Abstract
Hyper-homocysteinemia (HHcy) is associated with microalbuminuria and glomerular injury in general and diabetic populations. However, HHcy’s role in hypertensive patients was not studied. We investigated whether HHcy is an independent risk factor for renal function decline and development of chronic kidney disease (CKD) in hypertensive men and women. This was a community-based prospective cohort study of 2,387 hypertensive adults without CKD at baseline, with a mean follow-up of 4.4 years. Baseline and follow-up levels of plasma Hcy, folate, vitamin B12, blood pressure and other pertinent covariables were obtained. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/per 1.73 m2 and an eGFR decline rate >1 ml/min/per 1.73 m2/year. There was a graded association between Hcy tertiles and eGFR decline. Subjects in the 3rd tertile of Hcy levels had an accelerated rate of eGFR decline and an increased risk of incident CKD, as compared with those in the 1st tertile, after adjusting for age, gender, baseline diabetes, SBP, BMI, smoking, dyslipidemia, eGFR, folate and vitamin B12 levels. In conclusion, in this prospective cohort of Chinese hypertensive adults, elevated baseline plasma Hcy can serve as an independent biomarker to predict renal function decline and incident CKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a serious clinical and public health challenge globally1,2,3. In China, 147 million people are affected by CKD3. Hypertension is also one of the leading causes of end stage renal disease (ESRD). Several studies have shown that even modestly elevated blood pressure (BP) places individuals at increased risk of ESRD relative to those with normal BP4,5,6. Therefore, early intervention to prevent renal injury in hypertensive population is of immense significance to prevent progression to CKD and ESRD. In addition to the traditional risk factors, such as age, BP, diabetes, smoking and hyperlipidemia, elevated plasma homocysteine (Hcy) levels, termed as hyperhomocysteinemia (HHcy), has emerged as an independent risk factor for the progression of CKD7,8. Experimental studies suggest that HHcy induces glomerular injury through redox signaling pathways and DNA hypomethylation mechanisms9,10,11,12,13. More importantly, recent human studies have shown that HHcy is associated with microalbuminuria in the general population and in patients with diabetes8,14,15. However, the effect of HHcy on renal function decline and incident CKD in hypertensive adults has not been studied.
Hcy is a sulfur-containing amino acid and an intermediate metabolite in methionine metabolism. Deficiency in Hcy metabolic enzymes and its co-factors, such as folate and other B vitamins, could lead to increased Hcy levels in both humans and mice16. It is known that methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folic acid metabolism that provides a methyl group for Hcy remethylation. The MTHFR 677TT variant impairs MTHFR function leading to the development of HHcy17. Because folate and other B vitamins are cofactors in the remethylation and trans-sulfuration pathways of Hcy metabolism, the supplementation of these vitamins has been used as an effective and inexpensive strategy for Hcy-lowering therapy18,19.
It is estimated that hypertensive disease affects 266 million adults in China, affecting nearly 27% of the adult population20. The Chinese population has a high prevalence of HHcy, particularly in the northern rural areas21,22, mostly due to a combination of low dietary folate intake and a higher prevalence of MTHFR 677TT mutation than in western populations (25% vs. <10%)23. Those with a high prevalence of folic acid deficiency (~40%) and hyperhomocysteinemia (~28%)21,22 might be at a high risk of vascular and renal disease as posed by HHcy and may benefit more from B vitamins supplementation. Recently, we demonstrated the efficacy of folic acid supplementation in primary prevention of stroke among adults with hypertension in China24. Therefore, studies to investigate the relationship between HHcy, B vitamins levels, renal function decline and incident CKD in hypertensive adults would help to identify potential risk factors or protectors in the prevention and treatment of CKD in this high-risk population and are of great clinical and public health interest in both the Chinese population and populations elsewhere that share similar characteristics.
In this community-based prospective cohort study, we sought to investigate the independent effect of baseline Hcy, folate and vitamin B12 levels on renal function decline and incident CKD in Chinese hypertensive adults without baseline CKD.
Results
Cohort description
Data from 2,387 Chinese hypertensive adults with a mean age of 60 years (range: 45 to 76 years) and mean estimated glomerular filtration rate (eGFR) of 93.9 ml/min/per 1.73 m2 at baseline were analyzed. The mean blood pressure at baseline and at the end of the follow-up was 168 ± 98 and 152 ± 84 mmHg, respectively. Forty-four percent of participants received antihypertensive treatment. The percentage of participants achieving the target BP of <140/90 mmHg at the end of the follow-up was 21.2%. At baseline, men tended to be older, more likely to be a current smoker and had higher DBP and lower serum total cholesterol, triglycerides and eGFR levels (Table 1). The median of plasma Hcy at baseline was 12.1 μmol/L, with a significant difference between men and women (P < 0.001). Gender differences were also found for folate levels. Meanwhile, no significant differences were found for baseline vitamin B12 levels between genders.
Higher Hcy level is associated with increased risk for poor renal outcomes
During 10,554 person-years (mean follow-up: 4.4 years), 69 (2.9%) participants developed CKD. As shown in Fig. 1A, a higher Hcy level at baseline was associated with higher incident CKD in the spline analysis. However, folate and vitamin B12 were not significantly associated with incident CKD in the spline analysis (Fig. 1B,C).
Adjusted splines of baseline homocysteine, folate and vitamin B12 and renal outcome.
The solid line represents the spline of baseline homocysteine (A) folate (B) and vitamin B12 (C) and incident chronic kidney disease adjusted by age, gender, systolic blood pressure, diabetes, smoking, cholesterol, HDL-C, triglycerides, body mass index, estimated glomerular filtration rate (eGFR) and previous use of ACEIs/ARBs. Adjusted splines of baseline homocysteine (D), folate (E) vitamin B12 (F) and eGFR decline rate are also shown. Dotted lines represent the 95% confidence interval of the splines. Incident chronic kidney disease was defined as follow-up eGFR <60 ml/min/1.73 m2 and eGFR decline rate >1 ml/min/1.73 m2/year. eGFR decline rate was calculated as the difference between the follow-up eGFR and baseline eGFR, which was divided by follow-up duration and expressed as ml/min/per 1.73 m2/year. Negative eGFR change indicates a fall in eGFR during follow-up. Abbreviations: ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein cholesterol.
When analyzed as a continuous variable, higher log-transformed Hcy was associated with higher incident CKD (Table 2), even after adjusting for age, gender, diabetes, SBP, body mass index (BMI), smoking, cholesterol, high density lipoprotein cholesterol (HDL-C), triglycerides, eGFR and previous use of angiotensin converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs). The association between Hcy levels and incident CKD remained unchanged after further adjustment for SBP at the end of the follow-up (Table 2). In analyses that considered tertiles of Hcy, the odds ratio (OR) for incident CKD increased across tertiles of plasma Hcy level, reaching 2.46 (95% confidence interval [CI] 1.05–5.77) for the top tertile compared with the lowest tertile (p for trend 0.011) in fully adjusted models.
Folate and vitamin B12 were not significantly associated with incident CKD in either unadjusted or adjusted models, respectively (Table 2). When Hcy, folate and vitamin B12 were all included in the multivariate model, only Hcy was significantly associated with an increased risk for incident CKD (Table 2).
Higher Hcy level is associated with more rapid decline in renal function
The mean yearly change for eGFR in the cohort was −0.87 (Q1–Q3: −2.02 ~ 0.20) ml/min per 1.73 m2/year. As shown in Fig. 1D, a higher Hcy level at baseline was associated with faster rate of eGFR decline in the spline analysis. However, folate and vitamin B12 were not significantly associated with a faster rate of eGFR decline in the spline analysis (Fig. 1E,F).
Higher Hcy level was associated with a faster rate of eGFR decline in fully adjusted models (Table 3). The highest tertile of Hcy was also associated with a more rapid eGFR decline rate in multivariate models (Table 3). Folate was not significantly associated with a faster rate of eGFR decline in any of the models (Table 3). Higher levels of vitamin B12 were associated with a slower rate of eGFR decline (Table 3). However, when Hcy, folate and vitamin B12 were all included in the multivariate model, only Hcy was significantly associated with a faster rate of eGFR decline (Table 3).
Stratified analysis
The association between higher log-transformed Hcy and increased risk of incident CKD appeared to be consistent across the subgroups, although the magnitude of association varied slightly. For example, the association between Hcy and CKD was stronger among those with baseline eGFR between 60–90 ml/min/per 1.73 m2 as compared to those with eGFR>90 ml/min/per 1.73 m2. However, tests for interaction were not significant (p for interaction >0.05) (Table 4). In addition, these associations were also found among subgroups stratified according to age, gender, baseline SBP and eGFR (p for interaction >0.05) (Table 4). In addition, a higher level of Hcy was associated with a more rapid eGFR decline rate among subgroups stratified according to age, gender, baseline SBP and eGFR (p for interaction >0.05) (Table 4). However, folate and vitamin B12 were not significantly associated with higher incident CKD or a faster rate of eGFR decline among any of the subgroups.
Discussion
In this prospective study of 2,387 hypertensive adults, we identified a graded association between plasma Hcy levels and an increased risk of accelerated renal function decline and incident CKD. This association was independent of plasma folate and vitamin B12 levels and other traditional risk factors. Our findings suggest that elevated plasma Hcy levels, but not low folate and/or vitamin B12 levels, may predict progression of renal function decline and incident CKD in hypertensive adults.
Increased plasma levels of Hcy, termed hyperhomocysteinemia, is diagnosed when plasma Hcy levels are greater than 15 μmol/L. HHcy is recognized as a potent risk factor and predictor for cardiovascular disease (CVD) in general populations25,26,27,28. Previously, a dose-response relationship between Hcy and CVD was identified in a population with plasma Hcy levels starting from 10 μmol/L29,30,31,32,33. In meta-analyses, the OR for cardiovascular disease was calculated to be 1.6 for a 5 μmol/L increase in plasma Hcy concentration34,35. The present study found that plasma Hcy levels, in a range from 5 to 20 μmol/L, were associated with a rapid decline of renal function and incident CKD in a hypertensive population. Increased risk of composite renal outcomes was found across Hcy tertiles, with a significantly increased OR at Hcy levels of approximately 13 μmol/L (in women) and 16 μmol/L (in men). This data is consistent with previous studies showing an increased risk of incident CKD and microalbuminuria in the general population with circulating Hcy levels of approximately 8 ~ 10 μmol/L7,8. These results indicate that an elevated plasma Hcy level, even in the range below the cutoff for HHcy, is a risk factor for renal function and incident CKD in a hypertensive population.
Our study findings suggest that elevated Hcy levels might be a modifiable risk factor for CKD and that preventive interventions should be considered for hypertensive adults with increased plasma Hcy levels. A number of strategies, including dietary modification of food intake components and supplementation with folic acid and vitamins B6 and B12, have been reported to be effective at lowering Hcy levels and reducing cardiovascular risk36. Folic acid and B vitamin supplementation may be considered as a simple and safe intervention to prevent renal function decline in hypertensive adults. Additional study including clinical trials is warranted to determine whether Hcy-lowering therapy can reduce the risk of renal function decline and incident CKD in hypertensive adults.
It is to our surprise that plasma folate and vitamin B12 levels did not predict or correlate with the incident CKD and rapid decline of eGFR (Fig. 1). These findings suggest that HHcy in a hypertensive population is not directly caused by a folate and/or vitamin B12 deficiency. It is possible that hypertension may directly cause renal damage, which then leads to impaired Hcy excretion and elevated plasma Hcy, an inverse causality. On the other hand, folate and B12 insufficiency may lead to enzymatic impairment in Hcy metabolic pathways, including remethylation, transsulfuration and adenosylation that may be involved in hypertensive HHcy13,16. It is well established that MTHFR and cystathionine beta-synthase (CBS) are key enzymes for Hcy remethylation and transsulfuration and the major variants for human HHcy. Folic acid and B vitamin supplementation has been used as an effective strategy to lower plasma Hcy levels and reduce CVD risk in MTHFR and CBS variants37,38,39,40. Therefore, folic acid and B vitamin supplementation might still prove to be a useful therapeutic option to reduce CKD risk in hypertensive patients with HHcy.
Our study has some limitations. As it was conducted in Chinese hypertensive adults, caution is needed when generalizing our findings to other populations. This study did not have the data on microalbuminuria, an important marker for the development and progression of CKD, which limits our ability to estimate the true incidence of CKD in this study.
In conclusion, our study demonstrated that elevated plasma Hcy level is an independent predictor of rapid decline in renal function and incident CKD in a Chinese hypertensive population. Our findings, if further confirmed, may have important clinical and public health implications.
Methods
Study population
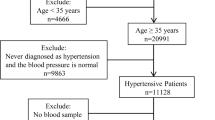
This community-based cohort study was approved by the Ethics Committees of the Institute of Biomedicine, Anhui Medical University. Investigation was performed in accordance with relevant guidelines and regulations. All participants were provided with written consent. The study was conducted in rural communities in Lianyungang, China. Eligible participants were those: (1) aged 45 to 75 years and (2) with hypertension (BP ≥ 140/90 mmHg or currently on antihypertensive therapy). Participants were excluded from the study if they had (1) confirmed CKD (i.e., glomerular filtration rate [GFR] <60 ml/min/per 1.73 m2) at time of screening; (2) diagnosed secondary hypertension; (3) a history of stroke, myocardial infarction, heart failure, coronary revascularization, and/or congenital or acquired heart diseases; (4) cancer; and/or (5) liver disease and/or severe mental disorders.
A total of 3,522 hypertensive adults were screened in 2008 and 2,518 (71.5%) were assessed at follow-up and participated in a subsequent scheduled visit after five years. Among the 2,518 participants, 131 were excluded from the analysis because of missing follow-up data. The final analysis included 2,387 participants.
Data collection
Data collection at baseline and at the follow-up visit were conducted by trained research staff according to a standard operating procedure.
Blood pressure measurement
Resting blood pressures were measured using a mercury sphygmomanometer in a seated position after ≥10 minutes of rest. The mean of the three readings on the first baseline screening day was recorded and confirmed by three measurements on the second baseline screening day with an interval of at least one day. The mean of the three BP measurements on the second baseline screening day was used as the baseline BP for analyses.
Questionnaires and medical history
Questionnaires were administered to collect information on socio-demographic status, medical history and medications. Diabetes was defined as physician-diagnosed diabetes, or currently receiving hypoglycemic therapy, or fasting glucose level higher than 7.0 mmol/L.
Anthropometry
Anthropometric measurements, including height and weight, were taken using a standard operating procedure.
Blood Sample
Venous blood was drawn after overnight fasting during the baseline visit and the follow-up visit. Both plasma and serum samples were collected and stored at −80 °C. The samples were shipped by commercial cold chain transportation and measured in a central laboratory.
Laboratory measurements
Measurement of plasma Hcy, folate and vitamin B12
Plasma Hcy, folate and vitamin B12 were measured by enzyme-cycling method using a commercial kit.
Measurement of estimated glomerular filtration
Serum creatinine was measured by enzymatic method (sarcosine oxidase-PAP) using a commercial kit (Beckman Coulter, Brea, CA, USA) according to the manufacturer’s protocol. The Beckman assay was calibrated to the Roche/Hitachi P module Creatinase Plus enzymatic assay (Roche Diagnostics, Basel, Switzerland), traceable to an isotope-dilution mass spectrometry assay at the National Institute of Standards and Technology41,42. eGFR was calculated using the two-level CKD-Epidemiology Collaboration (CKD-EPI) formula43.
Measurement of other parameters
Fasting glucose, total cholesterol, triglycerides and HDL-C were measured using a Hitachi 7020 Automatic Analyzer (Beckman Coulter, Brea, CA, USA).
Renal outcome definitions
Incident CKD
was defined as follow-up eGFR <60 ml/min/per 1.73 m2. To avoid excluding participants with minor fluctuations in eGFR that may be due to “noise”, the definition also included a >1 ml/min/per 1.73 m2/year decrease in eGFR44.
Rate of eGFR decline
was calculated as the difference between the follow-up eGFR and baseline eGFR, which was divided by follow-up duration and expressed as ml/min/per 1.73 m2/year. A negative eGFR change indicates a fall in eGFR.
Statistical analysis
All of the analyses were conducted using the statistical package R software, version 3.0.145. Mean and standard deviation or median (25th, 75th percentile) and percentages were calculated for the description of population characteristics by gender. The differences in population characteristics between men and women were compared using the Student’s t tests, signed rank tests, or chi-square test as appropriate. Multivariable logistic regression was used to assess the relations of Hcy, folate and vitamin B12 with incident CKD. Multivariate linear regression models were used to evaluate the associations between Hcy, folate and vitamin B12 with change in kidney function. In these analyses, Hcy, folate and vitamin B12 were modeled both as continuous variables and as categorical variables (categorized into tertiles). Hcy was natural log-transformed to normalize its skewed distributions. Because of gender differences in Hcy and folate distribution, gender-specific tertiles of Hcy and folate were used. The multivariate models were adjusted for age, gender, diabetes, SBP, BMI, smoking, cholesterol, HDL-C, triglycerides, eGFR and previous use of ACEIs/ARBs. Effect modification was tested using a multivariate regression by stratifying the potential risk factors for renal outcomes. Adjusted splines of generalized additive models were created to evaluate the associations between baseline Hcy, folate and vitamin B12 and the outcomes. All tests were two-tailed and P < 0.05 was considered significant.
Additional Information
How to cite this article: Xie, D. et al. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci. Rep. 5, 16268; doi: 10.1038/srep16268 (2015).
References
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
Zhang, Q. L. & Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 8, 117 (2008).
Zhang, L. et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379, 815–822 (2012).
Hsu, C. Y., McCulloch, C. E., Darbinian, J., Go, A. S. & Iribarren, C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165, 923–928 (2005).
Tozawa, M. et al. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 41, 1341–1345 (2003).
Klag, M. J. et al. Blood pressure and end-stage renal disease in men. N Engl J Med 334, 13–18 (1996).
Ninomiya, T. et al. Hyperhomocysteinemia and the development of chronic kidney disease in a general population: the Hisayama study. Am J Kidney Dis 44, 437–445 (2004).
Fox, C. S. et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol 21, 2143–2149 (2010).
Shastry, S., Ingram, A. J., Scholey, J. W. & James, L. R. Homocysteine induces mesangial cell apoptosis via activation of p38-mitogen-activated protein kinase. Kidney Int 71, 304–311 (2007).
Yi, F. et al. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int 70, 88–96 (2006).
Sethi, A. S., Lees, D. M., Douthwaite, J. A., Dawnay, A. B. & Corder, R. Homocysteine-induced endothelin-1 release is dependent on hyperglycaemia and reactive oxygen species production in bovine aortic endothelial cells. J Vasc Res 43, 175–183 (2006).
Yi, F. & Li, P. L. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 28, 254–264 (2008).
Lee, M. E. & Wang, H. Homocysteine and hypomethylation. A novel link to vascular disease. Trends Cardiovasc Med 9, 49–54 (1999).
Marti, F. et al. Hyperhomocysteinemia is independently associated with albuminuria in the population-based CoLaus study. BMC Public Health 11, 733 (2011).
Jager, A. et al. Serum homocysteine levels are associated with the development of (micro)albuminuria: the Hoorn study. Arterioscler Thromb Vasc Biol 21, 74–81 (2001).
Chen, N. C. et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J 24, 2804–2817 (2010).
Frosst, P. et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10, 111–113 (1995).
Stampfer, M. J. & Malinow, M. R. Can lowering homocysteine levels reduce cardiovascular risk? N Engl J Med 332, 328–329 (1995).
Rimm, E. B. et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 279, 359–364 (1998).
Gao, Y. et al. Prevalence of hypertension in china: a cross-sectional study. PLoS One 8, e65938 (2013).
Hao, L. et al. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12 and vitamin B-6 status. J Nutr 137, 407–413 (2007).
Hao, L. et al. Geographical, seasonal and gender differences in folate status among Chinese adults. J Nutr 133, 3630–3635 (2003).
Botto, L. D. & Yang, Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151, 862–877 (2000).
Huo, Y. et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 313, 1325–1335 (2015).
Veeranna, V. et al. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 58, 1025–1033 (2011).
Kang, S. S., Wong, P. W. & Malinow, M. R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 12, 279–298 (1992).
Stanger, O. et al. Clinical use and rational management of homocysteine, folic acid and B vitamins in cardiovascular and thrombotic diseases. Z Kardiol 93, 439–453 (2004).
Towfighi, A., Markovic, D. & Ovbiagele, B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: a nationwide study. J Neurol Sci 298, 153–157 (2010).
Bostom, A. G. et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med 131, 352–355 (1999).
Boushey, C. J., Beresford, S. A., Omenn, G. S. & Motulsky, A. G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274, 1049–1057 (1995).
Malinow, M. R., Nieto, F. J., Szklo, M., Chambless, L. E. & Bond, G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation 87, 1107–1113 (1993).
Nygard, O. et al. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 337, 230–236 (1997).
Wilcken, D. E. & Wilcken, B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis 20, 295–300 (1997).
den Heijer, M., Rosendaal, F. R., Blom, H. J., Gerrits, W. B. & Bos, G. M. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost 80, 874–877 (1998).
Wald, D. S., Law, M. & Morris, J. K. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325, 1202 (2002).
Wang, X. et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369, 1876–1882 (2007).
Jiang, X. et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol 25, 2515–2521 (2005).
Jamaluddin, M. D. et al. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood 110, 3648–3655 (2007).
Cheng, Z., Yang, X. & Wang, H. Hyperhomocysteinemia and Endothelial Dysfunction. Curr Hypertens Rev 5, 158–165 (2009).
Qin, X. et al. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr 32, 722–727 (2013).
Levey, A. S. et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53, 766–772 (2007).
Junge, W., Wilke, B., Halabi, A. & Klein, G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffe method. Clin Chim Acta 344, 137–148 (2004).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150, 604–612 (2009).
Madero, M. et al. Association of arterial rigidity with incident kidney disease and kidney function decline: the Health ABC study. Clin J Am Soc Nephrol 8, 424–433 (2013).
Ford, E. S. & Bowman, B. A. Serum and red blood cell folate concentrations, race and education: findings from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 69, 476–481 (1999).
Acknowledgements
This work was supported by the Major State Basic Research Development Program of China (973 program) (No. 2012CB517703), National Key Technologies R&D program (2011BAI10B02) and Major Scientific and Technological Planning Project of Guangzhou City (2010U1-E00821) to Dr. Fan Fan Hou and a National Nature and Science Grant (No. 81202280) and The PhD Start-up Fund of the Natural Science Foundation of Guangdong Province (S201204006973) to Dr. Di Xie.
Author information
Authors and Affiliations
Contributions
H.F.F. and X.X.P. designed research; X.D., Y.Y., G.J.N., Y.S.L., W.Q., L.Y.B., T.G.F., H.Y., D.G.P., W.S.J., W.B.Y., P.F. and Z.Q. conducted research; X.D., Q.X.H. and X.X. analyzed data; H.F.F., X.D., W.X.B. and W.H. wrote the paper; H.F.F. had primary responsibility for final content. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, D., Yuan, Y., Guo, J. et al. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci Rep 5, 16268 (2015). https://doi.org/10.1038/srep16268
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16268
This article is cited by
-
Association of hyperhomocysteinemia and chronic kidney disease in the general population: a systematic review and meta-analysis
BMC Nephrology (2023)
-
The relationship between homocysteine and cardiopulmonary exercise testing in patients with acute coronary syndrome after percutaneous coronary intervention
BMC Cardiovascular Disorders (2023)
-
Gender differences in risk factors for high plasma homocysteine levels based on a retrospective checkup cohort using a generalized estimating equation analysis
Lipids in Health and Disease (2021)
-
C677T MTHFR Gene Polymorphism is Contributing Factor in Development of Renal Impairment in Young Hypertensive Patients
Indian Journal of Clinical Biochemistry (2021)
-
Hyperhomocysteinemia and Peripheral Arterial Disease in Patients Undergoing Hemodialysis
Hellenic Journal of Surgery (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.