biochemistry and function 29, 636–640, doi: 10.1002/cbf.1799 (2011).
Ling, M. T. et al. Overexpression of Id-1 in prostate cancer cells promotes angiogenesis through the activation of vascular endothelial growth factor (VEGF). Carcinogenesis 26, 1668–1676, doi: 10.1093/carcin/bgi128 (2005).
Lyden, D. et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401, 670–677, doi: 10.1038/44334 (1999).
Yung, S., Li, F. K. & Chan, T. M. Peritoneal mesothelial cell culture and biology. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis 26, 162–173 (2006).
Catar, R. et al. The proto-oncogene c-Fos transcriptionally regulates VEGF production during peritoneal inflammation. Kidney international 84, 1119–1128, doi: 10.1038/ki.2013.217 (2013).
Gazvani, R. & Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 123, 217–226 (2002).
Ruzinova, M. B. & Benezra, R. Id proteins in development, cell cycle and cancer. Trends in cell biology 13, 410–418 (2003).
Fong, S., Debs, R. J. & Desprez, P. Y. Id genes and proteins as promising targets in cancer therapy. Trends in molecular medicine 10, 387–392, doi: 10.1016/j.molmed.2004.06.008 (2004).
Kang, Y., Chen, C. R. & Massague, J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Molecular cell 11, 915–926 (2003).
Li, Y., Yang, J., Luo, J. H., Dedhar, S. & Liu, Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. Journal of the American Society of Nephrology: JASN 18, 449–460, doi: 10.1681/ASN.2006030236 (2007).
Liang, Y. Y., Brunicardi, F. C. & Lin, X. Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell research 19, 140–148, doi: 10.1038/cr.2008.321 (2009).
Benezra, R., Rafii, S. & Lyden, D. The Id proteins and angiogenesis. Oncogene 20, 8334–8341, doi: 10.1038/sj.onc.1205160 (2001).
Tsunedomi, R. et al. Decreased ID2 promotes metastatic potentials of hepatocellular carcinoma by altering secretion of vascular endothelial growth factor. Clinical cancer research: an official journal of the American Association for Cancer Research 14, 1025–1031, doi: 10.1158/1078-0432.CCR-07-1116 (2008).
Fegan, K. S., Rae, M. T., Critchley, H. O. & Hillier, S. G. Anti-inflammatory steroid signalling in the human peritoneum. The Journal of endocrinology 196, 369–376, doi: 10.1677/JOE-07-0419 (2008).
Fassbender, A. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing and storage in endometriosis research. Fertility and sterility 102, 1244–1253, doi: 10.1016/j.fertnstert.2014.07.1209 (2014).
Acknowledgements
We are grateful to Prof Philippa Saunders for advice and guidance on the manuscript; Mrs Helen Dewart and Mrs Ann Doust for patient recruitment and sample collection; Dr Forbes Howie for assay development; Prof Steve Hillier for use of the MeT-5A cell line; Mr Bob Morris, Mrs Frances Collins, Ms Arantza Esnal-Zufiurre and Mrs Jean Wade for technical support and advice; Mrs Sheila Milne for secretarial support and Mr Ronnie Grant and Mr Jeremy Tavener for graphics support. Funding: This work was funded by a Wellbeing of Women research grant (R42533) awarded to AWH, JKB and WCD and an MRC Centre Grant G1002033. VJY receives grant support from Federation of Women Graduates (134225) and a PhD studentship from the College of Medicine and Veterinary Medicine at the University of Edinburgh.
Author information
Authors and Affiliations
Contributions
A.W.H., W.C.D. and V.J.Y. conceived and designed the project. V.J.Y. carried out the laboratory work. V.J.Y., S.F.A. and J.K.B. carried out the analysis. All authors contributed to the manuscript write up.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Young, V., Ahmad, S., Brown, J. et al. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci Rep 5, 16859 (2015). https://doi.org/10.1038/srep16859
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16859
This article is cited by
-
Trophic and immunomodulatory effects of adipose tissue derived stem cells in a preclinical murine model of endometriosis
Scientific Reports (2022)
-
Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls
BMC Women's Health (2020)
-
The Role of Ankaferd Blood Stopper and Oxytocin as Potential Therapeutic Agents in Endometriosis: A Rat Model
Current Medical Science (2020)
-
miR-17-5p mitigates endometriosis by directly regulating VEGFA
Journal of Biosciences (2020)
-
The inhibition of bone morphogenetic protein 1 attenuates endometriosis lesions in vivo and in vitro
Archives of Gynecology and Obstetrics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.