Abstract
The insect neuropeptide family FXPRLa, which carries the Phe-Xaa-Pro-Arg-Leu-NH2 sequence at the C-terminus, is involved in many physiological processes. Although ligand–receptor interactions in FXPRLa signaling have been examined using in vitro assays, the correlation between these interactions and in vivo physiological function is unclear. Diapause in the silkworm, Bombyx mori, is thought to be elicited by diapause hormone (DH, an FXPRLa) signaling, which consists of interactions between DH and DH receptor (DHR). Here, we performed transcription activator-like effector nuclease (TALEN)-based mutagenesis of the Bombyx DH-PBAN and DHR genes and isolated the null mutants of these genes in a bivoltine strain. All mutant silkworms were fully viable and showed no abnormalities in the developmental timing of ecdysis or metamorphosis. However, female adults oviposited non-diapause eggs despite diapause-inducing temperature and photoperiod conditions. Therefore, we conclude that DH signaling is essential for diapause induction and consists of highly sensitive and specific interactions between DH and DHR selected during ligand–receptor coevolution in Bombyx mori.
Similar content being viewed by others
Introduction
Neuropeptide signaling is functionally diverse as a result of specific peptide ligands that preferentially activate particular receptor subtypes to perform physiological and developmental functions in animals, a process that developed through ligand–receptor coevolution1,2. Insect FXPRLa-family neuropeptides, which carry the Phe-Xaa-Pro-Arg-Leu-NH2 sequence at the C-terminus, play a role in many physiological processes, including diapause induction, pheromone biosynthesis, cuticular tanning, myostimulation, pupariation behavior and termination of pupal diapause3. These FXPRLa neuropeptides are evolutionarily related to the vertebrate peptide neuromedin U (NMU) and G-protein coupled receptors (GPCRs) in the NMU receptor clade are activated by FXPRLa peptides4. The sensitivity and specificity of response of NMU receptors to individual FXPRLa peptides has been studied in detail for some insect species using in vitro assays2,4,5,6,7,8. The results suggest that the ligand–receptor interactions in FXPRLa signaling have two major characteristics—high specificity and pleiotropism, indicating that certain peptides activate their respective authentic receptor with high specificity and that closely related clusters of specific peptides activate related groups of receptors2. However, the relationship between these ligand–receptor interactions and in vivo physiological functions remains unclear.
In the silkworm (Bombyx mori) genome, FXPRLa neuropeptides are encoded by two genes: diapause hormone (DH)-pheromone biosynthesis activating neuropeptide (PBAN) DH-PBAN and capa (Supplementary Table S1)9. DH-PBAN encodes a polypeptide precursor consisting of five FXPRLa neuropeptides: DH, PBAN and α-, β- and γ-SGNPs (Subesophageal ganglion neuropeptides) (Fig. 1A)10. The capa gene encodes a polypeptide precursor consisting of three neuropeptides that contain an FXPRLa, CAPA-PK. The GPCRs related to the NMU receptor activated by these peptides are clustered in the phylogeny11. DH is well known as a neuropeptide hormone responsible for induction of embryonic diapause in Bombyx12. DH functions by acting on a GPCR, DH receptor (DHR; Fig. 1B) in the developing ovaries during pupal–adult development in females13. Previous studies showed that DH is a selective and sensitive ligand for DHR and is distinguished from other neuropeptides encoded by DH-PBAN8,13. Therefore, it was thought that diapause induction elicited by DH signaling consisted of interactions between DH and DHR. However, the relationship between DH–DHR interactions and diapause induction has not been investigated in vivo.
Construction of TALEN-based mutants of the DH-PBAN and DHR genes.
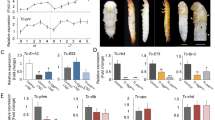
Schematic representations of the genes (top) and cDNA structures (bottom) of the DH-PBAN (A) and DHR (B) mutants. Shaded boxes and lines represent exons and introns, respectively. The cDNA of DH-PBAN encodes a signal sequence (S.S), DH, PBAN and α-, β- and γ-SGNPs, which carry the 5′ and 3′ untranslated regions (light-grey boxes on left and right sides, respectively). The cDNA of DHR consists of seven transmembrane domains (1–7), which carry the 5′ and 3′ untranslated regions (light-grey boxes on left and right sides, respectively). The FXPRLa and transmembrane domains are indicated using blue boxes. Orange triangles represent the TALEN binding sites. The sizes of exons and introns (in bp) are indicated by scales in each map. (C,D) The sequences of the TALEN target sites are indicated by orange boxes. Partial coding sequences corresponding to the DH N-terminus and transmembrane domain 1 of DHR are indicated using blue boxes. The deleted bases in spacer regions of each mutant are indicated by hyphens and identical bases are indicated by asterisks. The ΔDHP33, ΔDHP531 and ΔDHR96 are truncated proteins, which encode 9, 26 and 28 amino acids, respectively. Eight amino acids were missing from DHR111, which led to the production of a defective truncated protein in the extracellular domain at the N-terminus of DHR.
Bombyx embryonic diapause is a unique process of seasonal polyphenism that is induced transgenerationally as a maternal effect in the bivoltine strain. Progeny diapause is determined by the mother’s experience during embryonic development. When eggs are incubated at 25 °C under continuous darkness (25DD), the resultant female moths are able to lay diapause eggs. In contrast, incubation at 15 °C under continuous darkness (15DD) causes the resultant moths to lay non-diapause eggs. If eggs are incubated at 20 °C under continuous illumination (20LL) or darkness (20DD), the resultant moths produce diapause or non-diapause eggs, respectively14. Thus, progeny diapause is determined by environmental factors such as photoperiod and temperature during maternal embryogenesis. Recently, we showed that the embryonic Bombyx TRPA1 ortholog (BmTrpA1) acts as a thermosensitive channel that is activated at temperatures above ~21 °C and affects diapause induction through DH release during pupal–adult development15. Thus, some of the molecular mechanisms in the pathway leading from reception of environmental signals to expression of the diapause phenotype have been revealed.
Recently, genome editing strategies including transcription activator-like effector nucleases (TALENs) have advanced the efficiency of targeted gene mutagenesis in a wide variety of organisms including Bombyx mori16,17. In the TALEN strategy, errors in the nonhomologous end joining (NHEJ) repair of the targeted double-strand breaks result in the mutations usually consisting of small deletions and/or insertions, which cause frameshifts and truncations of open reading frames to disrupt the gene functions17. Thus, application of TALEN mutagenesis is suitable for analysis of in vivo physiological functions of Bombyx genes involved in diapause induction.
Here, we performed TALEN-based mutagenesis of Bombyx DH-PBAN and DHR to isolate the null mutants of those genes in a bivoltine strain. All mutant silkworms were fully viable and showed no abnormalities in the developmental timing of ecdysis and metamorphosis. However, female adults oviposited non-diapause eggs despite diapause-inducing temperature and photoperiod conditions. Therefore, we concluded that DH signaling is essential for diapause induction, which is independently accomplished by highly sensitive and specific interactions between DH and DHR selected through ligand–receptor coevolution in Bombyx mori.
Results
Construction of TALEN-based mutants of DH-PBAN and DHR
For mutagenesis of DH-PBAN, we selected a TALEN target in the sequences of the first exon that encoded DH (Fig. 1A,C). We isolated the two homozygous mutants containing 4- or 5-base deletions and designated the mutants as ΔDHP33 and ΔDHP531, respectively (Fig. 1C). These were considered null mutants, which could not translate the DH-PBAN preprohormone by frame-shift of DH-PBAN cDNA. We obtained two mutants of DHR, designated ΔDHR96 and ΔDHR111 (Fig. 1D), which carried a 7- or 24-base deletion, respectively, of the sequences in the anterior region encoding the transmembrane domain 1 (TM1) of the second exon (Fig. 1B). The ΔDHR96 mutant translated the truncated protein containing a partial DHR signal sequence and was considered the null mutant. The ΔDHR111 mutant was thought to cause an in-frame mutation; DHR111 was missing eight amino acids, which led to the production of truncated protein defective in the extracellular domain at the N-terminus of DHR. Thus, we isolated four mutants related to DH signaling.
DH and glycogen contents in ΔDHP and ΔDHR mutants
To confirm the null mutagenesis of DH-PBAN, we first investigated the immunoreactivity of DH and PBAN in pupal subesophageal ganglion (SG). Previous reports showed that DH-PBAN is exclusively expressed in seven pairs of neurosecretory cells (DH-PBAN-producing neurosecretory cells; DHPCs) located within the SG18,19. In wt (25DD) pupal SG, namely wt pupal SG that incubated at 25 °C under continuous darkness during embryogenesis, immunoreactive signals to anti-DH[N] antibody were detected in DHPC somata, including the SMd, SMx and SLb neuromeres along the ventral midline and in SL cells (Fig. 2A), similar to previously reported results20. Likewise, immunoreactive signals were detected in DHPCs by using the anti-PBAN[N] antibody, although no SLb and SL cells were observed (Fig. 2B). Fluorescence signals disappeared in ΔDHP33 and ΔDHP531, in which both anti-DH[N] and anti-PBAN[N] antibodies were used (Fig. 2C–F), suggesting that neither DH nor PBAN neuropeptides were produced in these mutants. In addition, pupal SG in ΔDHR96 and ΔDHR111 had immunoreactivity (Fig. 2G–J).
DH and glycogen contents in ΔDHP and ΔDHR mutants.
(A–J) Representative images of immunohistochemical staining of pupal subesophageal ganglion (SG) using anti-DH[N] (A,C,E,G,I) and anti-PBAN[N] (B,D,F,H,J). The pupal SG was dissected 4 d after pupation and immunostaining was performed in the wt (25DD) (A,B), ΔDHP33 (25DD) (C,D), ΔDHP531 (25DD) (E,F), ΔDHP96 (25DD) (G,H) and ΔDHR111 (25DD) (I,J). The DHPC somata (SMd, SMx, SLb and SL) were detected in wt and ΔDHR. (K) The DH titer in the hemolymph was measured 4 d after pupation, by using TR-FIA. Each bar represents the mean ± SD of 8 samples. N.D., not detected; **P < 0.01; ***P < 0.001 vs. wt (25DD). (L) Glycogen content was measured in ovaries just after eclosion. Each bar represents the mean ± SD of 8 pupae. n.s., not significant vs. wt (15DD); ***P < 0.001 vs. wt (25DD). (Scale bar, 100 μm).
To further accurately determine the levels of circulating DH in the hemolymph, we developed a new, sensitive time-resolved fluoroimmunoassay (TR-FIA). By using synthetic DH as a standard, we found the detection limit for a 150-μL hemolymph sample from one or two animals to be ≈0.57 pM (≈85.8 amol). We measured DH levels in the hemolymph of pupa at 4 days after pupation (Fig. 2K). In wt (25DD) pupa, DH titer was 8.85 ± 2.71 pM, which was two-fold higher than in wt (15DD), suggesting the active release of DH in wt (25DD). DH was undetectable in the ΔDHP33 and ΔDHP531 lines despite rearing under 25DD conditions. Furthermore, DH levels in DHR mutants were two-fold higher than those in wt (25DD). These results suggest changes in DH titer in diapause of wt; in addition, DH levels were affected by the disruption of DH and DHR, indicating that DH signaling itself regulates the hemolymph DH levels.
Bombyx DH stimulates transcription of the trehalase gene in ovaries, thereby increasing trehalase activity, which facilitates greater accumulation of glycogen in eggs—a prerequisite for diapause initiation21,22. The glycogen content in ovaries of wt (25DD) pupa was high compared with that in wt (15DD) (Fig. 2L). The four 25DD ΔDHP and ΔDHR mutants had similar glycogen content to wt (15DD), but glycogen differed significantly from that in the wt (25DD). These results suggested that DH signaling affects glycogen accumulation in ovaries during the preparative phase of diapause.
Phenotypic analyses of ΔDHP and ΔDHR mutants
In general, diapause eggs have dark brown pigmentation because of 3-hydroxykynurenine (3-OHK) in their serosa, whereas non-diapause eggs lack this pigment and thus, appear light yellow. Notably, DH was suggested to facilitate the accumulation of 3-OHK in pupal ovaries14. Progeny eggs of wt (25DD) showed light brown pigmentation on day 2 after oviposition, became dark brown on day 10 after oviposition, and, as they were diapause eggs, eventually failed to hatch (Fig. 3A, wt). Progeny eggs of ΔDHP33 and ΔDHR96 showed no pigmentation and the larvae hatched on day 10 after oviposition despite 25DD rearing conditions (Fig. 3A, ΔDHP33, ΔDHR96). The percentage of diapause eggs was counted in 50 batches of progeny eggs among the wt and four mutants (Fig. 3C and Supplementary Fig. S1A). In the wt, 25DD and 20LL adults oviposited diapause eggs and 15DD and 20DD eggs mostly became non-diapause eggs. However, all four mutant adults oviposited non-diapause eggs, mostly under 25DD, 15DD, 20LL and 20DD conditions (Fig. 3C). Thus, disruption of the DH signaling pathway appeared to block diapause induction in the mutants despite diapause-inducing temperature and photoperiod conditions.
Phenotypic analyses of ΔDHP and ΔDHR mutants.
(A) Representative images of eggs in wt (25DD), ΔDHP33 (25DD) and ΔDHP96 (25DD), 2 d or 10 d after oviposition. (B) Pigmented non-diapause eggs were observed in mutants. At 2 d after oviposition, pigmented eggs were observed (black asterisk); these eggs had delayed development (white asterisk). (C) Percentages of diapause eggs are shown for wt and four mutants incubated under various environmental conditions. The percentages of diapause eggs were estimated by counting the numbers of eggs in diapause and those not in diapause after non-diapause eggs hatched in 50 egg batches. (D) Morphological observation using thionin staining of egg. Embryos were collected 10 d after oviposition and thionin staining was performed. We observed arrested development at diapause stage in early embryonic development in wt and in ΔDHP33 (25DD) and ΔDHR96 (25DD). (E) Rescue experiments by injection of synthetic FXPRLa peptides (DH, α-, β- and γ-SGNPs and PBAN) into female pupa of mutants. The peanut oil (PO) or each peptide (10, 100 and 1000 pmol) were injected into wt (15DD), ΔDHP33 (25DD), ΔDHP531 (25DD), ΔDHR96 (25DD) and ΔDHR111 (25DD) and the diapause eggs inducing activity was measured. Each bar represents the mean ± SD of 6–10 animals. *P < 0.05; **P < 0.01. Asterisks indicate significant differences vs. PO injection of each strain. (F) Developmental timing of 100 larvae was observed in the wt and four mutants. The developmental period was considered the period when all larvae molted to the next instar (instars I–IV) and when all larvae initiated spinning (instar V). The number of parenthesis shows the number of larva died at this stage. (G–H) Eggs were incubated at 25 °C (25DD) or 15 °C (15DD) in the dark. Pupae (G) and cocoon-shell (H) weights after 4 d of pupation are shown in female. Each bar represents the mean ± SD of 35 animals. n.s., non-significant; *P < 0.05; ***P < 0.001. Orange asterisks indicate significant differences vs. wt (25DD); blue asterisks indicate significant differences vs. each 25DD strain. (Scale bar, 2 mm).
Interestingly, a few eggs in the mutants had light-brown pigmentation on day 2 after oviposition (Fig. 3B, black asterisk); these pigmented eggs hatched, but embryonic development was slightly delayed (Fig. 3B, white asterisk). Further, some of the pigmented eggs failed to hatch and embryonic development was arrested at a specific stage during embryogenesis, immediately after formation of the cephalic lobe and telson and after segmentation of mesoderm, known as the diapausing stage in wt (Fig. 3D)23. The resulting mutants oviposited diapause eggs at ratios of 0.03–0.50% (Fig. 3C and Supplementary Fig. S2A). Next, we attempted rescue experiments of mutant lines by injecting synthetic DH or other FXPRLa to confirm whether only DH was responsible for diapause induction (Fig. 3E). In wt (15DD), DH had a significant effect on diapause egg inducing activity, which increased in a dose-dependent manner at the range of 10–1000 pmol/pupa; however, PBAN showed diapause egg inducting activity only at 100 times the amount of DH. Furthermore, almost no activity was observed after injections of α-, β- and γ-SGNPs. In ΔDHP33 and ΔDHP531 lines, as well as in wt (15DD), high diapause eggs inducing activities were noted only after DH injection. In addition, almost no activity was observed in ΔDHR96 and ΔDHR111 even after DH injection.
Previous studies using in vitro assays showed that Bombyx DHR is also expressed in the prothoracic gland, the organ that synthesizes and releases the insect molting hormones (ecdysteroids), which may be activated by DH to function ecdysteroidogenesis in larval instars8. Therefore, we tested for effects on the developmental timing of ecdysis and metamorphosis in the mutant lines (Fig. 3F–H). Generally, wt (15DD) larvae spent less time in the larval period than did wt (25DD) larvae; wt (15DD) larvae initiated spinning earlier than did wt (25DD) larvae23, as indicated by the 3-day shift in wt (15DD) compared to that in wt (25DD) (Fig. 3F). We reared 100 larvae from each of the wt and four mutants, all under the same conditions and observed the time of molting and duration of the larval period. Most wt (25DD) larvae spent 5, 4, 5, 2.5 and 7.5 d in the 1st, 2nd, 3rd, 4th and 5th instar, respectively (Fig. 3F) and larvae initiated spinning 21 d after hatching, with a peak at 22 d (Supplementary Fig. S1B)—similar to that of the four mutants under 25DD conditions (Fig. 3F). Under 15DD conditions, the four mutants and wt showed similar developmental timing (Fig. 3F and Supplementary Fig. S1B). In addition, because it is known that wt (25DD) pupae have heavier bodies and cocoon shells23, we tested whether pupal and cocoon-shell weights are affected by DH signaling. Both female and male pupae incubated under 25DD conditions had bodies (Fig. 3G and Supplementary Fig. S1C) and cocoon shells (Fig. 3H and Supplementary Fig. S1D) that were heavier than those of wt (15DD) and that were similar in weight to those of all mutant pupae. Thus, we did not observe differences in the duration of the larval period or the weight of pupae and cocoons in DH-PBAN and DHR mutants, suggesting that DH signaling does not participate in ecdysteroidogenesis in vivo. Further, because the previous in vitro experiments used high concentrations of DH, we speculated that artificial effects were observed.
PBAN is known to stimulate the secretion of a sex pheromone, bombykol, from the pheromone gland in Bombyx3. Further, we observed a slight reduction in sexual behaviors such as flapping, orientation and attempted copulation in male ΔDHP33 and ΔDHP531 mutants but not in ΔDHR mutants, which suggests that pheromone production is suppressed by PBAN knockout in female. Each mutant eventually mated and oviposited eggs in similar numbers to the wt (Supplementary Fig. S1A).
Discussion
We clearly showed in this study that in vivo disruption of DH-PBAN and DHR blocked diapause induction in progeny embryos. As described previously, when expressed in a Xenopus oocyte system, DHR showed the highest affinity (EC50, ~70 nM) for DH compared with the other FXPRLa encoded by DH-PBAN8,13. Furthermore, an in vivo bioassay showed that synthetic DH was more effective than other FXPRLa at inducing diapause, with threshold levels less than 1/100 that of PBAN and other peptides encoded by DH-PBAN (Fig. 3E)10. In Orgyia thyellina, not only DH induced embryonic diapause in progeny, but also other FXPRLa encoded by DH-PBAN induced diapause in an in vivo bioassay24. Taken together, we conclude that DH signaling is essential for diapause induction and that a highly sensitive and specific interaction between DH and DHR is a result of ligand–receptor coevolution in Bombyx mori.
Extensive structural–functional studies of the Bombyx PBAN receptor (PBANR) have been performed using mutant receptors and in vitro assays25,26. These studies revealed a number of functional domains and sites that are crucial for receptor activation and regulation; thus, it has been suggested that the extracellular loops (regions between each transmembrane domain) of PBANR, DHR and related GPCRs function as a ligand-selection filter26. Therefore, the extracellular loop domains of DHR may have evolved to interact selectively with DH as well as to fulfill the functional requirements for diapause induction in Bombyx. The ΔDHR111 mutant, which was defective in eight amino acids of the extracellular N-terminus, had a similar phenotype as did the null mutant ΔDHR96. Since domain swaps in the Helicoverpa zea PBANR suggested roles for the N-terminus in ligand binding5, the ΔDHR111 mutant may be defective in ligand binding ability. Furthermore, we showed that the DH titer increased in DHR mutants compared to the wt. It is probable that these mutant receptors were unable to internalize the ligand, similar to that reported for PBANR27, or were unable to trigger negative feedback regulation of DH release, resulting in an abnormal increase in DH titer. Thus, we propose, based on our TALEN-mediated in vivo analysis, that the extracellular N-terminus is critical for Bombyx DHR function.
The structural similarity between DH and CAPA-PK, which carries the WFGPRLa sequences in the C-terminus (Supplementary Table S1), has been assumed to explain the highly sensitive cross-reactivity of CAPA-PK to DHR. We clearly demonstrated the role of DH in diapause induction. It may be likely that there are differences in spatiotemporal dynamics between DH and CAPA-PK. Therefore, CAPA-PK might not interact with DHR in ovaries during pupal–adult development.
In facultative diapause, the decision to enter diapause is generally determined by environmental factors such as photoperiod, temperature and nutrition received by that individual or its mother at an earlier developmental stage28. Although many links in the pathway leading from reception of environmental signals to expression of diapause phenotype remain poorly understood, it has been proposed that environmental information is stored, integrated and later translated into neuroendocrine functions in the form of diapause induction29. The duration over which the information is stored may span numerous developmental stages or even generations29. We demonstrated that this hypothesis is well adapted to embryonic diapause in Bombyx. Recently, we showed that the embryonic BmTRPA1 acts as a thermosensitive channel that is activated at temperatures above ~21 °C and affects diapause induction through DH release during pupal–adult development15. In this study, we demonstrated that both thermal and photoperiod information was stored until the mid-pupal stage and was integrated with DH signaling to determine diapause phenotype, although the molecular mechanism(s) participating in light (photoperiod)-sensing and storage and integration of information remain unknown (Fig. 4). Furthermore, because it has been speculated that innervation from the brain controls the release of DH30, integrated information may affect brain plasticity in the control of DH release. Here, we have attempted to resolve the molecular mechanisms described in Fig. 4 using TALEN-based mutagenesis.
Schematic drawing of relationship between temperature and photoperiod and diapause induction via a unique peptidergic signaling system, DH signaling.
Progeny diapause is determined by environmental temperature and photoperiod during maternal embryonic development. Silkworms incubated under 25DD and 20LL conditions lay pigmented diapause eggs. In contrast, incubation at 15DD and 20DD causes the resultant moths to lay non-diapause eggs. BmTRPA1 acts as a thermosensitive channel that affects diapause induction. We hypothesize that the links in the pathway from reception of environmental signals to expression of the diapause phenotype include storage, integration and later translation of information into DH signaling in the form of diapause induction. Non-diapause eggs complete their embryogenesis approximately 9 d after oviposition at 25 °C. In contrast, diapause eggs remain in the diapause stage.
Diapause is accompanied by complex physiological and biochemical changes (referred to as diapause syndrome) in which reserves are accumulated prior to diapause to enable survival during diapause and post-diapause development31. In Bombyx, there are dramatic metabolic differences between 25DD and 15DD during the preparative phase of diapause in the maternal generation. Namely, 25DD eggs accumulate greater glycogen and become pigmented. As suggested in previous reports14,21,22, we revealed that DH signaling alone facilitates greater accumulation of glycogen as well as accumulation of 3-OHK in 25DD eggs. Because the removal of SG from diapause-type animals in mid-pupal stages can induce the production of non-diapause eggs that are occasionally light pink32, the mutant phenotypes of light-colored pigmented eggs obtained here are consistent with the idea that the animals were deficient in DH signaling.
Although the DH signaling cascade was blocked in the DH-PBAN and DHR mutants under 25DD, 20LL and 20DD conditions, we obtained a few pigmented diapause eggs from these mutants. Largely unknown signaling pathway(s) may be active in the preparative phase of diapause induction during embryonic development at temperatures above 20 °C. For example, we observed extended developmental periods and heavier bodies and cocoon shells in 25DD silkworms, consistent with a previous report23. We suggest that the activation of BmTRPA1 signaling pathway(s) might affect the duration of growth in the larval period and the weight of the pupal body and cocoon shell. This signaling might be involved in preparing the diapause phenotype to facilitate entering diapause and it might participate in the storage and integration of information linked to DH signaling. Therefore, 25DD conditions increase the potential for silkworms to enter diapause. Occasionally, diapause may be accidentally induced in a subset of eggs, without DH signaling. Although DH signaling is essential for diapause induction, unknown signaling pathway(s) including the BmTRPA1 pathway may help in preparing for diapause induction at 25 and 20 °C during embryogenesis. These pathways might also participate in the storage and integration of environmental information linked to DH signaling in the maternal generation in order to induce diapause.
Materials and Methods
Silkworms
The bivoltine (Kosetsu) strain of Bombyx mori was used in these experiments. Eggs were incubated under four different conditions: (1) at 25 °C under continuous darkness (25DD) to obtain diapause eggs in the wild type (wt); (2) at 15 °C under continuous darkness (15DD) to obtain non-diapause eggs in the wt; (3) at 20 °C under continuous illumination (20LL) to obtain diapause eggs in the wt; or (4) at 20 °C under continuous darkness (20DD) to obtain non-diapause eggs in the wt. Larvae were then reared on an artificial diet (Kuwano-hana, JA Zennoh Gunma) at 25–27 °C under a 13-h light/11-h dark cycle (13L:11D) and relative humidity of 30–50%. The percentages of diapause eggs were estimated by counting the numbers of eggs in diapause and those not in diapause after non-diapause eggs hatched in 50 egg batches. The results are expressed as the average percent diapause in each egg batch14.
To screen the knockout strain, eggs were incubated at 25 °C under high humidity (approximately 80%) until hatching; larvae were reared at 25 to 27 °C under long-day conditions (20L:4D) on an artificial diet (Product No. 404110, Kyoto Institute of Technology) to induce non-diapause eggs despite 25DD conditions, as described previously33.
TALEN construction and screening of knockout silkworm
Construction of TALEN mRNAs and screening for germline mutants were performed according to Takasu et al.17. Briefly, TALEN targets were searched using TAL Effector Nucleotide Targeter 2.0 (https://tale-nt.cac.cornell.edu) in the coding regions of the DH-PBAN and DHR genes. DNA constructs containing the TAL segments were prepared using Golden Gate TALEN kit (Addgene). TALEN mRNAs were then synthesized using mMessage mMachine T7 Ultra kit (Ambion); mRNA of each TALEN was mixed at a concentration of 0.5 μg/μL for microinjection. Non-diapause eggs of the Kosetsu strain were collected within 1 h after oviposition during the syncytial blastoderm stage; the TALEN mRNA mixture was injected into the eggs using a glass needle (uMPm-02; Daiwa Union) attached to a manipulator (kaikopuchu-STDU1; Daiwa Union) and FemtoJet (Eppendorf).
For screening of germline mutagenesis, the G0 adults were mated with wt. The oviposited G1 eggs were collected and approximately 10 eggs from each brood were pooled for genomic DNA extraction using Nucleospin Tissue (Macherey-Nagel). The DNA fragment containing the targeted region of interest was amplified by PCR using Takara Ex Taq (Takara) (Supplementary Fig. S2A). To test for mutagenesis, the PCR products of DH-PBAN and DHR were digested with restriction enzymes Psp1406I (Takara) and FastDigest MnlI (Thermo), respectively; the presence of an undigested PCR product would suggest that the restriction site was disrupted by TALENs (Supplementary Fig. S2A,B). Mutated PCR products were subcloned using a TOPO TA cloning kit (Invitrogen) and checked by sequencing. The broods containing mutated sequences were reared and mutated G1 adults were crossed with the siblings that carried the same mutation. Homozygous mutants were obtained after confirmation by sequencing of the target region in the G2 or G3 egg genome.
Immunostaining
The immunostaining procedures were adapted from Hagino et al.20. Briefly, the primary antibodies, anti–DH[N] or –PBAN[N], which recognize a 12–amino acid sequence of the N–terminal region of each peptide, were used at a ratio of 1:2500, respectively, at 4 °C overnight. The signal was detected with Cy2–labeled goat anti–mouse IgG (Jackson ImmunoResearch Lab.) diluted to 1:1500 and was observed using an Olympus FV1000–D confocal microscope (Olympus). Confocal scans were performed under the same conditions for specimens in each mutant strain.
Time-resolved fluoroimmunoassay (TR-FIA)
We developed a new method for measurement of the hemolymph DH titers. Hemolymph was collected on ice from one or two pupae on day 4 after pupation into a microcentrifuge tube containing small amounts of sodium diethyldithiocarbamate, a phenoloxidase inhibitor and p-APMSF, a protease inhibitor. The final concentrations of the inhibitors were 5 mM and 20 μM, respectively. After centrifuging at 9,200 × g for 5 min to remove hemocytes, 150 μL of the hemolymph was added with 150 μL of 2% acetic acid and 300 μL of methanol, followed by boiling for 10 min. The mixture was centrifuged at 18,000 × g and 480 μl of the resulting supernatant was concentrated to approximately 100 μl by vacuum centrifugation for 1 h, followed by mixing with 1 ml of 1% trifluoroacetic acid (TFA). This mixture was applied to a Sep-Pak Vac 3cc C8 cartridge (Waters) equilibrated with 0.1% TFA. The cartridge was washed with 10% acetonitrile (ACN) containing 0.1% TFA and eluted with 40% ACN containing 0.1% TFA. The eluate was lyophilized and then dissolved with dilution buffer [TBS (50 mM Tris-HCl containing 0.9% NaCl) containing 0.5% BSA, 0.1% Tween-20 and 0.05% sodium azide] for use in DH determination by TR-FIA. The recovery rate of DH by this extraction method was estimated to be ≈80%.
TR-FIA was developed based on the method described by Mizoguchi et al.34. The wells of an RIA/EIA plate (Costar #3590) were filled with 80 μL each of anti-DH[N] mouse monoclonal antibody35 (1.5 μg/mL in TBS) and incubated overnight at 4 °C. After discarding the antibody solution, the wells were blocked with TBS containing 4% skimmed milk and 0.1% Tween-20 for 1 h at 25 °C. After washing three times with TBS-T (TBS containing 0.05% Tween-20), 100 μL of anti-DH[C] rabbit antibody35 diluted 1:300 with dilution buffer and either the standard hormone (chemically synthesized DH in 50 μL dilution buffer) or 50 μL of the test sample (hemolymph extract) were distributed into the wells and the plate was incubated for 2 h at 25 °C with shaking on a microplate shaker. The wells were then washed four times with TBS–T, filled with 50 μL of DELFIA Assay buffer (PerkinElmer) containing biotinylated anti–rabbit IgG antibody (Boehringer Mannheim) and europium–labeled streptavidin (PerkinElmer) at concentrations of 83 and 200 ng/mL, respectively and incubated for 1 h at 25 °C with shaking. After incubation, the wells were developed with DELFIA Enhancement solution and the fluorescence signals measured with an ARVO X4 plate reader (PerkinElmer).
Measurement of glycogen content
The ovaries were dissected out with phosphate-buffered saline (PBS) just after eclosion. One ovariole was thoroughly separated from each animal, blotted dry, weighed quickly and stored at –20 °C before use. Glycogen was extracted by digesting the homogenate with 30% (w/v) KOH in a boiling bath for 30 min; the glycogen was then precipitated with ethanol at 4 °C36 and measured by the phenol-sulfuric acid method37.
Thionin staining of embryo
The embryos were collected 10 d after oviposition and were stained with carbolic thionin solution according to An et al.38 with modifications; to facilitate dechorionization, the eggs were boiled in 80% ethanol for 5 min after fixation.
Rescue experiment
Synthetic peptides (DH, α-, β- and γ-SGNPs and PBAN) of 95% purity (HPLC area percentage) were obtained from Operon Biotechnologies. Each peptide was dissolved in peanut oil (Sigma-Aldrich) and 10 μl solutions of various doses were injected into pupa at 4 days after pupation. The diapause eggs inducing activity was estimated by counting the numbers of eggs in diapause and those not in diapause after the non-diapause eggs hatched. The results are expressed as the average percent diapause in each egg batch as described above.
Statistical analysis
Data were compared using Student’s t-tests. The significance of differences presented in Fig. 3E was evaluated using the Steel–Dwass test. Statistical analyses were performed in Excel 2011 (Microsoft) with the software add-in Toukei-Kaiseki Ver. 2.0 (Esumi).
Additional Information
How to cite this article: Shiomi, K. et al. Disruption of diapause induction by TALEN-based gene mutagenesis in relation to a unique neuropeptide signaling pathway in Bombyx. Sci. Rep. 5, 15566; doi: 10.1038/srep15566 (2015).
References
Darlison, M.G. & Richter, D. Multiple genes for neuropeptides and their receptors: co-evolution and physiology. Trends Neurosci. 22(2), 81–88 (1999).
Jiang, H., Wei, Z., Nachman, R.J., Adams, M.E. & Park, Y. Functional phylogenetics reveals contributions of pleiotropic peptide action to ligand-receptor coevolution. Sci Rep. 4, 6800 (2014).
Jurenka, R. & Nusawardani, T. The pyrokinin/pheromone biosynthesis-activating neuropeptide (PBAN) family of peptides and their receptors in Insecta: evolutionary trace indicates potential receptor ligand-binding domains. Insect Mol Biol. 20(3), 323–334 (2011).
Park, Y., Kim, Y.J. & Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA 99(17), 11423–11428 (2002).
Choi, M.Y., Fuerst, E.J., Rafaeli, A. & Jurenka, R. Role of extracellular domains in PBAN/pyrokinin GPCRs from insects using chimera receptors. Insect Biochem Mol Biol. 37(4), 296–306 (2007).
Nusawardani, T., Kroemer, J.A., Choi, M.Y. & Jurenka, R.A. Identification and characterization of the pyrokinin/pheromone biosynthesis activating neuropeptide family of G protein-coupled receptors from Ostrinia nubilalis. Insect Mol Biol. 22(3), 331–340 (2013).
Shalev, A.H. & Altstein, M. Pheromonotropic and melanotropic PK/PBAN receptors: Differential ligand-receptor interactions. Peptides 63C, 81–89 (2014).
Watanabe, K. et al. FXPRL-amide peptides induce ecdysteroidogenesis through a G-protein coupled receptor expressed in the prothoracic gland of Bombyx mori. Mol Cell Endocrinol. 273(1–2), 51–58 (2007).
Roller, L. et al. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem Mol Biol. 38(12), 1147–1157 (2008).
Sato, Y. et al. Precursor polyprotein for multiple neuropeptides secreted from the suboesophageal ganglion of the silkworm Bombyx mori: characterization of the cDNA encoding the diapause hormone precursor and identification of additional peptides. Proc Natl Acad Sci USA 90(8), 3251–3255 (1993).
Yamanaka, N. et al. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PloS One 3(8), e3048 (2008).
Yamashita, O. Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J Insect Physiol. 42, 669–679 (1996).
Homma, T. et al. G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem Biophys Res Commun. 344(1), 386–393 (2006).
Yamashita, O. & Hasegawa, K. Embryonic diapause In Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol 1 (eds Kerkut GA & Gilbert LI ) 407–434 (Pergamon Press, Oxford, 1985).
Sato, A. et al. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc Natl Acad Sci USA 111(13), E1249–1255 (2014).
Daimon, T., Kiuchi, T. & Takasu, Y. Recent progress in genome engineering techniques in the silkworm, Bombyx mori. Dev Growth Differ. 56(1), 14–25 (2014).
Takasu, Y., Tamura, T., Sajwan, S., Kobayashi, I. & Zurovec, M. The use of TALENs for nonhomologous end joining mutagenesis in silkworm and fruitfly. Methods 69(1), 46–57 (2014).
Sato, Y., Ikeda, M. & Yamashita, O. Neurosecretory cells expressing the gene for common precursor for diapause hormone and pheromone biosynthesis-activating neuropeptide in the suboesophageal ganglion of the silkworm, Bombyx mori. Gen Comp Endocrinol. 96(1), 27–36 (1994).
Shiomi, K. et al. The Pitx homeobox gene in Bombyx mori: regulation of DH-PBAN neuropeptide hormone gene expression. Mol Cell Neurosci. 34(2), 209–218 (2007).
Hagino, A., Kitagawa, N., Imai, K., Yamashita, O. & Shiomi, K. Immunoreactive intensity of FXPRL amide neuropeptides in response to environmental conditions in the silkworm, Bombyx mori. Cell Tissue Res. 342(3), 459–469 (2010).
Shiomi, K. et al. Induction of non-diapause eggs by injection of anti-diapause hormone rabbit serum into the diapause type of the silkworm, Bombyx mori. J Insect Physiol. 40(8), 693–699 (1994).
Su, ZH. et al. Molecular characterization of ovary trehalase of the silkworm, Bombyx mori and its transcriptional activation by diapause hormone. Biochim Biophys Acta. 1218(3), 366–374 (1994).
Yamashita, O. & Yaginuma, T. Silkworm eggs at low temperatures: Implication for sericulture In Insects at Low Temperature (eds Lee J. R. E, Denlinger D. L. ) 424–445 (Chapman and Hall, New York, 1991).
Uehara, H., Senoh, Y., Yoneda, K., Kato, Y. & Shiomi, K. An FXPRLamide neuropeptide induces seasonal reproductive polyphenism underlying a life-history tradeoff in the tussock moth. PloS One 6(8), e24213 (2011).
Hull, J.J., Lee, J.M. & Matsumoto, S. Identification of specific sites in the third intracellular loop and carboxyl terminus of the Bombyx mori pheromone biosynthesis activating neuropeptide receptor crucial for ligand-induced internalization. Insect Mol Biol. 20(6), 801–811 (2011).
Kawai, T. et al. Identification of functionally important residues of the silkmoth pheromone biosynthesis-activating neuropeptide receptor, an insect ortholog of the vertebrate neuromedin U receptor. J Biol Chem. 289(27), 19150–19163 (2014).
Hull, J.J. et al. Cloning and characterization of the pheromone biosynthesis activating neuropeptide receptor from the silkmoth, Bombyx mori. Significance of the carboxyl terminus in receptor internalization. J Biol Chem. 279(49), 51500–51507 (2004).
Denlinger, D.L., Yocum, G.D. & Rinehart, J.P. Hormonal control of diapause In Insect Endocrinology (ed Gilbert LI ) 430–463 (Academic Press, San Diego, 2012).
Tauber, M.J., Tauber, C.A. & Masaki, S. Seasonal Adaptations of Insects (Oxford University press, 1986).
Shimizu, I., Aoki, S. & Ichikawa, T. Neuroendocrine control of diapause hormone secretion in the silkworm, Bombyx mori. J Insect Physiol. 43(12), 1101–1109 (1997).
Hahn, DA. & Denlinger, D.L. Energetics of insect diapause. Annual review of entomology 56, 103–121 (2011).
Yamashita, O. & Hasegawa, K. Studies on the mode of action of diapause hormone in the silkworm, Bombyx mori. III. Effect of diapause hormone extract on 3-Hydroxykynurenine content in ovaries of silkworm pupae. J Seric Sci Jpn. 33(2), 115–123 (1964).
Tsuchida, K. & Yoshitake, N. Effect of environmental condition on the voltinism of the silkworm, Bombyx mori reared on artificial diets. J Seric Sci Jpn. 48, 469–472 (1979).
Mizoguchi, A., Ohashi, Y., Hosoda, K., Ishibashi, J. & Kataoka, H. Developmental profile of the changes in the prothoracicotropic hormone titer in hemolymph of the silkworm Bombyx mori: correlation with ecdysteroid secretion. Insect Biochem Mol Biol. 31(4–5), 349–358 (2001).
Kitagawa, N. et al. Establishment of a sandwich ELISA system to detect diapause hormone and developmental profile of hormone levels in egg and subesophageal ganglion of the silkworm, Bombyx mori. Zoolog Sci. 22(2), 213–221 (2005).
Yamashita, O. & Irie, K. Larval hatching from vitellogenin-deficient eggs developed in male hosts of the silkworm. Nature 283, 385–386 (1980).
Dubois, M., Gilles, K.A., Hamilton, J.K., Pebers, P.A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal Chem. 28, 350–356 (1956).
An, Y., Yamashita, T., Seino, A., Imai, K. & Suzuki, K. Functional mimicry of the silkworm diapause hormone by an insect paralytic peptide. J Insect Biotechnol Sericol. 76, 51–55 (2007).
Acknowledgements
We thank T. Ikeda for silkworm rearing. We also thank Drs. Y. An (Iwate University) and Y. Yasukochi (NIAS) for useful discussions. This research was funded by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan and supported partly by the Teimei Empress’s Memorial Grant of The Dainippon Silk Foundation. We are also indebted to the Division of Gene Research, Research Center for Human and Environmental Sciences, for providing facilities for these studies.
Author information
Authors and Affiliations
Contributions
K.S. designed research. K.S., Y.T., M.K., R.T., M.M., M.K., H.S., M.I(T). and A.M. performed research. K.S. and A.M. analyzed data. K.S. wrote the manuscript with support from all authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shiomi, K., Takasu, Y., Kunii, M. et al. Disruption of diapause induction by TALEN-based gene mutagenesis in relation to a unique neuropeptide signaling pathway in Bombyx. Sci Rep 5, 15566 (2015). https://doi.org/10.1038/srep15566
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15566
This article is cited by
-
Comparisons in temperature and photoperiodic-dependent diapause induction between domestic and wild mulberry silkworms
Scientific Reports (2021)
-
An insulin-like growth factor-like peptide promotes ovarian development in the silkmoth Bombyx mori
Scientific Reports (2019)
-
Identification of functional enolase genes of the silkworm Bombyx mori from public databases with a combination of dry and wet bench processes
BMC Genomics (2017)
-
Effects of transgenic overexpression of diapause hormone and diapause hormone receptor genes on non-diapause silkworm
Transgenic Research (2017)
-
Highly efficient and inducible DNA excision in transgenic silkworms using the FLP/FRT site-specific recombination system
Transgenic Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.