Abstract
This paper documents the key anatomical features during the development of P. armeniacum zygotic embryos and their ability to germinate asymbiotically in vitro. This study also examines the effect of media and seed pretreatments on seed germination and subsequent seedling growth. Seeds collected from pods 45 days after pollination (DAP) did not germinate while 95 DAP seeds displayed the highest seed germination percentage (96.2%). Most seedlings (50%) developed to stage 5 from 110 DAP seeds whose compact testa had not yet fully formed. Suspensor cells were vacuolated, which enabled the functional uptake of nutrients. The optimum basal medium for seed germination and subsequent protocorm development was eighth-strength Murashige and Skoog (1/8MS) for 95 DAP seeds and ¼MS for 110 DAP seeds. Poor germination was displayed by 140 DAP seeds with a compact testa. Pretreatment of dry mature seeds (180 DAP) with 1.0% sodium hypochlorite solution for 90 min or 40 kHz of ultrasound for 8 min improved germination percentage from 0 to 29.2% or to 19.7%, respectively. Plantlets that were at least 5 cm in height were transplanted to a Zhijing stone substrate for orchids and 85.3% of plantlets survived 180 days after transplanting.
Similar content being viewed by others
Introduction
Paphiopedilum is a rare orchid genus, which is comprised of about 96–100 species around the world1,2,3. The rarity, beauty and value of Paphiopedilum species, popularly known as slipper orchids, have always captured the interest of orchid growers and hobbyists. Wild populations of Paphiopedilum are under the constant threat of extinction due to overcollection for use as ornamental plants or as breeding parents. This loss of suitable habitats is caused exclusively by anthropogenic activity in response to the trade of this orchid. Incidentally, the trade of all Paphiopedilum species, which are listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Appendix I, is prohibited4,5,6. P. armeniacum S. C. Chen et F. Y. Liu is one of the most fascinating Paphiopedilum species because of its characteristic golden flowers. Currently (last assessed on September 6, 2015), 124 hybrids use P. armeniacum as the parent, 39 as seed parents and 85 as pollen parents7. P. armeniacum is a terrestrial or lithophytic orchid that is only distributed in the western part of Yunnan province, China, along the Nu River from Shidian county in the south to the North of Fugong county and the Southwest of Weixi county in the North and flowers from March to May in the wild2,8 (Fig. 1). P. armeniacum is currently listed as endangered in The IUCN Red List of Threatened Species, version 2014.3. (www.iucnredlist.org, 2015).
The success of tissue culture protocols based on ex vitro-derived Paphiopedilum explants is limited due to the rarity of materials, difficulties caused by bacterial and fungal decontamination and the poor development of explants that survive under in vitro conditions6,9,10. Asymbiotic seed germination is an efficient method for the large-scale propagation of orchids11. Several protocols for in vitro seed germination of Paphiopedilum species, including P. armeniacum, have been described6. The development of Paphiopedilum zygotic embryos during seed germination and protocorm development in vitro is stimulated by suitable pretreatment, including with sodium hypochlorite (NaOCl) or ultrasound5,12,13,14. P. armeniacum seed germination and subsequent protocorm development are significantly influenced by capsule maturity, seed pretreatment, medium composition, culture conditions, culture method, as well as other conditions6,13,14,15,16,17. In addition, asymbiotic seed germination percentage is subtantially lower (0–60%) than in other Paphiopedilum species while some experimental results, including seed germination percentage on the same medium, are inconsistent13,14. There are some reports on zygotic embryo development in Paphiopedilum species18,19,20,21,22. However, no analysis exists of the comparison between embryo/seed morphological characteristics, seed germination percentage and protocorm development. Moreover, no studies to date have used a pretreatment with NaOCl or ultrasound to facilitate seed germination and protocorm development of P. armeniacum.
Based on this background, there were three primary goals of this study. Firstly, to investigate the key anatomical features during zygotic embryo development of P. armeniacum in association with the ability of zygotic embryos to germinate asymbiotically in vitro. Secondly, to examine the effect of culture media and seed pretreatments on seed germination and subsequent seedling growth, with the ultimate purpose of increasing both. Thirdly, to establish an effective in vitro propagation system for the large-scale propagation of P. armeniacum to meet commercial needs and to eventually reestablish populations of this threatened orchid species back into the wild.
Results
Embryo development
The major microscopic structural events taking place in developing capsules of P. armeniacum after pollination are described in Table 1 and displayed in Fig. 2. At 45 days after pollination (DAP), about 80% of ovules had been fertilized and zygotic embryos began to develop, becoming elongated (Fig. 2a). The nucleus was localized toward the chalazal end and a prominent vacuole was found at the micropylar end. At 52 DAP the zygote divided into two cells (Fig. 2b) and derivatives of the basal cell gave rise to the suspensor, which only consisted of a single cell that was highly vacuolated. At 59 DAP a three-cell pre-embryo was observed (Fig. 2c). At 66 DAP the cell at the terminus divided anticlinally giving rise to a T-shaped pre-embryo with four cells (Fig. 2d). At 73 DAP the middle two cells divided forming a spherical six-cell pre-embryo (Fig. 2e). At 80 DAP a multi-cell early globular embryo displayed a curving suspensor (Fig. 2f). A globular embryo began to form at 87 DAP when the outer layer of cells of the outer integument began to dehydrate and the suspensor started to degenerate (Fig. 2g). At 94 DAP a globular embryo proper formed, the inner-layer cells of the outer integument began to dehydrate, the suspensor continued to degenerate and starch and lipid globules accumulated, events that epitomize the storage of nutrients (Fig. 2h). At 101 DAP the globular embryo had fully developed, the suspensor had fully degenerated and starch and lipid globules continued to accumulate (Fig. 2i). At 108 DAP the inner testa disappeared and there was additional accumulation of starch and lipid globules. At 115 DAP the cells of the inner integument degenerated and the globular embryo was very close to forming a testa rich in starch and lipid globules (Fig. 2j). By 122 DAP the embryo had fully matured, evidenced by a compact dark testa and no further morphological changes took place (Fig. 2k). At 140 DAP mature seed had desiccated and at 150 DAP seeds were fully mature. At 220 DAP, the ripe capsule split.
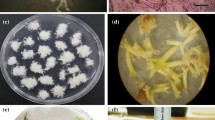
Histological study of embryo development in P. armeniacum.
(a) Zygote (Z), soon after fertilization at 45 days after pollination (DAP); (b) two-cell pre-embryo (arrowhead) at 52 DAP; (c) three-cell pre-embryo at 59 DAP; (d) T-shaped pre-embryo with four cells at 66 DAP; (e) six-cell pre-embryo at 73 DAP; (f) early globular embryo with a curving suspensor (S) at 80 DAP; (g) globular embryo at 87 DAP, the outer layer cells of the outer integument begin to dehydrate and the suspensor degenerates; (h) globular embryo at 94 DAP, the inner layer cells of the outer integument begin to dehydrate and starch and lipid globules (LG) accumulate; (i) the globular embryo at 101 DAP with a degenerated suspensor; (j) the globular embryo is very close to the seed coat (SC) at 115 DAP; (k) the embryo has matured with a compact SC and no further morphological changes at 122 DAP; (l) the SC is very compact and thick at 180 DAP. E: embryo; II, inner integuments; OI, outer integuments. Scale bar = 50 μm.
Effect of the degree of seed maturity on TTC staining and in vitro germination
Seed germinated at 120 days after culture. 2,3,5-Triphenyl tetrazolium chloride (TTC) staining percentage was significantly affected by the degree of seed maturity (Table 2). Pre-embryos at 45 DAP did not stain or germinate. There were no significant differences between seed germination and TTC staining percentage in 55 or 65 DAP pre-embryos. TTC staining percentage was significantly higher than seed germination percentage at 75 or 85 DAP. Highest seed germination percentage (96.9%) and TTC staining percentage (95.7%) were observed in 95 DAP seeds. These values were significantly higher than during all other collection periods. However, TTC staining percentage (82.3%) was still significantly higher (66.8%) in 110 DAP seeds. At 120 DAP TTC staining percentage was lower than seed germination percentage in any collection period. Although highest seed germination percentage was observed in 100 DAP seeds, 140 DAP seeds developed faster and some protocorms could develop to stage 4. A correlation (Pearson’s R = 0.684; P = 0.05) was found between TTC embryo staining and seed germination.
At 180 days after culture, 95 DAP seeds showed highest germination percentage (96.2%), which was significantly higher than all other collection periods, but the percentage of protocorm necrosis was also highest (20.0%). Although the next highest seed germination percentage was 75.0% in 110 DAP seeds, a value that was also significantly higher than all other collection periods, 50% of the protocorms developed to stage 5. This value was significantly higher than all other collection periods (Table 3). Therefore, 110 DAP seeds were assumed to be most suitable for protocorm development.
Effect of basal media on germination in vitro
Seeds of 95 or 110 DAP showed a different response to most basal media at 180 days after culture (Table 4 and Table 5). Highest total seed germination percentage of 95 DAP seeds was observed on quarter-strength Murashige and Skoog23 (¼MS) and on eighth-strength ( MS) basal media relative to the other eight tested basal media. However,
MS) basal media relative to the other eight tested basal media. However,  MS was most suitable for subsequent protocorm development. When
MS was most suitable for subsequent protocorm development. When  MS was used, seed germination percentage at stage 5 was 40.5%, which was significantly higher than the other nine tested basal media, including 30.1% seed germination on ¼MS medium. This latter medium was most suitable for germination of 110 DAP seeds and subsequent protocorm development, resulting in the highest total seed germination percentage (75.0%) with 50% of protocorms developing to stage 5. These values were significantly higher than on the other nine tested basal media. Therefore,
MS was used, seed germination percentage at stage 5 was 40.5%, which was significantly higher than the other nine tested basal media, including 30.1% seed germination on ¼MS medium. This latter medium was most suitable for germination of 110 DAP seeds and subsequent protocorm development, resulting in the highest total seed germination percentage (75.0%) with 50% of protocorms developing to stage 5. These values were significantly higher than on the other nine tested basal media. Therefore,  MS medium proved to be the most appropriate basal medium for seed germination and protocorm development of 95 DAP seeds while ¼MS was most suitable for 110 DAP seeds.
MS medium proved to be the most appropriate basal medium for seed germination and protocorm development of 95 DAP seeds while ¼MS was most suitable for 110 DAP seeds.
Effect of NaOCl on TTC staining and germination of mature seeds
Microscopic observations showed that the dark testa of P. armeniacum seeds became greyish white after treatment with NaOCl. TTC did not stain fully mature (180 DAP) seed, which germinated on ¼MS medium supplemented with 0.5 mg l−1 α-naphthaleneacetic acid (NAA), 10% coconut water (CW) and 1.0 g l−1 activated charcoal (AC) at 180 days after culture. The transparency of the testa increased as the exposure period to NaOCl was prolonged. TTC staining, seed germination percentage and protocorm development were affected by the concentration and duration of NaOCl treatment. TTC staining and seed germination percentage were highest when 180 DAP seeds were pretreated with NaOCl containing 1.0% available chlorine for 90 min. These values were significantly higher than all other treatments and these processes were accompanied by the development of most protocorms to stages 4 and 5 (Table 6). However, when seeds were pretreated with the lowest NaOCl concentration (0.5% available chlorine) and for the shortest exposure period (30 min), or the highest NaOCl concentration (1.5% available chlorine) and for the longest exposure period (120 min), seed germination percentage (5.7% and 5.5%, respectively) and TTC staining percentage (2.5% and 3.8%, respectively) were significantly lower than all other treatments (Table 6). After NaOCl pretreatment, a strong correlation (Pearson’s R = 0.939; P = 0.01) was found between TTC embryo staining and seed germination.
Effect of ultrasonication pretreatment on TTC staining and germination of mature seeds
TTC staining and germination percentage gradually increased when the seeds were ultrasonicated for 2 to 8 min but decreased when ultrasonication exceeded 10 min. However, highest TTC staining (19.7%) and germination percentage (25.4%) were observed after 8 min of ultrasonication, which was accompanied by the simultaneous development of most protocorms to stages 4 and 5 (Table 7). After ultrasonication pretreatment, a strong correlation (Pearson’s R = 0.989; P = 0.01) was found between TTC embryo staining and seed germination.
Effect of organic amendments on plantlet growth in vitro
The seedlings arising from seed that germinated on ¼MS medium supplemented with 0.5 mg l−1 NAA, 10% CW and 1.0 g l−1 AC after 180 days were first subcultured on ¼MS medium supplemented with 1.0 mg l−1 NAA, 10% CW, 1.0 g l−1 peptone and 1.0 g l−1 AC. These seedlings could grow to over 2 cm in height following 90 days of culture (Fig. 3h, data not shown). When plantlets 2 cm in height were transferred to Hyponex N026 medium supplemented with 1.0 g l−1 peptone, 1.0 mg l−1 NAA and 1.0 g l−1 AC and different types and concentrations of organic amendments, plantlet growth in vitro was significantly affected (Table 8). Following the assessment of all growth parameters (mean number of shoots per seedling, height of tallest shoots, number of leaves in tallest shoots and length and diameter of longest roots), Hyponex N026 medium supplemented with 50 g l−1 banana homogenate (BH) was found to be most suitable for plantlet growth in vitro since it resulted in a favorable number of shoots, tallest shoots, most leaves and moderate root length and diameter. These are aspects that are appropriate for transplanting and that ensure effective ex vitro plantlet growth (Fig. 3i).
In vitro seed germination and seedling development of P. armeniacum.
(a) Dry mature seed of P. armeniacum observed by scanning electron microscopy; (b) stage 0, swelling seeds, ungerminated; (c) stage 1, testa ruptured; (d) stage 2, appearance of the shoot; (e) stage 3, appearance of the shoot and rhizoids; (f) stage 4, emergence and elongation of first leaf; (g) stage 5, presence of two or more leaves; (h) development of seedlings on ¼MS medium supplemented with 1.0 mg l−1 NAA, 10% CW, 1.0 g l−1 peptone and 1.0 g l−1 AC; (i) seedling growth on Hyponex N026 medium supplemented with 1.0 mg l−1 NAA, 1.0 g l−1 peptone, 50 g l−1 BH and 1.0 g l−1 AC; (j) transplanted plantlets after 6 months’ acclimatization in the greenhouse. Scale bars: (a) 100 μm, (b), (c) and (d) 0.2 mm, (e) 0.5 cm, (f) and (g) 1.0 cm, (h) and (i) 1.5 cm, (j) 3.0 cm.
Greenhouse acclimatization
Plantlets grew vigorously 30 days after transplanting. After 90 days of transplanting, the highest percentage of plantlet survival (90.7%) was observed on Chilean sphagnum moss which was significantly higher than on sieved peat, commercial sand for orchids or substrate mixture 2 (Table 9). The lowest plantlet survival percentage (74.3%) was observed on commercial sand for orchids, which was not significantly different to sieved peat or substrate mixture 2. After 180 days of transplanting, the highest plantlet survival percentage (85.3%) was observed on Zhijing stone for orchids (Fig. 3j), which was also significantly higher than on sieved peat, commercial sand for orchids or substrate mixture 2, but the lowest plantlet survival percentage (63.7%) was observed on commercial sand for orchids. This latter substrate performed most poorly among all tested substrates. Relative to plantlet survival percentage on the same substrate at 90 and 180 days after transplanting, both values were not significantly different on Zhijing stone for orchids, substrate mixture 1 or substrate mixture 3; all three substrates included Zhijing stone for orchids (Table 9). Since the roots of transplanted seedlings almost never elongated on Chilean sphagnum moss, Zhijing stone for orchids is most suitable for transplanting seedlings. About 5000 plantlets from all treatments were successfully acclimatized to greenhouse conditions and can be used for ornamental, ecorehabilitation and conservation purposes.
Discussion
Many reports have indicated that Paphiopedilum seed germination in vitro is significantly affected by the degree of seed maturity6. Asymbiotic seed germination of fully mature Paphiopedilum orchid seeds is often difficult and thus immature seeds need to be germinated more readily; moreover, even though mature seeds are suitable for storage, seed germination of mature dry seeds following storage has not yet been reported5,6,12,13,14,19,21. There are some reports related to zygotic embryo development in Paphiopedilum18,19,20,21,22. However, analyses between embryo/seed morphological characteristics and seed germination percentage or protocorm development do not exist. Lee et al.21 reported that the suspensor is the major site of nutrient uptake for the developing zygotic embryo. In the in vitro culture of early zygotic embryos, the suspensor may remain the major site of nutrient uptake. These absorbed nutrients are necessary for the further in vitro development of the embryo and for protocorm formation and development. In this study, germination percentage of 95 DAP seed was highest and at this stage, the suspensor had only started to degenerate, making the pathway to active nutrient uptake a realistic option. However, at 95 DAP, the embryo had not yet fully matured and the accumulation of starch and lipid globules was insufficient to sustain subsequent seedling development, resulting in the death of 20% of germinated protocorms. Seed collected from 110 DAP capsules had the next highest seed germination percentage and most seedlings developed to stage 5. In this developmental stage, although the suspensor degenerated and the embryo had fully formed, the compact testa did not form; this state may have permitted the penetration and absorption of water and nutrients21. At 120 DAP, seed germination decreased sharply from 66.9% to 37.5% at 110 DAP; at this developmental stage, the embryo was fully mature with a compact and fully formed testa which may have prevented the absorption and permeation of water and nutrients21. At 130 DAP, seed germination percentage decreased further, possibly because of additional development of the impermeable testa caused by cutinization and lignification24,25, the presence of chemical inhibitors such as abscisic acid (ABA), or the lack of certain germination-promoting hormones26. Lee found that the endogenous ABA content of Cypripedium formosanum, which is also commonly referred to as a slipper orchid, was low at 60 DAP but increased rapidly during 120–150 DAP27. A high level of ABA accumulated on the surface wall of the embryo proper and the shrivelled inner integument of mature C. formosanum seeds, which coincided with a rapid decrease in seed germination.
Seed germination and seedling development of Paphiopedilum is affected by the choice of medium5,6,13. Most Paphiopedilum species prefer a low salt medium for seed germination6,13,14,16,28. However, some experimental results in the literature, such as the germination percentage of seeds of the same species on the same medium, are inconsistent or even contradictory13,14. Chen et al.13 reported that Robert Ernst medium (RE) was most suitable for the germination of 120 DAP seed of P. armeniacum and P. micranthum and significantly higher than on MS, ½MS, Knudson’s C (KC) and Hyponex media. In contrast, Ding et al.14 indicated that 1/5MS medium was most suitable for 112 DAP seed germination of P. armeniacum and P. micranthum and higher than on RE, ½MS, 1/10MS, KC and Hyponex media. In this study, we found differences in the most appropriate medium for the germination of P. armeniacum seed at different stages of seed maturity. The most appropriate medium for younger seeds (95 DAP) was  MS (94.3% seed germination) or ¼MS (96.2% seed germination), but
MS (94.3% seed germination) or ¼MS (96.2% seed germination), but  MS was more suitable for subsequent protocorm development. The percentage of seedlings that developed to stage 5 on
MS was more suitable for subsequent protocorm development. The percentage of seedlings that developed to stage 5 on  MS was 40.5%, which was significant higher than 30.1% on ¼MS. 110 DAP seeds preferred a higher salt medium (¼MS). This preference may be because younger seeds were sensitive to a higher salt concentration in the medium. This result might also explain why seed germination percentage of some Paphiopedilum species is different on the same medium. This is most likely because the collecting period (i.e., age) of seeds is different and even though the collecting period may be consistent, their maturity may be inconsistent in different growing environments or seed harvest years29.
MS was 40.5%, which was significant higher than 30.1% on ¼MS. 110 DAP seeds preferred a higher salt medium (¼MS). This preference may be because younger seeds were sensitive to a higher salt concentration in the medium. This result might also explain why seed germination percentage of some Paphiopedilum species is different on the same medium. This is most likely because the collecting period (i.e., age) of seeds is different and even though the collecting period may be consistent, their maturity may be inconsistent in different growing environments or seed harvest years29.
TTC staining is the most widely used biochemical method to evaluate seed viability. Compared to direct germination assays, this method has the advantage of being rapid and suitable for controlled conditions30,31,32. This test has been successfully used with some orchids, including Cypripedium33,34,35,36. Lanzer et al.33 reported that the germination percentage of C. acaule was always lower than that of embryos stained with TTC. Lee et al.34 and Zhang et al.35 obtained the same result for C. formosanum. However, Yamazaki and Miyoshi25 reported that the mature seeds (140 DAP) of Cephalanthera falcata were not stained by TTC. These results are difficult to interpret because mature C. formosanum and C. falcata seeds have two compact testas, thus cutinization and lignification of the inner integument (often been referred to as the ‘carapace’) is postulated to strengthen the inhibition of embryo growth by mechanical restriction37,38 or by chemical reactions25,34,35. The seed germination and TTC staining percentage of Paphiopedilum SCBG Red Jewel were significantly affected by the degree of seed maturity, but no correlation (Pearson’s R = 0.086) was found between TTC embryo staining and seed germination (Zeng et al., unpublished data). In this study, before seed matured, TTC staining percentage was higher than germination percentage but after seed matured, TTC staining of seeds was lower than germination percentage (Table 2). However, in this study, strongly positive correlations (Pearson’s R = 0.939 or 0.989 respectively), were found between TTC embryo staining and seed germination from seeds at different developmental stages or from mature seeds following pretreatment with NaOCl or ultrasonication. These results may reflect differences in the inherent characteristics of different Paphiopedilum species or varieties. Nevertheless, mature Paphiopedilum seeds have a single testa19,20,21,22 and germination percentage is usually higher than Cypripedium seeds6,36, but we found that the TTC staining percentage of Paphiopedilum seeds tends to be lower or not stained at all. This difference in TTC staining and seed germination between Paphiopedilum and Cypripedium could be attributed not only to the permeability of the testa, but may also depend on the reaction of different tissues to TTC dyes39,40, because the TTC staining assay is based on the activity of seed dehydrogenases30.
Mature orchid seeds may have a greater potential for propagation and storage because of a fuller testa and lower water content22,35. Mature seeds may also assist their survival in harsh climates. Under natural conditions, seasonal changes in temperature, wetting and drying, mechanical abrasion or digestion by mycorrhizal fungi may damage the testa and promote seed germination34. However, in this study, 1.3% of fully mature (180 DAP) P. armeniacum seeds obtained directly from wet capsules were stained by TTC, while displaying a 4.5% seed germination percentage after culture for 120 days. In contrast, fully mature (180 DAP) dry stored P. armeniacum seeds could not be stained by TTC or germinate (data not shown), although germination and protocorm development could be stimulated by suitable pretreatment with NaOCl or ultrasonication, as was also observed in previous reports for orchids, including Paphiopedilum5,12,33,34,38,41. The TTC staining of seeds was not studied when they were pretreated with NaOCl or ultrasonication. Pretreatment of NaOCl may erode the testa and disrupt cell wall integrity, thus increasing the permeability of the seed to oxygen and nutrients24,42,43,44,45, while inhibitors such as ABA may become reduced within seeds12,26. However, this hypothesis needs to be tested further. The use of ultrasound had been used to increase the germination of mature orchid, possibly because embryos of treated seeds result in cavitation and acoustic microstreaming, two processes that can modify cellular ultrastructure, enzyme stability and cell growth and can also cause breaks in extracellular polymers, release DNA from the nucleus, decrease cell stability, alter cell membrane permeability and modify charges on the surfaces of cells or stimulate protein synthesis in plant cells and protoplasts46,47. However, in this study, when mature dry seeds (180 DAP) were pretreated with ultrasound, seed germination percentage was higher than TTC staining percentage for all treatments, but when they were pretreated with NaClO containing 1.0% available chlorine for 30, 60 or 90 min or 0.5% available chlorine for 120 min, TTC staining percentage was higher than seed germination percentage. Conversely, in other treatments, an opposite result was observed and TTC staining percentage of seed treated with ultrasound was lower than with NaClO pretreatment (Table 6; Table 7). This may be caused by some inhibitors that prevent staining in the testa but that can be eliminated by suitable NaOCl pretreatment.
Orchid seed germination and protocorm development are stimulated by organic amendments5,11,48,49,50,51,52. Organic amendments include, among others, CW, carrot homogenate (CH), BH, potato homogenate (PH), tryptone or peptone5,6 but only CH, BH and PH are used for Paphiopedilum seedling growth and rooting6. Therefore, short, 2-cm tall seedlings were initially subcultured on ¼MS medium supplemented with 1.0 mg l−1 NAA, 10% CW, 1.0 g l−1 peptone and 1.0 g l−1 AC without CH, BH, PH. In all culture stages, CW can facilitate seed germination and protocorm development possibly because CW contains many different types of biochemicals, including amino acids, vitamins, sugar and plant growth regulators, as well as various inorganic ions such as phosphorus, magnesium, potassium and sodium53,54. The inhibitory effect of a high BH concentration on seedling growth may be caused by excess carbohydrates or other nutrients in the medium which can prevent plantlet growth in vitro6.
A high survival of in vitro Paphiopedilum plantlets was possible when they were transplanted to Chilean sphagnum moss5. However, the roots of the transplanted plantlets elongated slowly and few new roots formed. Therefore, Chilean sphagnum moss is an appropriate substrate only for temporary planting of Paphiopedilum plantlets derived from in vitro. Two to three months after transplanting, plantlets must be transplanted once again to another substrate. In this study, Zhijing stone for orchids was the most suitable substrate, together with two other substrate mixtures 1 and 3, which contain Zhijing stone for orchids, while commercial sand for orchids was least effective. This may be because Zhijing stone for orchids has good water holding capacity and water permeability, commercial sand for orchids has good water permeability and bad water holding capacity, while Chilean sphagnum moss has good water holding capacity and bad water permeability (data not shown).
In conclusion, the present study documents, for the first time and in considerable detail, the degree to which seed maturity of P. armeniacum is a critical factor for seed germination and subsequent seedling development in vitro and analyzes the relationships between embryo morphology, seed germination and TTC staining, factors that have to date not yet been studied in this genus. The optimum procedure for seed germination and subsequent development of P. armeniacum is to sow 95 to 110 DAP seeds aseptically on  MS or ¼MS media. Mature dry seeds need to be pretreated with 1.0% NaOCl solution for 90 min or with 40 kHz of ultrasound for 8 min to enhance seed germination. This procedure might also serve as a useful method to improve seed germination in other terrestrial orchid genera.
MS or ¼MS media. Mature dry seeds need to be pretreated with 1.0% NaOCl solution for 90 min or with 40 kHz of ultrasound for 8 min to enhance seed germination. This procedure might also serve as a useful method to improve seed germination in other terrestrial orchid genera.
Materials and Methods
Plant material
About 1000 P. armeniacum S. C. Chen et F. Y. Liu plants were maintained in a glass greenhouse with a water curtain and blowers for cooling and ventilation in the South China Botanical Garden, Guangzhou, China. The plants were potted in a substrate of Zhijing stone for orchids (Northridge Enterprise Co. Ltd., Taiwan) under no more than 800 μmol m−2 s−1 natural light maintained by a sunshade net. The key characteristics of Zhijing stone for orchids are: pH 5.7, EC 88 μs cm−1, unit weight 0.89 g cm−3, total porosity 69.2% and water-holding porosity 32.6%. The average temperature and relative humidity ranged from 10–32 °C and 70–98%, respectively. Initial trials indicated that the setting percentage and seed viability from self-pollination were not significantly different to cross-pollination: setting percentage exceeded 90% in both cases. Therefore, the flowers from three-year-old adult plants were labeled and artificially self-pollinated by transferring pollen onto the stigma of the same flower as they became fully opened in March to April. For the experiments, 800 capsules were obtained and approximately 100,000 seeds/capsule.
Histological and histochemical studies
Capsules of different developmental stages from 38 to 122 DAP were collected at 7-day intervals. Capsules are sliced horizontally and fixed in a solution of FAA (50% ethanol: acetic acid: formalin; 89:6:5, v/v) at 4 °C overnight. After fixation, samples were rinsed six times within 2 h with distilled water, then dehydrated in a graded ethanol series: 30% ethanol for 20 min, 50% ethanol for 20 min, 70% ethanol at 4 °C overnight, 80% ethanol for 20 min, 90% ethanol for 20 min, 100% ethanol for 30 min while the last step was repeated once more. After dehydration, the samples were immersed in epoxy propane for 30 min then re-immersed for the same time period. Samples were transferred to Spurr’s resin for 6 h at room temperature then immersed in fresh Spurr’s resin at 4 °C overnight. Finally, the materials were embedded in Spurr’s resin and baked at 70 °C for 48 h. Semi-thin sections (2 μm thick) were cut with glass knives on a LKB-11800 microtome (LKB Ltd., Uppsala, Sweden). The sections were stained with 1 mg ml−1 toluidine blue (Sigma Chemical Co., St. Louis, MO, USA) for 10 min, then washed in distilled water and mounted in water containing 0.1% n-propyl gallate (Sigma Chemical Co.), an antifading compound, as detailed by O’Brien and McCully55. The fluorescence pattern of toluidine blue was examined using an epifluorescence microscope (Axioskop 2, Carl Zeiss AG, Oberkochen, Germany) equipped with a Zeiss filter set 15 (546/12 nm excitation filter and 590 emission barrier filter)56. The sections were observed and images were captured digitally using a CCD camera attached to a light microscope (Axioskop 2) to observe the microscopic structural features of seeds, including starch and lipid globules.
Mature seed morphology (Fig. 3a) was observed by scanning electron microscopy (SEM) using the method reported in Zeng et al.5 Samples were fixed in 3% (v/v) glutaraldehyde in 0.1 phosphate buffer (pH 7.0) for 12 h and dehydrated in an ethanol series [30%–50%–70%–80%–90%–100% (v/v) in water for 10 min each step], followed by treatment three times with tert-butanol, for 10 min each time. The samples were dried with a JFD-310 freeze dryer, affixed to aluminium stubs and coated with gold palladium using a JFC-1600 Fine Coater. The samples were examined with a JSM-6360LV scanning electron microscope (JEOL, Tokyo, Japan).
Effect of the degree of seed maturity on TTC staining and in vitro germination
To determine the influence of the degree of seed maturity on TTC staining and seed germination, 10 seed capsules were collected at different developmental stages – every 10 days from 45 to 180 DAP, except for a 15-day interval from 95 to 110 DAP during which laboratory staff was limited. A similar experimental design had been used by Zeng et al.5 Seed capsules were surface sterilized by dipping into 75% (v/v) ethanol for 2 min, followed by agitation for 15 min in 1 g l−1 mercuric chloride (HgCl2) and 0.05% (w/v) Tween 20, after which the capsules were rinsed four times with sterile distilled water (SDW). TTC staining used ca. 300 seeds from each collection date that were soaked in filtered TTC solution (1 g in 100 ml phosphate buffer, pH 7.0) for 24 h in the dark at 25 °C and rinsed five times in SDW. Seeds were viewed using a Leica S8APO microscope (Wetzlar, Germany). Embryos that were completely colored pink to red were considered to be viable while seeds that were partially colored, white, yellow or brown were assumed to not be viable.
Based on initial trials, quarter-strength MS medium (one quarter of macro- and micronutrients; ¼MS) medium23 supplemented with 0.5 mg l−1 NAA, 10% CW (v/v) and 1.0 g l−1 activated charcoal (AC) was suitable for seed germination. Therefore, at each collection date, surface-disinfected capsules were cut open longitudinally, seeds were scooped out with sterile forceps and placed on this medium. For each treatment, ca. 300 seeds from five capsules were cultured in a 500-ml culture flask containing 90 ml of medium. All experiments consisted of three independent replicates with 10 culture flasks per replicate. Cultures were observed every 30 days for signs of germination and subsequent protocorm development using a Leica S8APO microscope. Developmental stages (Fig. 3b–g) were adapted from Zeng et al.6, as follows: “(1) Stage 0, ungerminated seed with embryo and not rupturing the testa; (2) stage 1, rupture of the testa by enlarging embryo; (3) stage 2, appearance of the shoot (=protomeristem) and/or rhizoids; (4) stage 3, appearance of the shoot and rhizoids; (5) stage 4, emergence and elongation of first leaf or more roots present; (6) stage 5 presence of two or more leaves and roots (=seedling).” The percentage of seed/protocorms/seedlings at each developmental stage was calculated by dividing the number of seed/protocorms/seedlings in each stage by the total number of cultured seeds in each flask ×100 in each flask at 120 days and 180 days. In this equation, seeds included seeds with and without an embryo. Germination was considered to have occurred only if a swollen embryo was present and if the testa had ruptured (Stage 1).
Effect of basal media on germination in vitro
To study the effect of inorganic salts on seed germination and subsequent protocorm development at different collection times, 95 and 110 DAP seeds from surface-disinfected capsules were placed onto 10 basal sowing media: (1) MS23, (2) half-strength MS (½ MS macro- and micronutrients), (3) ¼MS, (4)  MS, (5) Knudson’s C (KC)57, (6) Vacin and Went (VW)58, (7) Robert Ernst (RE)59, (8) Thomale GD60, (9) Hyponex N02652, (10) Hyponex N0165. All basal media were supplemented with 1.0 g l−1 AC, 20 g l−1 sucrose, 10% CW, 0.5 mg l−1 NAA and 5.2 g l−1 agar (Huankai Microbial Sci. & Tech, Co., Ltd., Guangzhou, China).
MS, (5) Knudson’s C (KC)57, (6) Vacin and Went (VW)58, (7) Robert Ernst (RE)59, (8) Thomale GD60, (9) Hyponex N02652, (10) Hyponex N0165. All basal media were supplemented with 1.0 g l−1 AC, 20 g l−1 sucrose, 10% CW, 0.5 mg l−1 NAA and 5.2 g l−1 agar (Huankai Microbial Sci. & Tech, Co., Ltd., Guangzhou, China).
Effect of NaOCl treatment on TTC staining and germination of maturity seeds
To evaluate the effect of NaOCl on seed germination percentage, mature seeds (180 DAP) dried on silica gel in a sealed glass jar showing highest levels of germination were soaked in 1 g l−1 HgCl2 for 10 min (control), then in NaOCl containing 0.5%, 1.0% or 1.5% available chlorine for 30, 60, 90 and 120 min. After these pretreatments, seeds were rinsed three times with sterilized water (filtered through sterilized filter paper; Hangzhou WoHua Filter Paper Co., Ltd., Hangzhou, China) and then placed on ¼MS medium containing 10% CW, 0.5 mg l−1 NAA and 1.0 g l−1 AC or stained by TTC and viewed using a Leica DFC 450 stereomicroscope. For each treatment, ca. 300 seeds were cultured in a 500-ml culture flask containing 90 ml of medium. All experiments consisted of three independent replicates with 10 culture flasks per replicate.
Effect of ultrasonication pretreatment on TTC staining and germination of maturity seeds
The sterilized capsules were split with a sterilized scalpel after disinfection and mature seed (180 DAP) dried on silica gel in a sealed glass jar were placed into a 50-ml aseptic conical tube. Seeds were suspended by adding 10 ml of SDW and ultrasonicated with an Ultrasonic Cleaning Machine (SB-5200 DTN, Scientz, Ningbo, China) at 40 kHz for 2, 4, 6, 8, or 10 min at room temperature. Ultrasonicated seeds were stained by TTC or transferred onto ¼MS medium as described above. For each treatment, ca. 300 seeds were cultured in a 500-ml culture flask containing 90 ml of medium. All experiments consisted of three independent replicates with 10 culture flasks per replicate.
Effect of organic amendments on plantlets growth in vitro
Following initial trials, ¼MS medium supplemented with 1.0 mg l−1 NAA, 1.0 g l−1 peptone and 1.0 g l−1 AC was found to be suitable for the growth of plantlets shorter than 2 cm in the first subculture while Hyponex N026 medium supplemented with 1.0 mg l−1 NAA, 1.0 g l−1 peptone, 1.0 g l−1 AC and appropriate organic amendments were beneficial for the growth of plantlets about 2-cm in height with 2–3 leaves and 2–3 roots. The status of plantlet growth (mean number of shoots per seedling, height of tallest shoot, number of leaves of tallest shoot, number of roots, length and width of longest root) were assessed on plantlets growing on Hyponex N026 media containing 50, 100, 150 g l−1 CW, CH, PH, or 25, 50, 100 g l−1 BH). The CW used in these experiments was obtained from 6- to 7-month-old green coconuts from Hainan province, China and was filtered through one sheet of filter paper. The fruits for the three organic amendments (CH, PH and BH) were purchased from a local supermarket and the homogenates were obtained after peeling, then homogenizing. Fruits purchased for the three organic amendments from different commercial sources had no significant influence on the efficiency of the culture medium. All experiments consisted of three independent replicates with 10 culture flasks per replicate, with 20 plantlets in each flask.
Greenhouse acclimatization
In vitro propagated plantlets 5-cm in height or taller were transferred to natural conditions for acclimation for 7 days, then transplanted into pots with Chilean sphagnum moss, sieved peat, Zhijing stone for orchids or mixed medium 1 [Zhijing stone for orchids: sieved peat: shattered fir bark; 2: 1: 1 (v/v)], mixed medium 2 [commercial sand for orchids: sieved peat: shattered fir bark; 2: 1: 1 (v/v)], or mixed medium 3 [Zhijing stone for orchids: coconut bran: shattered fir bark; 2: 1: 1 (v/v)] between April and May. The transplanted plantlets were grown in a greenhouse with a water curtain and blowers for cooling and ventilation under no more than 800 μmol m−2 s−1 natural light with sunshade nets (Zhejiang Luqiao Fangyuan Mesh Factory, Taizhou, China). Plantlets were watered at 1–2-day intervals. Average temperatures ranged from 20 to 32 °C and humidity levels ranged from 70 to 98%. The percentage of plantlet survival was recorded 90 and 180 days after transplanting. Each experiment consisted of three independent replicates with 100 plantlets per replicate.
Culture conditions
Whenever no special illumination requirements existed, then all cultures were incubated in 500-ml conical flasks closed with perforated rubber stoppers and plugged with cotton. Each flask contained 90 ml of medium that in turn contained 20 g l−1 sucrose and 5.2 g l−1 agar. Medium pH was adjusted to 5.5 with 56.11 g l−1 (1 mol l–1 KOH) and 36.46 g l−1 (1 mol l–1) HCl before autoclaving at 121 °C for 18 min at 1.06 kg cm–2. The CW used in these experiments was the same as that obtained above. The cultures were incubated at 25 ± 1 °C with a 16-h photoperiod under cool white fluorescent lamps delivering a photosynthetic photon flux density of ca. 45 μmol m–2 s–1.
Data analysis
All experiments were established in a completely randomized design. The data were analyzed with SPSS 17.0 for Windows (Microsoft Corp., Washington, USA) and expressed as means ± standard error (SE) using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) at P = 0.05. Correlations were determined as Pearson’s correlation coefficient at P = 0.05 or P = 0.01.
Additional Information
How to cite this article: Zhang, Y.-Y. et al. Embryo development in association with asymbiotic seed germination in vitro of Paphiopedilum armeniacum S. C. Chen et F. Y. Liu. Sci. Rep. 5, 16356; doi: 10.1038/srep16356 (2015).
References
Cribb, P. In The Genus Paphiopedilum. 2nd edn 48–396 ( Cribb, P. ., National History Publications, Borneo, 1998).
Wu, Z. Y., Raven, P. H. & Hong, D. Y. eds. In Flora of China. Vol. 25 (Orchidaceae) 33–44 ( Liu, Z. J. et al.Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, 2009).
World Checklist of Selected Plant Families. http://apps.kew.org/wcsp/home.do.Date of access: 25/09/2015.
CITES. http://www.cites.org/eng/app/appendices.php. Appendices I, II and III. (valid from 12 June 2013). Date of access: 11/06/2015.
Zeng, S. J. et al. Asymbiotic seed germination, seedling development and reintroduction of Paphiopedilum wardii Sumerh., an endangered terrestrial orchid. Sci Hortic 138, 198–209 (2012).
Zeng, S. J. et al.In vitro propagation of Paphiopedilum orchids. Crit Rev Biotechnol 10.3109/07388551.2014.993585 (2015).
Royal Horticultural Society (RHS) Horticultural Database. http://www.rhs.org.uk/plants/index.asp. The International Orchid Register. Date of access: 25/09/2015.
Chen, S. C. & Liu, F. Y. Notes on some species of Paphiopedilum from Yunnan. Acta Bot Yunnan 4, 163–167 (1982).
Stewart, J. & Button J. Tissue culture studies in Paphiopedilum. Amer Orchid Soc Bull 135, 88–95 (1975).
Huang, L. C. A procedure for asexual multiplication of Paphiopedilum in vitro. Amer Orchid Soc Bull 57, 274–278 (1988).
Hossain, M. M. et al. The application of biotechnology to orchids. Critical Rev Plant Sci 32, 69–139 (2013).
Lee, Y. I. The asymbiotic seed germination of six Paphiopedilum species in relation to the time of seed collection and seed pretreatment. Acta Hortic 755, 381–385 (2007).
Chen, Z. L., Ye, X. L., Liang, C. Y. & Duan J. Seed germination in vitro of Paphiopedilum armeniacum and P. micranthum. Acta Hortic Sin 31, 540–542 (2004).
Ding, C. C., Wu, H. & Liu, F. Y. Factors affecting the germination of Paphiopedilum armeniacum. Acta Bot Yunnan 26, 673–677 (2004).
Ding, C. Q., Yu, H., Liu, H. Y. & Wei, X. Q. Embryo culture and rapid propagation of Paphiopedilum armeniacum. Plant Physiol Commun 41, 55 (2005).
Long, B., Niemiera, A. X., Cheng, Z. Y. & Long, C. L. In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue Organ Cult 101, 151–162 (2010).
Zhang, J. J., Yan, N. & Hu, H. The seed development of three Paphiopedilum species in relation to asymbiotic germination. Plant Div Res 35, 33–40 (2013).
Zinger, N. V. & Poddubnaya-Arnoldi, V. Application of histochemical techniques to the study of embryonic processes in certain orchids. Phytomorphology 16, 111–124 (1966).
Nagashima, T. Studies in the seed germination and embryogenesis in Cymbidium goeringii Rchb. f. and Paphiopedilum insigne var. sanderae Rchb. J Jpn Soc Hortic Sci 51, 94–105 (1982).
Ren, L. & Wang, F. H. Embryological studies of Paphiopedilum godefroyae Stein. Acta Bot Sin 29, 14–22 (1987).
Lee, Y. I., Yeung, E. C., Lee, N. & Chung, M. C. Embryo development in the lady’s slipper orchid, Paphiopedilum delenatii, with emphasis on the ultrastructure of the suspensor. Ann Bot 98, 1311–1319 (2006).
Zhang, Y., Lee, Y. I., Deng, L. & Zhao, S. W. Asymbiotic germination of immature seeds and the seedling development of Cypripedium macranthos Sw., an endangered lady’s slipper orchid. Sci Hortic 164, 130–136 (2013).
Murashige, T. & Skoog, F. A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15, 473–497 (1962).
van Waes, J. M. & Debergh, P. C. In vitro germination of some Western European orchids. Physiol Plant 67, 253–261 (1986).
Yamazaki, J. & Miyoshi K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann Bot 98, 197–1206 (2006).
van der Kinderen, G. Abscisic acid in terrestrial orchid seeds: a possible impact on their germination. Lindleyana 2, 84–87 (1987).
Lee, Y. I. Growth periodicity, changes of endogenous abscisic acid during embryogenesis and in vitro propagation of Cypripedium formosanum Hay. In PhD dissertation ( Lee, Y. I., National Taiwan Uni., Taipei, Taiwan, 2003).
Pierik, R. L. M., Sprenkels, P. A., Vanderharst, B. & Vandermeys, Q. G. Seed germination and further development of plantlets of Paphiopedilum ciliolare Pfitz in vitro. Sci Hortic 34, 139–153 (1988).
De Pauw, M. A. & Remphrey, W. R. In vitro germination of three Cypripedium species in relation to time of seed collection, media and cold treatment. Can J Bot 71, 879–885 (1993)
Mackay, D. B. In Viability of seeds, 172–208 ( Mackay, D. B., New York: Syracuse Uni. Press, Syracuse, 1972).
Singh, F. Differential staining of orchid seeds for viability testing. Amer Orchid Soc Bull 50, 416–418 (1981).
Shoushtari, B. D., Heydary, R., Jogson, G. L. & Arditti, J. Germination and viability staining of orchid seed following prolonged storage. Lindleyana 9, 77–84 (1994).
Lauzer, D., St.-Arnaud, M. & Barabe, D. Tetrazolium staining and in vitro germination of mature seeds of Cypripedium acaule (Orchidaceae). Lindleyana 9, 197–204 (1994).
Lee, Y. I., Lee, N., Yeung, E. C. & Chung, M. C. Embryo development of Cypripedium formosanum in relation to seed germination in vitro. J Amer Soc Hortic Sci 130, 747–753 (2005).
Zhang, Y., Zhang, Q. X., Zhao, S. W. & Ling C. Y. Morphological characteristics and viability testing of Cypripedium macranthos seed. J Beijing For Univ 32, 69–73 (2010).
Zeng, S. J. et al. Seed biology and in vitro seed germination of Cypripedium. Crit Rev Biotechnol 34(4), 358–371 (2014).
Miyoshi, K. & Mii, M. Stimulatory effects of sodium and calcium hypochlorite, pre-chilling and cytokinins on the germination of Cypripedium macranthos seed in vitro. Physiol Plant 102, 481–486 (1998).
Miyoshi, K. & Mii, M. Ultrasonic treatment for enhancing seed germination of terrestrial orchid, Calanthe discolor, in asymbiotic culture. Sci Hortic 35, 127–130 (1998).
Pritchard, H. W. Determination of orchid seed viability using fluorescein diacetate. Plant Cell Environ 8, 727–730 (1985).
Vierheiling, H., Coughlan, A. P., Wyss, U. & Pichea, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64, 5004–5007 (1998).
Shin, Y. K., Baque, M. A., Elghamedi, S., Lee, E. J. & Paek, K. Y. Effects of activated charcoal, plant growth regulators and ultrasonic pre-treatments on in vitro germination and protocorm formation of Calanthe hybrids. Austr J Crop Sci 5, 582–588 (2011).
Frank, A. B. & Larson, K. L. Influence of oxygen, sodium hypochlorite and dehulling on germination of green needle grass seed (Stipa viridula Trin). Crop Sci 10, 679–682 (1970).
Kaneko, Y. & Morohashi, Y. The effect of sodium hypochlorite treatment on the development of α-amylase activity in mung bean cotyledons. Plant Sci 164, 287–292 (2003).
Bae, K. H., Kim, C. H., Sun, B. Y. & Choi, Y. E. Structural changes of seed coats and stimulation of in vitro germination of fully mature seeds of Cypripedium macranthos Swartz (Orchidaceae) by NaOCl pretreatment. Propag Ornam Plants 10, 107–113 (2010).
Piao, R. Z., Wang, Y. L. & Zhao, H. Y. Preliminary exploration on in vitro seed culture conditions of Cypripedium macranthos Sw. J Anhui Agri Sci 39, 18428–18429, 18445 (2011).
Joersbo, M. & Brunstedt, J. Protein synthesis stimulated in sonicated sugar beet cells and protoplasts. Ultrasound Med Biol 16, 719–724 (1990).
Teixeira da Silva, J. A. & Dobránszki, J. Sonication and ultrasound: impact on plant growth and development. Plant Cell, Tissue Organ Cult 117, 131–143 (2014).
Harvais, G. Growth requirements and development of Cypripedium reginae in axenic culture. Can J Bot 51, 327–332 (1973).
Chu, C. C. & Mudge, K. W. Effects of prechilling and liquid suspension culture on seed germination of the Yellow Lady’s Slipper orchid, Cypripedium calceoclus var. pubescens. Lindleyana 9, 153–159 (1994).
DeMarie, E., Weimer, M. & Mudge, W. In vitro germination and development of ‘Showy Lady’ slipper orchid (Cypripedium reginae Walt.) seeds. HortScience 26, 272 (1991).
Teixeira da Silva, J. A., Singh, N. & Tanaka, M. Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae) and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tiss Organ Cult 84, 119–128 (2006).
Zeng, S. J. et al. Asymbiotic seed germination, induction of calli and protocorm-like bodies and in vitro seedling development of the rare and endangered Nothodoritis zhejiangensis, a Chinese orchid. HortScience 46, 460–465 (2011).
Raghavan, V. Diets and culture media for plant embryos. in RCC Handbook Series in Nutrition and Food, 361–413 ( Rechcigl, M. J. ed., Taylor & Francis Publisher, London, 1977).
Yong, J. W. H., Ge, L., Ng, Y. F. & Tan, S. N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 14, 5144–5164 (2009).
O’Brien, T. P. & McCully, M. E. In The study of plant structure: principles and selected methods. ( O’Brien, T. P. & McCully, M. E., Termarcarphi Pty. Ltd., Melbourne, 1981).
Zhang, X. H. et al. Endogenous hormone levels and anatomical characters of haustoria in Santalum album L. seedlings before and after attachment to the host. Plant Physiol 169, 859–866 (2012).
Knudson, L. A new nutrient solution for the germination of orchid seeds. Amer Orchid Soc Bull 15, 214–217 (1946).
Vacin, E. & Went F. W. Some pH changes in nutrient solutions. Bot Gaz 110, 605–613 (1949).
Arditti, J. In Orchid biology: Reviews and perspective II, 352 ( Arditti, J. et al., Cornell Univ. Press Ithaca, New York, 1982).
Thomale, H. In Die Orchideen, 62–88 (Thomale, H., Eugen Ulmer, Stuttgart, 1954).
Acknowledgements
This study was supported by the Shanghai Administration of Greenery and Cityscape Program (G142426), the Dongguan Social Development Program (20131081030), the Guangdong Key Technology Research and Development Program (2013B020503055, 2014B09091051) and the Guangzhou Key Technology Research and Development Program (Y233051001).
Author information
Authors and Affiliations
Contributions
Y.Y., S.Z., J.A.T.d.S., W.C. and J.D. conceived and designed the experiments; Y.Y., S.Z. and R.F. performed the experiments; Y.Y., S.Z. and J.X. analyzed the data; K.W. and J.D. contributed reagents/materials/analysis tools; Y.Y., S.Z. and J.A.T.d.S. wrote the manuscript; All authors reviewed the manuscript, approved all edits in the final version and take public responsibility for the content.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, YY., Wu, KL., Zhang, JX. et al. Embryo development in association with asymbiotic seed germination in vitro of Paphiopedilum armeniacum S. C. Chen et F. Y. Liu. Sci Rep 5, 16356 (2015). https://doi.org/10.1038/srep16356
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16356
This article is cited by
-
In vitro rapid propagation technology system of Dendrobium moniliforme (L.) Sw., a threatened orchid species in China
Plant Biotechnology Reports (2023)
-
In vitro optimization of seed germination and protocorm development in Gastrochilus japonicus (Makino) Schltr. (Orchidaceae)
Plant Growth Regulation (2023)
-
Characterization of phytohormone and transcriptome profiles during protocorm-like bodies development of Paphiopedilum
BMC Genomics (2021)
-
Transcriptome analysis provides insights into the non-methylated lignin synthesis in Paphiopedilum armeniacum seed
BMC Genomics (2020)
-
Highly competent in vitro propagation of Thrixspermum japonicum (Miq.) Rchb.f., a rare epiphytic orchid
In Vitro Cellular & Developmental Biology - Plant (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.