Abstract
Obesity is often associated with irregular dietary habits and reduced physical activity. Regular exercise induces a metabolic response that includes increased expression of various cytokines, signaling proteins and hormones and reduced adipocyte size. In this study, mice performed a swimming exercise for 10 min/day, 5 days/week for 3 weeks. We then investigated the effect of this exercise regimen on inflammation induced by infection with drug-resistant Staphylococcus aureus strain 3089 (DRSA). In humans, DRSA causes dermatitis and pneumonitis. Similarly, DRSA induced inflammatory pneumonitis in both no-exercise (No-EX) and swim-trained (SW-EX) ICR mice. Regular exercise increased levels of the pro-inflammatory cytokines TNF-α and IL-1β and nitric oxide in both serum and whole lung tissue in SW-EX, as compared to No-EX control mice. Moreover, levels of the antimicrobial peptide cathelicidin were significantly increased in visceral adipose tissue and whole lung tissue in the SW-EX group and this was accompanied by a reduction in the size of visceral adipocytes. In addition, levels of the inflammation marker peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) were not increased in the lung tissue of SW-EX mice. These findings suggest that in these model mice, regular exercise strengthens immune system responses, potentially preventing or mitigating infectious disease.
Similar content being viewed by others
Introduction
In recent years, rapid improvements in industrial and informational technology have increased the quality of life for many1. On the other hand, there has been an increase in environmental pollution and people tend to do less regular exercise and suffer greater amounts of stress2. These conditions have led to an increased incidence of obesity and greater exposure to bacterial and viral diseases3,4,5,6,7,8. Moreover, overuse of antibiotics has led to the development of drug-resistant microorganisms such as methicillin-resistant staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and other multi-drug resistant (MDR) strains, which are the source of potentially life-threatening infections in the absence of a robust immune response9. This is particularly true in hospitals, where recent reports indicate that the drug resistance of various strains has increased the death rates from nosocomial infections10,11.
Obesity is associated with chronic low-grade inflammation, an increased risk of atherosclerosis, type 2 diabetes, neurodegeneration, tumor growth, reduced functional capacity and reduced longevity in humans3,12. Cytokines are signaling molecules secreted by various cell types, which control immune responses and are involved in the regulation of cell proliferation and differentiation and antitumor activity, among other functions12,13,14. A lack of physical activity has been associated with increased production of pro-inflammatory cytokines, adipokines (a subgroup of cytokines produced by adipose tissue), triglycerides and LDL-cholesterol, as well as overexpression of Toll-like receptors (TLRs), including TLR2 and TLR415,16,17,18,19,20,21,22. Conversely, regular exercise reportedly increases production of various cytokines and myokines (a subgroup of cytokines produced in skeletal muscle) that strengthen the immune system, while stimulating increases in adipose tissue oxidation, insulin sensitivity, anti-inflammatory activity, anti-tumor defense, pancreatic function and brown fat metabolism for muscle growth23,24,25,26.
The secretion of antimicrobial peptides (AMPs) such as cathelicidin and defensin by immune cells is one of the ways in which the body protects itself from disease, including antibiotic-resistant bacterial infections27. In particular, cathelicidin secreted by macrophages and polymorphonuclear leukocytes (PMNs) is well known to exert strong antimicrobial effects27. Given the beneficial effects of the exercise on immune system function summarized above, we hypothesized that a lack of exercise increases the potential for serious organ damage as well as increased morbidity and mortality among those who become ill. Consistent with that idea, regular exercise appears to increase immunity and reduce metabolic syndrome disease acitivity28,29,30. Furthermore, we suggest that regular exercise increases cathelicidin production by immune cells, which, in the event of an infection, would reduce the growth and destructive effects of the microorganism.
To test those ideas, we applied swim training to ICR mice and compared the responses to infection by drug-resistant Staphylococcus aureus strain 3089 (DRSA) in no-exercise (No-EX) and swim-trained (SW-EX) groups. Susceptibility to DRSA and manifestation of its symptoms (e.g., dermatitis and pulmonitis) reflect the host’s ability to mount an appropriate immune response31. We anticipated that regular exercise would increase immune control of the DRSA infection and, in turn, diminish DRSA-associated responses. We used swimming as the regular exercise because it exerts the same or similar effects as jogging, walking and other aerobic exercises (such as treadmill exercise)28. Moreover, it activates the body’s muscles better than treadmill exercise while exerting a smaller weight load on the joints.
DRSA is associated with chronic obstructive pulmonary disease (COPD)32, the production of inflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β)33, as well as the inflammatory marker proteins nitric oxide (NO)34 and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α)35. We studied changes in cell morphology and the expression levels of inflammatory marker proteins, cytokines and cathelicidin AMP in whole blood, adipocytes and whole lungs using ELISA assays, hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC), NO assays and western blot analysis.
Results
Levels of TNF-α and IL-1β in whole blood following DRSA infection
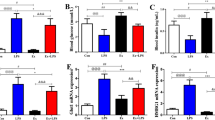
The effects of swimming exercise (SW-EX) (10 min/day, 5 days/week for 3 weeks) on induced inflammation were examined using ICR mice infected with DRSA (Fig. 1). Within 6 h after intraperitoneal injection of DRSA (1 × 107 cfu/ml), blood levels of two pro-inflammatory cytokines, TNF-α and IL-1β, were significantly higher in No-EX + DRSA mice than in the No-EX group, but were not significantly elevated in SW-EX or SW-EX + DRSA groups (Fig. 2). Moreover, serum TNF-α and IL-1β levels were lower in SW-EX + DRSA mice than SW-EX mice.
TNF-α, IL-1β and cathelicidin levels in visceral fat
Obesity-induced inflammation within organ tissues serves as the basis for metabolic syndrome36,37. In addition obesity increases ones susceptibility infection drug-resistant bacteria and the associated pathology31,32. Figure 3 shows the effect of intraperitoneal injection of DRSA on expression of TNF-α, IL-1β and cathelicidin in the visceral fat of No-EX and SW-EX mice. Immunostaining for TNF-α, IL-1β and cathelicidin shows that within 6 h after injection of DRSA, expression of all three proteins was increased in both No-EX and SW-EX mice. In the SW-EX mice, however, the adipocytes were smaller in size and appeared to express higher levels of TNF-α, IL-1β and cathelicidin than No-EX mice.
Adipocyte morphology and expression of cathelicidin, IL-1β and TNF-α in visceral adipose tissue following induced inflammation.
Visceral fat was extracted from No-EX and SW-EX mice, sectioned in paraffin, stained with H&E and subjected to immunohistochemistry. (A) No-EX, (B) No-EX + DRSA, (C) SW-EX, (D) SW-EX + DRSA.
Pulmonary production of nitric oxide (NO)
To evaluate inflammation of the lungs (pneumonitis) of ICR mice infected with DRSA. We measured NO levels in lung tissue extracts as an index of pneumonitis in No-EX and SW-EX mice (Fig. 4). The results show that intraperitoneal injection of DRSA strongly increased NO production in No-EX mice, but had little or no effect on pulmonary NO production in SW-EX mice. It thus appears that regular exercise may exert a protective effect against pneumonitis induced by DRSA infection in ICR mice.
Pulmonary expression of pro-inflammatory cytokines and inflammation markers
Intraperitoneal injection of DRSA led to substantial upregulation of pulmonary TNF-α and IL-1β expression in No-EX mice. By contrast, DRSA infection had little effect on pulmonary expression of TNF-α and IL-1β in SW-EXE mice, although the baseline levels of the two cytokines were already higher than in No-EX mice. Similarly, DRSA infection enhanced pulmonary expression of PGC-1, a protein marker expressed by macrophages and PMNs during inflammatory responses27,28, in No-EX but not SW-EX mice (Fig. 5). DRSA infection also sharply increased cathelicidin expression in both No-EX and SW-EX mice, as compared with untreated controls.
Evaluation of morphological changes and expression of cathelicidin in the lungs
Finally, we examined the histological changes in the lungs of mice following intraperitoneal injection of DRSA. We found that in lung tissue from No-EX mice, the induced inflammation led to hemorrhage, infiltration of inflammatory cells (e.g., macrophages), alveolar swelling and production of cathelicidin (Fig. 6A,B). These inflammatory changes were not seen in lung tissue from SW-EX mice, although levels of cathelicidin were strongly upregulated.
Expression of cathelicidin in lung tissue following induced inflammation.
Shown are paraffin-sections of lungs from No-EXE and SW-EX mice collected 6 h after infection with DRSA. The sections were stained with H&E and subjected to immunohistochemistry. (A) No-EX, (B) No-EXE + DRSA, (C) SW-EX, (D) SW-EX + DRSA.
Discussion
It is now recognized that the chronic inflammation associated with obesity is mediated in part by various chemokines, hormones and cytokines (such as TNF-α and IL1-β) and that it plays a key role in a constellation of diseases that includes hypertension, type 2 diabetes and cardiovascular disease and is often referred to as metabolic syndrome38,39,40,41,42,43. Obesity thus appears to weaken the immune system while inducing a chronic inflammatory response. A key mediator of chronic inflammatory disease is elevated triglyceride (TG) within adipocytes36. TG is cleaved by lipases secreted from the pancreas into free fatty acids (FFAs) and glycerol37. FFAs induce inflammation by binding to TLR-2 and TLR-4 receptors on immune cells (e.g., macrophages) in blood vessels and stimulating the release of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-644. On the other hand, regular exercise improves immunity29 through moderate induction of pro-inflammatory cytokines (e.g., TNF-α, IL-1β and IL-6) in skeletal muscle45. Reactive oxidative species (ROS) are also produced46 during exercise, which in turn activate c-Jun N-terminal kinases (JNK)47. Collectively, these processes are associated with skeletal muscle injury and tissue repair30, which induces myofibrillar protein synthesis and muscle hypertrophy through activation of mammalian target of rapamycin complex 1 (mTORC1)48 and promotes organ function49,50. Regular exercise also appears to shrink adipocytes and reduce obesity by inducing β-oxidation51.
Infectious microorganisms also elicit acute inflammatory responses mediated by cytokines secreted from immune cells, akin to inflammation associated with obesity52. In this study, we examined the effect of 3 weeks of exercise (swimming) on the immune response induced by infection with DRSA. Our findings show that regular exercise suppresses infection-induced inflammation, as indicated by reductions in the levels in two inflammatory cytokines (TNF-α and IL1-β) and two inflammation markers (NO and PGC-1) and it increases cathelicidin levels in the blood, adipose tissue and whole lung tissue. Earlier reports indicate that production of TNF-α and IL1-β is significantly increased by regular exercise53,54,55, resulting in improved immune responses, prevention of various diseases, increased signal transduction and reduced body mass through β-oxidation56,57. Secretion of large amounts of cytokines stimulated by infectious pathogens results in strong activation of immune cells58. TNF-α and IL-1β, in particular, are key mediators of the signal transduction initiating immediate immune cell activity upon release from the site of infection59,60. We observed that serum TNF-α and IL-1β levels were lower in SW-EX and SW-EX + DRAS mice than No-EX + DRSA mice and were even lower in SW-EX + DRSA than SW-EX mice. We also observed that production of TNF-α and IL-1β was reduced in exercising mice, while cathelicidin production was increased, suggesting that suppression of the infection by cathelicidin led to a decrease in cytokine production. We therefore suggest that the immediate action (autocrine and/or paracrine) of cathelicidin on the infected tissues contributed to a reduction in serum cytokine levels.
On the other hand, SW-EX mice exhibited higher levels of TNF-α and IL-1β in adipose tissue. This is thought to reflect increased proliferation and/or recruitment of macrophages (including a switch from the M2 to the M1 phenotype) to the area around adipocytes as a result of β-oxidation of free fatty acids accomplished through regular exercise58,60. Regular exercise would also be expected to increase expression of cathelicidin by infiltrating macrophages in adipose tissue. Consistent with that idea, we observed significantly greater cathelicidin production in both uninfected and infected SW-EX mice than in No-EX mice. Furthermore, the cathelicidin production would also be expected to influence whole-body immune responses via the lymphatic system and blood vessels following infection with DRSA. This is perhaps reflected by pulmonary NO production, which is known to be enhanced by inflammation caused by infectious disease and/or obesity38. When we measured pulmonary NO following DRSA infection of No-EX and SW-EX mice, we observed that although NO levels did not differ between the two groups under basal conditions, they were significantly higher in No-EX mice infected with DRSA suggesting lack of exercise may increase the likelihood of adverse whole-body immune responses that can underlie septic shock.
It was previously reported that pulmonary levels of TNF-α, IL-1β and the inflammation marker PGC-1 are all increased following infection with microorganisms such as S. aureus, often leading to sepsis and death61,62,63,64,65. However, cathelicidin produced by macrophages and PMNs, along with other AMPs such as defensin and histatin, exert strong antimicrobial effects against microorganisms such as S. aureus and Pseudomonas aeruginosa27,54. When we compared the production of TNF-α and IL1-β, PGC-1 and cathelicidin in whole lung tissue from No-EX and SW-EX ICR mice following DRSA infection, we found that DRSA infection had little effect on pulmonary expression of TNF-α and IL-1β in SW-EXE mice, though their baseline levels were already higher than in No-EX mice. Likewise, DRSA infection had little effect on pulmonary expression of PGC-1. By contrast, cathelicidin levels were increased to a substantially greater (more than 3-fold) degree in SW-EX than No-EX mice following DRSA infection. These findings suggest that regular exercise increases production of cathelicidin, thereby enhancing the endogenous antimicrobial response against bacterial infection and, in turn, suppressing production of inflammatory cytokines and inflammation markers.
We propose that regular exercise increases cathelicidin production by immune cells in adipose and lung tissues, which also produce TNF-α and IL1-β. However, the increased cathelicidin prevents the inflammation-induced secretion of TNF-α, IL1-β, PGC-1 from immune cells by disrupting bacterial membranes and binding to lipoteichoic acid (LTA) on the bacterial cell surface. In addition, it was previously reported that activation of skeletal muscle during regular exercise leads to increases in the serum levels of various cytokines66, which in turn mediate other inflammatory responses32,33,67. By contrast, we found that irrespective of DRSA infection-induced inflammation, serum cytokine levels in ICR mice subjected to regular exercise (SW-EX + DRSA and SW-EX) were lower than in infected, unexercised control mice (No-EX + DRSA). In fact, serum TNF-α and IL-1β levels in the SW-EX + DRSA and SW-EX groups did not significantly differ from those in the uninfected and unexercised control group (No-EX mouse). Notably, TNF-α and IL-1β levels appeared to be even lower in SW-EX + DRSA than SW-EX mice (Fig. 2). We think that the lower serum levels of pro-inflammatory cytokines reflects the immediate stimulus and engagement (autocrine and/or paracrine) of the immune cell receptors (such as macrophages) by the cytokines in the infected animals and that the skeletal muscle damage caused by the exercise enhanced that effect68. Ultimately, the effective immune cell response to the infectious microorganism (DRSA), including release of cathelicidin, led to rapid mitigation of cytokine release and lower serum levels than in the uninfected control.
β-oxidation of free fatty acids not only leads to shrinkage of adipocytes during regular exercise, but reduces inflammation, which has a preventive effect on diseases such as diabetes, cardiovascular disease and stroke, among others60,69 (Fig. 7) and it also improves immune system defenses and decreases susceptibility to infection.
Methods
Experimental design and swimming exercise training
Male ICR mice were used for this study. The ambient air temperature was maintained at 22–25 °C with 40–60% humidity and a 12 h dark-light cycle. The swimming exercise procedure conformed to the “Resource Book for the Design of Animal Exercise Protocols” and was approved by the American Physiological Society Committee to Develop an APS Resource Book for the Design of Animal Exercise Protocols. When handling mice, we followed the established guidelines.
Eight-week-old ICR mice were assigned to a no-exercise (No-EX, n = 7) or swim-trained (SW-EX, n = 7) group. The exercise training protocol for the swimming group was as follows: prior to the initiation of training, the mice were allowed access to the swimming pool for 3 min/day for 5 days, which enabled them to become familiar with the environment. After finishing each exercise session, the mice were completely dried to prevent hypothermia. The rectangular tank was 15–25 cm deep, 40 cm long and 25 cm wide. During training, mice in the SW-EX group swam for 10 min/day, 5day/week for 3 weeks. We monitored the mice during the swim to prevent them from climbing, diving, bobbing or floating. Mice in the No-EX group were treated the same in all other regards, but did not undergo swim training70,71,72.
To elicit changes in cytokine, cathelicidin and inflammatory protein expression, inflammation was induced by infecting mice through intraperitoneal injection of DRSA (1 × 107 cfu/ml)19,25,26. Negative control mice (No-EX and SW-EX) were administered PBS buffer without bacteria. After 6 h, experimental mice were anesthetized using 0.5–2% isoflurane (the procedure conformed to the “Korean College of Laboratory Animal Medicine”, KCOLAM) and whole blood samples were collected from the heart and lung and adipose tissue samples were collected through abdominal incisions.
Measurement of serum TNF-α and IL-1β in ICR mice
TNF-α and IL-1β expression was measured in whole blood from No-EX and SW-EX mice following induction of inflammation with DRSA. Whole blood samples were collected 6 h after the mice were injected with bacteria or vehicle (control). The samples were then centrifuged at 2000 g for 10 min at 4 °C to remove the red blood cells and the serum was retained. Levels of TNF-α and IL-1β were measured using specific ELISA kits (Koma Biotech, Seoul, Korea). Absorbance was measured at 450 nm using a microplate reader63.
Immunohistochemistry (IHC) and hematoxylin & eosin staining
Lungs and visceral fat were extracted from mice, washed once with phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde for 24 h at 4 °C. The fixed tissue was then dehydrated through a 50–100% ethanol series (2 h at each step) and 3 incubations (1 h each) in xylene, embedded in paraffin and cut into 4-μm-thick sections using a microtome. Sections were incubated for 30 min at room temperature with primary antibody in 5% bovine serum albumin (BSA). The primary antibodies used were polyclonal mouse anti-cathelicidin (ABfrontier, AB93357), monoclonal mouse anti-TNF-α (ABfrontier, AB1793) and polyclonal mouse anti-IL-1β (ABfrontier, AB1413). The samples were washed with tris-buffered saline containing tween (TBST buffer), incubated with secondary antibody (goat anti-mouse IgG (HRP) LF-SA5001-conjugated) and stained with hematoxylin and eosin (H&E). Stained sections were examined under a fluorescence microscope (Ix71, Olympus, Tokyo, Japan)64.
Measurement of nitric oxide (NO) in lungs
After injecting mice with DRSA or vehicle, samples of whole lung tissues were collected from the No-EX and SW-EX groups and homogenized in one volume of PRO-PREPTM protein extraction solution (iNtRON Biotechnology, Seoul, Korea). After homogenization and centrifugation at 12,000 g for 10 min to remove floating contaminants, the supernatant was retained for NO assays. To determine NO levels, Griess reagent was added to the samples, which were then mixed with equal volumes of sulfanilic acid (1% in phosphoric acid) and N-(1-naphthyl) ethylenediamine dihydrochloride (0.1% in DW). Aliquots of the resultant mixture (50 μl) were incubated for 30 min at room temperature with mixing, after which absorbance was measured at 548 nm using a microplate reader. NO levels were calculated against a standard curve constructed using sodium nitrite73.
Western blot analysis
For western blot analysis, proteins collected from whole lung tissue were separated by SDS-PAGE in a 15% polyacrylamide gel for 3 h. The separated proteins were then transferred to PVDF membranes (Bio-Rad, USA) for 1 h at 90 volts. After blocking the membranes overnight at 4 °C with 5% skim milk, they were probed using anti-GAPDH (Santa Cruz Biotechnology, LF-PA0018), polyclonal mouse anti-NF-κB (Santa Cruz Biotechnology, SC-71675), monoclonal mouse anti-TNF-α (ABfrontier, AB1793), polyclonal mouse anti-IL-1β (ABfrontier, AB1413), polyclonal mouse anti-Cathelicidin (ABfrontier, AB93357) and polyclonal mouse anti-PGC-1 (Millipore AB3242). The membranes were then washed with TBST buffer and incubated with secondary antibodies (goat anti-mouse IgG (HRP) LF-SA5001-conjugated). Finally, the blots were developed using a western blot detection kit (LF-QC0103, Abfrontier)74.
Statistical analysis
The data were analyzed using SPSS version 20.0 (Chicago, IL, USA). Values are presented as means ± SD. One-way ANOVA was used to compare TNF-α, IL-1β and NO levels between the No-EX and SW-EX groups. P values < 0.05 were considered significant.
Additional Information
How to cite this article: Lee, J.-K. et al. Effect of Regular Exercise on Inflammation Induced by Drug-resistant Staphylococcus aureus 3089 in ICR mice. Sci. Rep. 5, 16364; doi: 10.1038/srep16364 (2015).
References
Vyncke, V. et al. Does neighbourhood social capital aid in levelling the social gradient in the health and well-being of children and adolescents? A literature review. BMC Public Health. 13, 65, doi: 10.1186/1471-2458-13-65 (2013).
Schüle, S. A. & Bolte, G. Interactive and independent associations between the socioeconomic and objective built environment on the neighbourhood level and individual health: a systematic review of multilevel studies. PLoS One. 10, e0123456,doi: 10.1371/journal.pone.0123456 (2015).
Frimel, T. et al. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med. Sci. Sports Exerc. 40, 1213–1219 (2008).
Alford, L. What men should know about the impact of physical activity on their health. Int. J. Clin. Pract. 64, 1731–1734 (2010).
Herman, K. M. et al. Self-rated health and life satisfaction among Canadian adults: associations of perceived weight status versus BMI. Qual. Life Res. 22, 2693–2705 (2013).
Casey, J. A. et al. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001-2010. Epidemiol. Infect. 29, 1–14 (2012).
Hegde, V. & Dhurandhar, N. V. Microbes and obesity-interrelationship between infection, adipose tissue and the immune system. Clin. Microbiol. Infect. 19, 314–320 (2013).
Lumeng, C. N. Innate immune activation in obesity. Mol. Aspects Med. 34, 12–29 (2013).
Chan, C. Y., St, John, A. L. & Abraham, S. N. Plasticity in mast cell responses during bacterial infections. Curr. Opin. Microbiol. 15, 78–84 (2012).
Jarvis, W. R., Jarvis, A. A. & Chinn, R. Y. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am. J. Infect. Control. 40, 194–200 (2012).
Farrell, D. J. et al. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010). Clin. Infect. Dis. 3, 206–214 (2012).
Hojman, P. et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 301, E504–510 (2011).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 65, 732 741
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 (2012).
Zbinden-Foncea, H. et al. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med. Sci. Sports Exerc. 44, 1463–1472 (2012).
Oliveira, M. & Gleeson, M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 109, 251–257 (2010).
Zhu, J. & Mohan, C. Toll-like receptor signaling pathways-therapeutic opportunities. Mediators Inflamm. 781235, doi: 10.1155/2010/781235 (2010).
Simpson, R. J. et al. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain. Behav. Immun. 23, 232–239 (2009).
Gleeson, M., McFarlin, B. & Flynn, M. Exercise and Toll-like receptors. Exerc. Immunol. Rev. 12, 34–53 (2006).
Shechter, R. et al. Hypothalamic neuronal toll-like receptor 2 protects against age-induced obesity. Sci. Rep. 3, 1254, doi: 10.1038/srep01254 (2013).
Shields, R. K. et al. Staphylococcus aureus infections in the early period after lung transplantation: epidemiology, risk factors and outcomes. J. Heart Lung Transplant. 31, 1199–1206 (2012).
Ding, Y. et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 1596–1604 (2012).
Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615 (2011).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Walsh, N. P. et al. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 17, 64–103 (2011).
Bar-Shai, M. et al. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free. Radic. Biol. Med. 44, 202–14 (2008).
Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 7, 545–594 (2014).
Pellegrin, M. et al. Swimming prevents vulnerable atherosclerotic plaque development in hypertensive 2-kidney, 1-clip mice by modulating angiotensin II type 1 receptor expression independently from hemodynamic changes. Hypertension. 53, 782–789 (2009).
Walsh, N. P. et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 17, 6–63 (2011).
Walsh, N. P. et al. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 17, 64–103 (2011).
Krishna, S. & Miller, L. S. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin. Immunopathol. 34, 261–280 (2012).
Linge, H. M. et al. Midkine is expressed and differentially processed during chronic obstructive pulmonary disease exacerbations and ventilator-associated pneumonia associated with Staphylococcus aureus infection. Mol. Med. 30, 314–323 (2013).
Browne, S. K. & Holland, S. M. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. Lancet. Infect. Dis. 10, 875–885 (2010).
van Sorge, N. M. et al. Methicillin-resistant Staphylococcus aureus bacterial nitric-oxide synthase affects antibiotic sensitivity and skin abscess development. J. Biol. Chem. 288, 6417–6426 (2013).
Li, J. et al. Positive correlation between PPARgamma/PGC-1alpha and gamma-GCS in lungs of rats and patients with chronic obstructive pulmonary disease. Acta. Biochim. Biophys. Sin (Shanghai). 42, 603–14 (2010).
Börgeson, E. et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 22. 125–137 (2015).
Kratky, D. et al. Pleiotropic regulation of mitochondrial function by adipose triglyceride lipase-mediated lipolysis. Biochimie. 96, 106–112 (2014).
Pilon, G. et al. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO- induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS One. 5, e15912, doi: 10. 1371/journal. pone. 0015912 (2010).
Ye, Z. et al. Obesity induced by neonatal overfeeding worsens airway hyper responsiveness and inflammation. PLoS One. 7, e47013, doi: 10. 1371/journal. pone. 0047013 (2012).
Piya, M. K., McTernan, P. G. & Kumar, S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J. Endocrinol. 216, T1–T15 (2013).
Slentz, C. A., Houmard, J. A. & Kraus, W. E. Exercise, abdominal obesity, skeletal muscle and metabolic risk: evidence for a dose response. Obesity (Silver Spring). 3, 27–33 (2009).
Oda, E. Metabolic syndrome: its history, mechanisms and limitations. Acta. Diabetol. 49, 89–95 (2012).
Mancuso, P. Obesity and respiratory infections: Does excess adiposity weigh down host defense? Pulm. Pharmacol. Ther. 26. 214–419 (2012).
Yin, J. et al. Toll-like receptor 2/4 links to free fatty acid-induced inflammation and β-cell dysfunction. J. Leukoc. Biol. 95,47–52 (2014).
Peake, J. M. et al. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 21, 8–25 (2015).
Powers, S. K. & Jackson, M. J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276 (2008).
Passos, E. et al. Role of physical exercise on hepatic insulin, glucocorticoid and inflammatory signaling pathways in an animal model of non-alcoholic steatohepatitis. Life Sci. 123, 51–60 (2015).
Watson, K. & Baar, K. mTOR and the health benefits of exercise. Semin. Cell. Dev. Biol. 36, 130–139 (2014).
Halmos, T. & Suba, I. The secretory function of skeletal muscles and its role in energy metabolism and utilization. Orv. Hetil. 155, 1469–1477 (2014).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Lancaster, G. I. & Febbraio, M. A. The immunomodulating role of exercise in metabolic disease. Trends. Immunol. 35, 262–269 (2014).
Giebelen, I. A. et al. Endogenous beta-adrenergic receptors inhibit lipopolysaccharide-induced pulmonary cytokine release and coagulation. Am. J. Respir. Cell. Mol. Biol. 39, 373–379 (2008).
Yu, F. S. et al. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J. Immunol. 185, 1142–1149 (2010).
Vandamme, D., Landuyt, B., Luyten, W. & Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 280, 22–35 (2012).
Walsh, N. P. et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 17, 6–63 (2011).
Martin, S. A., Pence, B. D. & Woods, J. A. Exercise and respiratory tract viral infections. Exerc. Sport Sci. Rev. 37, 157–164 (2009).
Moreira, A. et al. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 90, 111–131 (2009).
Benichou, G. et al. Innate immunity and resistance to tolerogenesis in allotransplantation. Front. Immunol. 19, 73 (2012).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Kawasaki, N. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2. 799, doi: 10. 1038/srep00799 (2012).
Hong, C. & Tontonoz, P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 18, 461–467 (2008).
Castillo-Quan, J. I. From white to brown fat through the PGC-1α-dependent myokine irisin: implications for diabetes and obesity. Dis. Model. Mech. 5, 293–295 (2012).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481, 463–468 (2012).
Arany, Z. GC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 18, 426–434 (2008).
Li, J. et al. Positive correlation between PPAR gamma/PGC-1alphaandgamma-GCS in lungs of rats and patients with chronic obstructive pulmonary disease. Acta. Biochim. Biophys. Sin (Shanghai). 42, 603–614 (2010).
Jaume adilla. et al. Vascular Effects of Exercise: Endothelial Adaptations Beyond Active Muscle Beds. Physiology Published. 3, 132–145 (2011).
Fabiana, B. et al. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nature reviews rheumatology. 11, 86–97 (2015).
Charlotte D’Mello & Mark, G. Swain . Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol 301, G749–G761 (2011).
Clària, J. et al. New insights into the role of macrophages in adipose tissue inflammation and Fatty liver disease: modulation by endogenous omega-3 Fatty Acid-derived lipid mediators. Front. Immunol. 2, 49, doi: 10. 3389/fimmu. 2011. 00049 (2011).
Papareddy, P. et al. C-terminal peptides of tissue factor pathway inhibitor are novel host defense molecules. J. Biol. Chem. 285, 28387–28398 (2010).
Takaoka, Y. et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci. Rep. 6, 5204; doi: 10. 1038/srep05204 (2014).
Hu, L. et al. Lipopolysaccharide neutralization by a novel peptide derived from phosvitin. Int. J. Biochem. Cell Biol. 45, 2622–2631 (2013).
Kim, I. S. et al. PhedranninA and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor-κB activation in RAW264.7 macrophages. Int. Immunopharmacol. 10, 1616–1625 (2010).
Gounder, S. S. et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 7, e45697, doi: 10. 137/journal. Pone. 0045697 (2012).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST; No. 2011-0017532) and a Global Research Laboratory (GRL) Grant (NRF-2014K1A1A2064460).
Author information
Authors and Affiliations
Contributions
Y.P. planned the research project, J.K.L. performed the experiments, analysis and prepared the figures, Y.P., T.L. and J.K.L. wrote the manuscript, Y.P. rationally designed and all authors contributed to the interpretation and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, JK., Luchian, T. & Park, Y. Effect of Regular Exercise on Inflammation Induced by Drug-resistant Staphylococcus aureus 3089 in ICR mice. Sci Rep 5, 16364 (2015). https://doi.org/10.1038/srep16364
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16364
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.