Abstract
Vitamin D deficiency, a major public-health worldwide, is associated with hyperuricemia but casual association is questioned. The study was conducted to determine potential causal associations between 25-hydroxy vitamin D (25(OH)D) and uric acid (UA). A cross-sectional study of the Electricity Generating Authority of Thailand (EGAT3) cohort was conducted. Subjects (n = 2,288) were used to genotype the group-specific component (GC) at rs2282679 and ATP-binding cassette subfamily G member 2 (ABCG2) at rs2231142. Mediation analysis with 1000-replication bootstrap was applied to construct causal pathways i.e., rs2282679 → 25(OH)D → UA and rs2231142 → UA → 25(OH)D: The mediator (i.e., 25(OH)D and UA) was firstly regressed on the studied gene (i.e., rs2282679 and rs2231142). A potential causal effect of C allele on UA through 25(OH)D was −0.0236 (95% CI: −0.0411, −0.0058), indicating every minor C allele resulted in decreasing the 25(OH)D and then significantly decreased the UA by 0.0236 unit. For the second pathway, the mediation effect was 0.0806 (95% CI: 0.0107, 0.1628); every T allele copy for rs2231142 increased UA and thus increased 25(OH)D by 0.0806 unit. Our study suggested potential causal associations between the GC gene and UA through the 25(OH)D mediator and the ABCG2 and the 25(OH)D through the UA mediator but the absolute effects are very clinically small.
Similar content being viewed by others
Introduction
Vitamin D deficiency and hyperuricemia are recognized as the major public health concerns worldwide. Prevalence of hyperuricemia has been increasing in both developed and developing countries1,2. High uric acid (UA) induces urate crystallization in many organs and causes gout, urolithiasis and acute and chronic nephropathy. In addition, hyperuricemia was also associated with diseases including hypertension3,4, metabolic syndrome5, diabetes mellitus6,7 and cardiovascular disease5,8,9. Over 1 billion population across the world have been diagnosed with vitamin D insufficiency or deficiency10, which caused both skeletal (i.e. rickets11 and osteoporosis12) and extra-skeletal diseases (i.e. diabetes mellitus13 and cardiovascular disease14).
Evidences from genome-wide association studies (GWAS) suggested that inherited characteristics play roles in UA and vitamin D metabolism pathways, in which approximately 40% to 60% and 29% to 80% for UA15,16 and 25-hydroxy vitamin D (25(OH)D) variations17,18,19 could be explained by the genetic background, respectively. About 75% of UA is excreted in proximal tubules of kidneys and the rest is eliminated via the gastrointestinal tract20,21. Most genes involve in excretion of UA via the urate transporters, which include solute carrier family 2-member (SLC2A9 and SLC22A11) and ATP-binding cassette subfamily G member 2 (ABCG2)22,23, but the ABCG2 loci were the strongest influence in Asian population22. This finding was replicated by individual studies, which found that ABCG2 rs2231142 C > A increased serum UA concentration24 by decreasing the urate transportation rate in proximal tubules25.
GWASs also discovered 4 loci which were involved in vitamin D synthesis pathway, in which the group-specific component (GC) at rs4588 and rs2282679 and the cytochrome P450 IIR-1 gene (CYP2R1) at rs10766197 were most significantly associated with serum vitamin D level. This finding was confirmed by individual studies in Asian population26,27,28.
Both vitamin D deficiency and hyperuricemia are associated with the risk of occurrence of chronic diseases, i.e. diabetes mellitus and cardiovascular disease. A number of animal and human studies have suggested that vitamin D and UA metabolism pathways are related. For example, induction of increased circulating UA was found to suppress 1α-hydroxylase leading to lower 1,25(OH)2D and increased PTH in rats29. Likewise, in humans, administration of allopurinol reduces serum UA with a concurrent increase in 1,25(OH)2D and a reduction in PTH30,31. On the other hand, previous studies in humans suggested negative association between parathyroid hormone (PTH) and serum UA32,33, which corresponded to findings from a study in postmenopausal women given teriparatide34. Low level of vitamin D could leads to hyperuricemia from PTH stimulation. This hypothesis was contradicted by evidences which found positive associations of UA on vitamin D related phenotypes such as bone mineral density (BMD)35, dementia36 and Parkinson’s disease37. It is therefore unclear if vitamin D reciprocally influences UA metabolism and thus constitutes a negative feedback loop commonly found in homeostatic system38. Toward this end, we assessed birectional causal pathways of vitamin D and serum UA using a mediation analysis with accounting for GC and ABCG2 polymorphisms.
Results
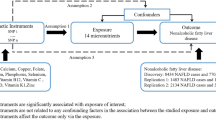
A total of 2288 out of 2592 of the EGAT cohort had genotypic data for GC at rs2282679 and ABCG2 at rs2231142 SNPs. The mean age and body mass index (BMI) were respectively 39.9 (SD = 6.6) years and 23.9 (SD = 3.8) with male gender was majority (74.3%), see Supplementary Table 1. Lipid profiles were measured with mean cholesterol, triglyceride, HDL and LDL of 216.7 (SD = 38.8), 129.5 (SD = 89.9), 51.5 (SD = 12.3) and 148.3 (SD = 36.9) mg/dL, respectively. In addition, mean total 25(OH)D and UA were 25.1 (SD = 6.8) ng/mL and 5.6 (SD = 1.5) mg/dL, respectively. Potential causal associations between the 2 SNPs, intermediate phenotypes and outcomes were assessed as follows:
GC rs2282679-25(OH)D-UA
Potential causal relationships between rs2282679, 25(OH)D and UA were assessed following a causal diagram in Fig. 1a. Two equations (i.e., rs2282679 → 25(OH)D and 25(OH)D → UA) were constructed with adjustment for covariables, see Table 1. The results suggested that every one minor C allele in the rs2282679 → 25(OH)D path would significantly decrease 25(OH)D by 2.4306 (95% confidence interval (CI): −2.8512, −2.0101) ng/mL, see Fig. 1b. The 25(OH)D → UA path suggested that increasing one unit of 25(OH)D would significantly increase UA by 0.0097 (95% CI: 0.0025, 0.0169) mg/dL see Fig. 1b. A bootstrap with 1000 replications yielded the potential causal effect of C allele on UA mediated through 25(OH)D by −0.0236 (95% CI: −0.0411, −0.0058), see Table 2. This could be interpreted that every minor C allele would result in decreased 25(OH)D level and then significantly decreased UA by 0.0236 unit. The minor allele C was also directly associated with UA but this was non-significant after correction for bias (coefficient = 0.0766, 95% CI: −0.0004, 0.1474). The percentage of gene effect contributed by mediation effect (a1b1 path) was 27.9% (95% CI: 24.9%, 30.9%).
ABCG2 rs2231142 → UA → 25(OH)D
The 2 equations (rs2231142 → UA and UA → 25(OH)D) were constructed with adjusted covariables as displayed in Fig. 2 and Table 3. For the first path, every T allele of rs2231142 would significantly increase UA by 0.2726 (95% CI: 0.2020, 0.3431) mg/dL. The UA was also significantly correlated with 25(OH)D in the second path, i.e. one unit of UA increase would significantly increase 25(OH)D by 0.2956 (95% CI: 0.0521, 0.5392) ng/mL. A 1000-replication bootstrap suggested the mediation effect of 0.0806 (95% CI: 0.0107, 0.1628), from which could be interpreted that every one copy of minor T allele would increase UA and thus increase 25(OH)D level by 0.0806 unit, see Table 4. The percentage of gene effect contributed through UA mediator (a1b1 path) was 29.5% (95% CI: 28.2%, 30.8%). This minor T allele was also directly associated with 25(OH)D, but this was not significant (coefficient = −0.2471, 95% CI: −0.6371, 0.1606).
Sensitivity analysis
A robustness of the results was explored if sequential ignorability (SI) assumptions were violated, see Supplementary Table 2. The proportions of original variances  that could be explained if we took into account for invalid SI assumptions were 0.0014 and 0.0012 for casual pathways 1 and 2, respectively. In addition, the unexplained variances that might be explained by unobserved confounders were 0.003 and 0.0025 for these corresponding pathways, indicating very mild effects if the assumptions were violated.
that could be explained if we took into account for invalid SI assumptions were 0.0014 and 0.0012 for casual pathways 1 and 2, respectively. In addition, the unexplained variances that might be explained by unobserved confounders were 0.003 and 0.0025 for these corresponding pathways, indicating very mild effects if the assumptions were violated.
Discussion
Previous evidences have shown a reverse association between vitamin D status and serum UA in various situation including postmenopausal women30, patients with diabetes31, or stable renal failure39. The causal role of vitamin D in this regard is still unclear. In vivo, hyperuricemia has been shown to suppress 1-α hydroxylase and hence lower 1,25(OH)2D with subsequent activation of parathyroid glands. This is in keeping with findings in humans which demonstrated the increased odds of hyperparathyroidism by hyperuricemia29. In the present study, we further delineated the interrelationship between vitamin D level and serum UA by determining the potential causal associations of GC rs2282679 → 25(OH)D → UA and ABCG2 rs2231142 → UA → 25(OH)D pathways. The findings suggested a potential causal effect of 25(OH)D on UA, in which decreasing vitamin D level from carrying minor allele C would result in decreasing serum UA. In addition, a ABCG2 rs2231142 → UA → 25(OH)D pathway indicated increasing UA from carrying a minor T allele would lead to increase vitamin D level.
It has been found that hyperuricemia, gout and primary hyperparathyroidism are associated40,41,42. Moreover, the causal role of parathyroid hormone (PTH) in causing hyperuricemia is suggested by the increase in serum uric levels in patients treated with teriparatide34. Nevertheless, the underlying mechanism of the observation is unclear. It is likely that PTH possesses a direct effect on UA metabolism, but it is also conceivable that PTH may influence UA through other mediators. Patients with primary hyperparathyroidism are at increased risk of vitamin deficiency43 and our finding of the likely causal effect of 25(OH)D on UA suggested vitamin D status as one of the mediators. However, our finding indicated a positive association between vitamin D status and UA which is opposite to the occurrence of lower vitamin D status and hyperuricemia in primary hyperparathyroidism or teriparatide treatment. Clinical trials to investigate the effect of vitamin D supplementation on serum UA are therefore necessary and monitoring of serum UA after vitamin D supplementation to avoid hyperuricemia may be warranted. With regard to the negative causal influence of UA on 25(OH)D suggested by our analyses, studies have shown an inversed association between serum UA and 1,25(OH)2D30,31. Moreover, administering allopurinol to lower serum UA in patients with gout resulted in an increase in 1,25(OH)2D. However, no change in 25(OH)D or PTH were demonstrated44. It therefore still remains to be determined if UA directly affects 25(OH)D as suggested by our study.
High level of UA, the end product of purine metabolism, is known as a cause of lowering kidney function41 and gouty arthritis40,42. Increasing UA level may also induce endothelial dysfunction and thus increase risk to develop diseases such as cardiovascular disease45,46,47, or insulin resistance48. Contrastingly, with its antioxidant property, high level of UA was found to increase BMD for all sites and thus decreased the odds of fractures35, decreased risk of dementia36 and Parkinson’s disease37. This might be explained in that UA itself directly affected these clinical outcomes, or its effect was mediated by other intermediate phenotype. A proper cohort studies (i.e., free from clinical endpoint at baseline, measure UA prior to intermediate phenotype/s and measure the intermediate phenotype/s prior to the interested outcome) are still required to assess a causal effects of UA on clinical endpoints.
Mendelian randomization approach using instrumental variable analysis has been used to assess a causal association pathway between gene and outcome through an intermediate phenotype49,50. This approach could be not applied to our data because the rs2282679 polymorphism itself was also directly associated with UA. This was consistent with a finding by Davies et al.51, in which the studied gene was independently associated with survival in melanoma patients.
Our study has some strengths. We have mapped two causal association of vitamin D and UA pathways using the data from EGAT cohort. A mediation analysis was applied to determine mediation effects of vitamin D on UA and vice versa. This method, also known as process analysis, is the only one of a few statistical methods that have been used to determine a potential causal mechanism or process of how one variable affects the outcome52. However, the mediation analysis requires a few important SI assumptions to yield valid results as follows53,54. First, the studied gene should be ignorable from the outcome and mediator given observed and unobserved confounding factors. Second, the observed mediators (i.e., 25(OH)D for path 1 and UA for path 2) should be independent (i.e. ignorable) from the outcome, given the gene status, pre-observed and unobserved confounding factors. The two SI assumptions could not be checked directly, but performing a sensitivity analysis would lead to estimate unexplained variance that may be explained by unobserved confounders; which were very low, i.e., 0.3% and 0.25% for pathway 1 and pathway 2. In addition, the first SI assumption should be able to be met because the two studied polymorphisms were randomly allocated since conception as for the Mendelian randomization approach49. As a result, violation of the SI assumption should have less effect on our mediation models.
However, our study has some weak points. First, our outcomes of interest are still surrogate or intermediate outcomes, so more clinical endpoints should be followed and assessed. Time of measurements for these intermediate variable/s and end outcomes should be well planned, i.e., the intermediate variables should be measured prior to the occurrence of the end outcome. There may be some other genetic instruments for vitamin D and UA but we had focused only on GC rs2282679 for vitamin D55 and ABCG2 rs2231142 for UA56. Considering those genes together as allelic score, either for instrumental variable or mediation analysis, should be better in explaining outcomes than considering only one polymorphism57. Finally, some other important confounders (e.g., dietary intake, sun exposure, vitamin D supplement, etc.) were not considered in our analysis because data were not available. This should be kept in mind that our potential causal effects might be confounded by these unobserved confounders, although the results from sensitivity analysis showed small effects.
In summary, our evidence has suggested a causal associations between GC(rs2282679) → 25(OH)D → UA and ABCG2(rs2231142) → UA → 25(OH)D pathways, but the effects are very clinically small. The genes contributed approximately 27.9% and 29.5% of total effects on UA and 25(OH)D, respectively. Further cohorts with long term follow up for clinical endpoints and sequential measurement of mediators should be conducted to confirm these findings.
Methods
This cross-sectional study was baseline data of the Electricity Generating Authority of Thailand (EGAT 3) cohort58. Subjects aged 24–54 regardless their disease status were recruited from the headquarters of EGAT in the Bangkok metropolitan area in the year 2009. The extended cohort was aimed to determine genetic factors and markers which were associated with metabolic disorders and bone health. Data collection was performed using a self-administered questionnaire, physical examination, electrocardiography, chest radiography and blood tests. The study was approved by the Institutional Review Board of the Faculty of Medicine at Ramathibodi Hospital and it was carried out in accordance with the approved guidelines. Written informed consents were obtained from every subject.
Serum 25-hydroxyvitamin D (25(OH)D) measurement
Serum 25(OH)D2 and 25(OH)D3 were analyzed by LC-MS/MS with an Agilent 1200 Infinity liquid chromatograph (Agilent Technologies, Waldbronn, Germany) coupled to a QTRAP® 5500 tandem mass spectrometer (AB SCIEX, Foster City CA, USA) using a MassChrom® 25-OH-Vitamin D3/D2 diagnostics kit (ChromSystems, Munich, Germany). The 25(OH)D assay was performed according to the manufacturer’s instructions. This method used a deuterated 25(OH)D3 as an internal standard to correct for sample and instrument variability. Samples were analyzed using an atmospheric pressure chemical ionization source for maximum sensitivity. The 25(OH)D separation was performed using Chromsystems precipitation reagent and trap column in conjunction with Agilent 1200 HPLC system configured for on-line sample preparation according to the configuration included in the documentation with this method. Briefly, 25(OH)D3 and 25(OH)D2 were extracted by mixing 100 μl of serum sample with 25 μl precipitation reagent and 200 μl of the internal standard solution. The mixture was vortexed for 20 seconds and incubated for 10 minutes at 4 °C. After the mixture was centrifuged for 5 minutes at 9,000 g, the upper layer was transferred to an autosampler vial and 5 μl was injected to the LC-MS/MS. The summation of serum 25(OH)D2 and 25(OH)D3 was used to reflect vitamin D status. The inter-assay and intra-assay coefficients of variation of total serum 25(OH)D level were 6.3% and 5.0%, respectively.
Uric acid measurement
Serum uric acid levels were determined using uricase method (Siemens Healthcare Diagnostics Inc., Newark DE, USA). The assay range was 0–20 mg/dl with reference ranges of 2.6–6.0 mg/dl and 3.5–7.2 mg/dl for females and males, respectively. The intra- and inter-assay coefficients of variation were respectively 1.4% and 1.4% at uric acid level 5.1 mg/dl; 1.2% and 1.3% at uric acid level 9.0 mg/dl.
Genotyping
A standard phenol-chloroform method was used to extract genomic DNA from peripheral bloodleukocytes. The GC rs2282679 (OMIM: 139200) and ABCG2 rs2231142 (OMIM: 603756) polymorphisms were genotyped using a TaqMan® assay with allele-specific probes on the ABIPrism 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA). The genotyping call rate was higher than 99%. When the single-nucleotide polymorphism calling was in doubt, direct sequencing was used to provide the correct genotypes.
Statistical analysis
Data were described using mean and frequency for continuous and categorical data, respectively. Genotyping frequencies for rs2282679 and rs2231142 polymorphisms were checked whether their distributions complied with Hardy–Weinberg Equilibrium using an exact test.
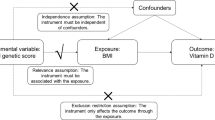
Mediation analysis for continuous data54,59 was applied by constructing two causal pathways, i.e., rs2282679 → 25(OH)D → UA (see Fig. 1a) and rs2231142 → UA → 25(OH)D (see Fig. 2). For the former pathway, rs2282679 was fitted as independent variable, 25(OH)D was mediator and UA was the outcome of interest. For the later pathway, rs2231142 was the independent variable, UA was the mediator and 25(OH)D was the outcome of interest. Causal equations for both pathways were constructed as follows: The mediator for each pathway was firstly regressed on the studied gene (called path a1, see Fig. 1a). The outcome variable was then regressed on mediator and studied gene (path b1). The 2 studied genes were fitted in the equations as additive effects by assigning 0, 1 and 2 for major homozygous, heterozygous and minor homozygous genotypes, respectively. The equations for these paths are as follows:


where xi = 0, 1, 2 for major homozygous, heterozygous and minor homozygous genotypes; m = 25(OH)D and UA for pathway 1 and 2, respectively; zk = confounders.
Confounders including age, gender, BMI and triglyceride were included in the two pathways. Triglyceride was considered instead of other lipid profiles because this variable was the most significantly associated with mediator and outcome and to avoid colinearity among them if they were included in the same model. A potential causal mediation effect was then estimated using the product-of-coefficient method, i.e., a1b152,60,61. A bootstrap analysis with 1,000 replications was then applied to estimate average causal mediation effects without requiring the assumption of normality59,62. For each bootstrap, the causal mediation effect was estimated, averaged across 1000 replications and its corresponding 95% CI was then determined using bias-corrected bootstrap technique.
A sensitivity analysis was performed to determine robustness of effects if the SI assumptions were violated63. The proportion of unexplained variances  that were explained by unobserved confounders and the proportions of original variances
that were explained by unobserved confounders and the proportions of original variances  which were explained by unobserved confounders in the mediator and outcome models were then estimated. Analyses were performed using STATA 13.0 software. STATA commands used for all analyses were provided in the Supplement document. A P-value < 0.05 was considered statistically significant.
which were explained by unobserved confounders in the mediator and outcome models were then estimated. Analyses were performed using STATA 13.0 software. STATA commands used for all analyses were provided in the Supplement document. A P-value < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Thakkinstian, A. et al. Potential causal associations between vitamin D and uric acid: Bidirectional mediation analysis. Sci. Rep. 5, 14528; doi: 10.1038/srep14528 (2015).
References
Liu, H., Zhang, X.-M., Wang, Y.-L. & Liu, B.-C. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. Journal of Nephrology, 10.1007/s40620-014-0082-z (2014).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 63, 3136–41 (2011).
Grayson, P. C., Kim, S. Y., LaValley, M. & Choi, H. K. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 63, 102–10 (2011).
Zhang, W. et al. Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clin Chem 55, 2026–34 (2009).
Choi, H. K. & Ford, E. S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 120, 442–7 (2007).
Bhole, V., Choi, J. W., Kim, S. W., de Vera, M. & Choi, H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med 123, 957–61 (2010).
Chien, K. L. et al. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem 54, 310–6 (2008).
Kim, S. Y. et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum 61, 885–92 (2009).
Krishnan, E., Svendsen, K., Neaton, J. D., Grandits, G. & Kuller, L. H. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med 168, 1104–10 (2008).
Holick, M. F. Vitamin D deficiency. N Engl J Med 357, 266–81 (2007).
Wharton, B. & Bishop, N. Rickets. Lancet 362, 1389–400 (2003).
Sahota, O. Osteoporosis and the role of vitamin D and calcium-vitamin D deficiency, vitamin D insufficiency and vitamin D sufficiency. Age and Ageing 29, 301–304 (2000).
Mathieu, C., Gysemans, C., Giulietti, A. & Bouillon, R. Vitamin D and diabetes. Diabetologia 48, 1247–1257 (2005).
Wang, T. J. et al. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation 117, 503–511 (2008).
Nath, S. D. et al. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol 18, 3156–63 (2007).
Yang, Q. et al. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism 54, 1435–41 (2005).
Shea, M. K. et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr 63, 458–64 (2009).
Arguelles, L. M. et al. Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent twins. J Clin Endocrinol Metab 94, 3273–81 (2009).
Wjst, M., Altmuller, J., Braig, C., Bahnweg, M. & Andre, E. A genome-wide linkage scan for 25-OH-D(3) and 1,25-(OH)2-D3 serum levels in asthma families. J Steroid Biochem Mol Biol 103, 799–802 (2007).
Mount, D. B. The kidney in hyperuricemia and gout. Current Opinion in Nephrology and Hypertension 22, 216–223, 10.1097/MNH.0b013e32835ddad2 (2013).
Bobulescu, I. A. & Moe, O. W. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis 19, 358–71 (2012).
Yang, B. et al. A genome-wide association study identifies common variants influencing serum uric acid concentrations in a Chinese population. BMC Med Genomics 7, 10 (2014).
Kottgen, A. et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet 45, 145–54 (2013).
Stiburkova, B., Pavlikova, M., Sokolova, J. & Kozich, V. Metabolic syndrome, alcohol consumption and genetic factors are associated with serum uric acid concentration. PLoS One 9, e97646 (2014).
Woodward, O. M. et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA 106, 10338–42 (2009).
Yoshida, S. et al. A GC polymorphism associated with serum 25-hydroxyvitamin D level is a risk factor for hip fracture in Japanese patients with rheumatoid arthritis: 10-year follow-up of the Institute of Rheumatology, Rheumatoid Arthritis cohort study. Arthritis Res Ther 16, R75 (2014).
Cheung, C. L., Lau, K. S., Sham, P. C., Tan, K. C. & Kung, A. W. Genetic variant in vitamin D binding protein is associated with serum 25-hydroxyvitamin D and vitamin D insufficiency in southern Chinese. J Hum Genet 58, 749–51 (2013).
Zhang, Z., He, J. W., Fu, W. Z., Zhang, C. Q. & Zhang, Z. L. An analysis of the association between the vitamin D pathway and serum 25-hydroxyvitamin D levels in a healthy Chinese population. J Bone Miner Res 28, 1784–92 (2013).
Chen, W. et al. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism 63, 150–60 (2014).
Peng, H. et al. Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS One 8, e61159 (2013).
Yilmaz, H., Kaya, M., Sahin, M. & Delibasi, T. Is vitamin D status a predictor glycaemic regulation and cardiac complication in type 2 diabetes mellitus patients? Diabetes Metab Syndr 6, 28–31 (2012).
Hui, J. Y. et al. The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res Ther 14, R56 (2012).
Dalbeth, N. et al. The effect of calcium supplementation on serum urate: analysis of a randomized controlled trial. Rheumatology (Oxford) 48, 195–7 (2009).
Miller, P. D., Schwartz, E. N., Chen, P., Misurski, D. A. & Krege, J. H. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18, 59–68 (2007).
Nabipour, I. et al. Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J Bone Miner Res 26, 955–64 (2011).
Euser, S. M., Hofman, A., Westendorp, R. G. & Breteler, M. M. Serum uric acid and cognitive function and dementia. Brain 132, 377–82 (2009).
de Lau, L. M., Koudstaal, P. J., Hofman, A. & Breteler, M. M. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58, 797–800 (2005).
Jr, J. E. Feedback loops and reciprocal regulation: recurring motifs in the systems biology of the cell cycle. Curr Opin Cell Biol 25, 676–86 (2013).
Vanholder, R., Patel, S. & Hsu, C. H. Effect of uric acid on plasma levels of 1,25(OH)2D in renal failure. J Am Soc Nephrol 4, 1035–8 (1993).
Reginato, A. M., Mount, D. B., Yang, I. & Choi, H. K. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol 8, 610–21 (2012).
Okada, Y. et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet 44, 904–9 (2012).
So, A. & Thorens, B. Uric acid transport and disease. J Clin Invest 120, 1791–9 (2010).
Tassone, F. et al. Vitamin D status in primary hyperparathyroidism: a Southern European perspective. Clin Endocrinol (Oxf) 79, 784–90 (2013).
Takahashi, S. et al. Decreased serum concentrations of 1,25(OH)2-vitamin D3 in patients with gout. Metabolism 47, 336–8 (1998).
Grassi, D., Desideri, G. & Ferri, C. New Insight into Urate-Related Mechanism of Cardiovascular Damage. Curr Pharm Des 7, 6089–6095 (2014).
Kanbay, A. et al. Uric acid as a potential mediator of cardiovascular morbidity in obstructive sleep apnea syndrome. Eur J Intern Med 25, 471–6 (2014).
Qin, L. et al. Association between serum uric acid levels and cardiovascular disease in middle-aged and elderly Chinese individuals. BMC Cardiovasc Disord 14, 26 (2014).
Zhu, Y. et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun 447, 707–14 (2014).
Davey Smith, G. & Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32, 1–22 (2003).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27, 1133–63 (2008).
Davies, J. R. et al. An inherited variant in the gene coding for vitamin D-binding protein and survival from cutaneous melanoma: a BioGenoMEL study. Pigment Cell Melanoma Res 27, 234–43 (2014).
MacKinnon, D. P., Fairchild, A. J. & Fritz, M. S. Mediation analysis. Annu Rev Psychol 58, 593–614 (2007).
Tingley, D., Yamamoto, T., Keele, L. & Imai, K. Mediation: R Package for causal mediation analysis. Journal of Statistical Software inpress (2013).
Imai, K., Keele, L. & Tingley, D. A general approach to causal mediation analysis. Psychol Methods 15, 309–34 (2010).
Vimaleswaran, K. S. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10, e1001383 (2013).
Palmer, T. M. et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ 347, f4262 (2013).
Burgess, S. & Thompson, S. G. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol 42, 1134–44 (2013).
Vathesatogkit, P. et al. Cohort profile: the electricity generating authority of Thailand study. Int J Epidemiol 41, 359–65 (2012).
Bruin, J. Newtest: command to compute new test. UCLA:Statistical Consulting Group. (2006). http://www.ats.ucla.edu/stat/stata/ado/analysis/. (Accessed:04/05/2014).
Kenny, D. A. Mediation. (2012). http://davidakenny.net/cm/mediate.htm. (Accessed:15/01/2014).
MacKinnon, D. P., Lockwood, C. M., Hoffman, J. M., West, S. G. & Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 7, 83–104 (2002).
Preacher, K. J. & Hayes, A. F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40, 879–91 (2008).
Hicks, R. & Tinggley, D. Causal mediation analysis. The STATA Journal 11, 605–619 (2011).
Acknowledgements
This work was supported by the project for Higher Education Research Promotion and National Research University Development, Office of the Higher Education Commission of Thailand and the Thailand Research Fund.
Author information
Authors and Affiliations
Contributions
A.T. conception and design, acquisition of data, analysis and interpretation, wrote the main the manuscript. T.A. data analysis and wrote the manuscript. L.C. genotyping data and lab tests. W.R. acquisition of data and critically comment. S.Y. conception and design and acquisition of data. P.S. conception and design and acquisition of data. B.O. conception and design, acquisition of data, interpretation, wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Thakkinstian, A., Anothaisintawee, T., Chailurkit, L. et al. Potential causal associations between vitamin D and uric acid: Bidirectional mediation analysis. Sci Rep 5, 14528 (2015). https://doi.org/10.1038/srep14528
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14528
This article is cited by
-
Vitamin D in pediatric patients with obesity and arterial hypertension
Scientific Reports (2021)
-
Association between vitamin D deficiency and lipid and non-lipid markers of cardiovascular diseases in the middle east region
European Journal of Clinical Nutrition (2019)
-
The role of uric acid in mineral bone disorders in chronic kidney disease
Journal of Nephrology (2019)
-
Association between serum uric acid and bone health in adolescents
Osteoporosis International (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.