Abstract
Research is crucial to implement evidence-based health interventions for control of non-communicable diseases (NCDs). This study aims to assess main features of randomized controlled trials (RCTs) for control of NCDs and to identify gaps in clinical research on NCDs between high-income and less developed countries. The study included 1177 RCTs in 82 Cochrane Systematic reviews (CSRs) and evaluated interventions for adults with hypertension, diabetes, stroke, or heart diseases. Multivariate logistic regression analyses were conducted to explore factors associated with risk of bias in included RCTs. We found that 78.2% of RCTs of interventions for major NCDs recruited patients in high-income countries. The number of RCTs included in the CSRs was increasing over time and the increasing speed was more noticeable for RCTs conducted in middle-income countries. RCTs conducted in less developed countries tended to be more recently published, less likely to be published in English, with smaller sample sizes and at a higher risk of bias. In conclusion, there is still a lack of research evidence for control of NCDs in less developed countries. To brace for rising NCDs and avoid waste of scarce research resources, not only more but also higher quality clinical trials are required in low-and-middle-income countries.
Similar content being viewed by others
Introduction
Non-communicable diseases (NCDs) are leading causes of mortality, morbidity and disability globally and the burden of NCDs is rising rapidly in low-and-middle-income countries (LMICs)1,2. The myth that NCDs affect mainly people in high income countries is consistently dismissed by available evidence. According to the World Health Organization, NCDs caused 38 million of global deaths in 2012, with 74% occurring in LMICs3. In addition, NCDs were responsible for more than 40% of premature deaths under age 70 years and 82% of the premature deaths occurred in LMICs3. Therefore, the United Nations held a high-level meeting on NCDs in 2013 and recommended a shift of global priority from infectious to non-infectious diseases4.
Research is crucial to develop and implement evidence-based health interventions for the prevention and control of NCDs in LMICs, as in high-income countries5,6. It is well known that most available evidence is from research conducted in high-income countries7,8. An analysis of Cochrane reviews found that only a very small proportion of trials of interventions for NCDs were conducted in LMICs9. Evidence from research in high-income countries may not be directly applicable to LMICs10,11. For example, empirical data indicated that effect sizes in clinical trials from more developed countries may be different from less developed countries12.
High quality randomized controlled trials (RCTs) provide the most valid evidence for the prevention and control of NCDs13. Although previous studies considered the amount and effect sizes of RCTs conducted in LMICs9,12, RCTs conducted in high-income countries and in LMICs have not been comprehensively compared in terms of sample sizes, publication languages and risk of bias. The purpose of this study is to assess main features of RCTs for the control of NCDs and to identify gaps in clinical research on NCDs between high-income and less developed countries.
Methods
Eligibility criteria
We included recently updated (since 2010) Cochrane Systematic reviews (CSRs) that evaluated treatment interventions for adult patients with the following chronic conditions: hypertensive disorders, Type 2 diabetes mellitus, stroke, or heart diseases. We exclude CSRs that evaluated interventions exclusively in children, infants or pregnant women. We also excluded CSRs of interventions primarily for the prevention of chronic conditions. There was no restriction on the primary outcome measures and the length of follow up.
Selection and data extraction
We searched Cochrane Database of Systematic Reviews in Cochrane Library (Issue 4 of 12, 2014) to identify eligible CSRs. The search strategy included a combination terms of “hypertension OR hypertensive OR diabetes OR diabetic OR stroke OR cardiovascular OR cerebrovascular” in Title, Abstract, or Keywords. Using this search strategy, we searched the Cochrane Database and transferred the initial yield into a bibliographic database (Endnotes). One researcher (HF) applied the inclusion and exclusion criteria to identify relevant CSRs and a second reviewer (FS) was involved when it was difficult to decide the eligibility of a CSR.
Data extraction was conducted by one researcher (HF) and then checked by a second researcher (FS). Discrepancy was addressed by discussion. The following data were obtained from the included CSRs: year as up-to-date, country of the corresponding author of CSRs, language restrictions for study inclusion and chronic conditions addressed. From RCTs included in the CSRs, we extracted data on types of interventions, year of publication, sample size, country origin, publication language and results of risk of bias assessment.
Quality of all RCTs included in CSRs was assessed using the Cochrane Collaboration’s tool for assessing risk of bias13. Specifically, the Cochrane quality parameters for risk of bias are designed to answer the following six questions. (1) Was the allocation sequence adequately generated? (2) Was allocation adequately concealed? (3) Was knowledge of the allocated intervention adequately prevented during the study? (4) Were incomplete outcome data adequately addressed? (5) Are reports of the study free of suggestion of selective outcome reporting? (6) Was the study apparently free of other problems that could put it at a high risk of bias? For each of these questions, systematic reviewers’ answers may be ‘Yes’, ‘No’ or ‘Unclear’, based on information available from included RCTs. If the answer is ‘Yes’, it indicates a low risk of bias. In this study, we used results of risk of bias assessment for the first five questions, because risk of other biases (the last question) was inconsistently assessed in the included CSRs.
Data synthesis and analysis
Data extracted from the included CSRs and RCTs were summarized by tabulations. RCTs included in the relevant CSRs were categorized by year and language of publication, country in which they were conducted and risk of bias. The definition of high-income, middle-income and low-income country was accordance with the World Bank’s country classification14. Mixed-effects, multivariate logistic regression analyses were also conducted to explore factors associated with risk of bias of included RCTs15.
Results
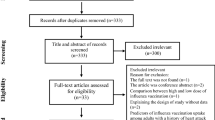
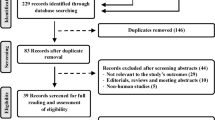
A total of 498 reviews from 8440 records were retrieved on April 2014. We screened 493 reviews after removal of duplicates. Based on their titles and abstracts, a total of 82 reviews met the inclusion criteria and made up the data set16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97. The 82 relevant CSRs included 1177 RCTs. Figure 1 shows the process of the CSRs selection.
The main characteristics of the included CSRs are summarized in Table 1. Corresponding authors of the CSRs were mostly from institutions in high-income countries (75.6%). The institutional affiliations of corresponding authors were in an upper-middle-income country for 19 CSRs and in a lower-middle-income country for only one CSR. Of the 82 CSRs, stroke or related vascular conditions were addressed in 36 (43.9%), hypertension disorders in 17 (20.7%), heart disease in 13 (15.9%) and diabetes in 16 (19.5%). Twelve point two percent of the included CSRs were updated as to 2010, 29.3% up to 2011, 34.1% up to 2012 and 24.4% up to 2013 or 2014. The median number of RCTs included in these CSRs was 9 (interquartile 4 to 17). Language restriction was explicitly not used in 75 (91.5%) CSRs and it was unclear in six CSRs. Only one CSR explicitly applied language restriction to included trials published in European languages (including English, German, Dutch, French, Italian, Portuguese or Spanish).
Randomised controlled trials included
The main characteristics of the 1177 RCTs are shown in Table 2. Most of the RCTs (78.2%) were conducted in high-income countries and only 18.3% of the 1177 RCTs recruited patients in middle-income countries (none from low-income countries). The proportion of RCTs in mixed-income countries (multiple countries belonging to different income groups) was 3.5%. The total number of patients in the 1177 RCTs was 511307; 76.3% were recruited in high-income countries, 19.1% in mixed countries and only 4.6% in middle-income countries. The sample size of individual RCTs ranged from 4 to 22576 (median 85, interquartile range: 40 to 198). RCTs with a sample size ≥500 accounted for 9.8% of RCTs in high-income countries and only 1.9% in middle-income countries (Table 2).
Of the 1177 RCTs, most were published in English (89.8%) and the proportion of RCTs published in Chinese was 8%, while other languages accounted less than 3%. When comparing the language of publication of RCTs conducted in different countries, except China, English dominated the language of publication in most of the countries. For the 124 RCTs conducted in China, 92 (74%) were published in Chinese language (including one published in both English and Chinese).
The included RCTs were published from 1962 to 2013, although most were published since 2000 (67.5%). The number of RCTs included in the CSRs was increasing over time and the increasing speed was noticeable for RCTs conducted in middle-income countries and mixed-income countries. The ratio of RCTs conducted in middle-income countries to RCTs conducted in high-income countries was only 4.4% (9:203) for RCTs published from 1990 to1999 and it increased to 36% (200:556) for those published since 2000 (Figure 2). Twenty-nine of the 626 RCTs published from 2000 to 2009 were conducted in multiple income groups of countries, while there were only two such RCTs published from 1990 to 1999 and none before 1990 (Fig. 2).
Quality of included RCTs
Of the 1177 RCTs, the proportion of RCTs with a low risk of bias was 45.0% in terms of sequence generation, 33.2% regarding allocation concealment, 37.2% regarding blinding, 57.6% regarding incomplete outcome and 44.2% about reporting bias (Table 3). The validity of the included RCTs tended to improve over time, although the proportion of low risk of bias was higher in RCTs published before 1980 compared with RCTs published from 1980 to 1989.
The proportion of low risk of bias was higher for RCTs conducted in high-income countries than in middle-income countries and the proportion of low risk of bias was highest in RCTs conducted in mixed-income countries. In addition, RCTs with larger sample sizes tended to have a low risk of bias (Table 3). The proportion of RCTs with low risk of bias was highest in RCTs published in English and lowest in RCTs published in Chinese. In terms of allocation concealment, for example, the frequency of low risk of bias was 36.1% for RCTs published in English, 3.3% for RCTs published in Chinese and 20.7% for RCTs published in languages other than English or Chinese. Regarding reporting bias, the proportion of low risk of bias was 47.4% for RCTs published in English, 8.7% for RCTs published in Chinese and 37.9% for RCTs published in other languages (see Table 3).
The results of mixed-effects, multivariate, logistic regression analysis are presented in Table 4. The dependent variable used in the analysis was a dummy variable for high study validity, defined as at least four of the five bias items were judged to be low. The results indicated that the study validity was high (that is, at low risk of bias) in RCTs with larger sample sizes, published in English and more recently. The study validity was relatively low in RCTs conducted in middle-income countries (P = 0.005). After adjusting for other variables, the difference in study validity was statistically non-significant between RCTs conducted in mixed-income countries and those in high-income countries (P = 0.11).
Discussion
The results of the current study are consistent with findings from previous studies, indicating that clinical research of interventions for NCDs has been conducted mainly in high-income countries and there is a lack of research evidence from LMICs7,8,9,11. We found that 78.2% of RCTs of treatment interventions for major NCDs recruited patients in high-income countries, 18.3% in middle-income countries, 3.5% in mixed-income countries and none in low-income countries. In the current study, we also systematically examined the main features of individual RCTs by income group, including sample size, year and language of publication and risk of bias. Compared with RCTs conducted in high-income countries, RCTs conducted in middle-income countries tended to be more recently published, less likely to be published in English, with smaller sample sizes and at a higher risk of bias.
Although the proportion of research evidence from LMICs is still very low, the number of RCTs on NCDs treatment interventions has been increasing in middle-income countries, which was similar to findings from other studies. A study reported an 18% increase in the proportion of health-related publications with authors in upper-middle-income countries between 2002 and 2011 and an 8% decreased in the proportion of publications with authors in high-income countries during the same period8.
It is important to note that the quality of clinical trials in middle-income countries is generally lower compared with clinical trials in high-income countries. This was in line with findings from previous studies98. In addition, RCTs conducted in middle-income countries were more likely to be published in languages other than English and studies published in languages other than English may be less likely to be included in systematic reviews99. Scarce resources for research in developing countries would be wasted if findings from many clinical trials there conducted could not be trusted or included in systematic reviews due to poor design and reporting quality100,101. Therefore, the quality of clinical research in LMICs needs to be appropriately addressed. Not only more but also high quality trials are required in less developed countries.
There was recently an increase in the number of multi-national studies conducted in mixed-income countries. Studies in mixed-income countries tended to have large sample sizes and a low risk of bias. Because collaboration between researchers in more and less developed countries will usually be required to conduct studies in mixed-income countries, such studies should be promoted to overcome problems caused by limited research capacity in less developed countries.
Generalizability of evidence from high-income countries to LMICs
Currently, high quality research evidence on the effectiveness of interventions for the control of NCDs is mainly from high-income countries. To tackle NCDs cost-effectively in LMICs, policy and practice decisions will often have to be based on research evidence generated from high-income countries102. However, evidence from high-income countries may not be generalizable to LMICs10.
Empirical evidence indicated the existence of country specific effects of medical interventions. For example, studies conducted in the United States (US) on average reported smaller treatment effects of cardiorenal drugs compared with studies in the non-US countries103. A meta-epidemiological study found that RCTs conducted in less developed countries tended to report more favourable results than trials in more developed countries12. The genuine difference in treatment effects between countries could not be ruled out in many cases. For example, it has been suggested that some interventions may be no more effective than usual care in high-income countries, but may be more effective than usual care in less developed countries104.
Limitations
RCTs that were not included in the Cochrane reviews could not be considered in this study. It is possible that some trials published in languages other than English may have not been included in the relevant Cochrane systematic reviews. Therefore, the proportion of RCTs conducted in LMICs may have been under-estimated in the current study.
We did not include CSRs that evaluated interventions for primary prevention, or interventions exclusively in children, infants or pregnant women. Therefore, findings from the current study may not be generable to trials of interventions for primary prevention or trials that exclusively included children or pregnant women.
The treatment effects of interventions were not compared in the current study, which was examined in a recent meta-epidemiological study12. Given the limited resources and time, we only included CSRs that evaluated treatment interventions for patients with stroke, hypertension, heart disease and diabetes.
Conclusions
Clinical research of interventions for NCDs has been conducted mainly in high-income countries and there is a lack of research evidence from LMICs. RCTs conducted in LMICs were less likely to be published in English, with smaller sample sizes and at a higher or unclear risk of bias than trials in high-income countries. To brace for rising NCDs and avoid waste of scarce research resources, not only more but also higher quality clinical trials are required in LMICs.
Additional Information
How to cite this article: Fan, H. and Song, F. An assessment of randomized controlled trials (RCTs) for non-communicable diseases (NCDs): more and higher quality research is required in less developed countries. Sci. Rep. 5, 13221; doi: 10.1038/srep13221 (2015).
References
Di-Cesare, M. et al. Inequalities in non-communicable diseases and effective responses. Lancet 381, 585–597, 10.1016/s0140-6736(12)61851-0 (2013).
Cerqueira, M. T. et al. Global response to non-communicable disease. BMJ 342, d3823, 10.1136/bmj.d3823 (2011).
WHO. Global status report on noncommunicable diseases 2014. (2014) Available at: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1. (Accessed: 24th June 2015).
Cao, X. A call for global research on non-communicable diseases. Lancet 385, e5–6, 10.1016/s0140-6736(14)62383-7 (2015).
Wise, J. Research network aims to tackle chronic disease in developing world. BMJ 338, b2440, 10.1136/bmj.b2440 (2009).
Smith, R. A global research network for non-communicable diseases. Lancet 383, 1446–1447, 10.1016/s0140-6736(13)61808-5 (2014).
Mendis, S., Yach, D., Bengoa, R., Narvaez, D. & Zhang, X. Research gap in cardiovascular disease in developing countries. Lancet. 361, 2246–2247, 10.1016/s0140-6736(03)13753-1 (2003).
Rottingen, J. A. et al. Mapping of available health research and development data: what’s there, what’s missing and what role is there for a global observatory? Lancet 382, 1286–1307, 10.1016/s0140-6736(13)61046-6 (2013).
Heneghan, C. et al. Evidence for non-communicable diseases: analysis of Cochrane reviews and randomised trials by World Bank classification. BMJ open 3, 10.1136/bmjopen-2013-003298 (2013).
Miranda, J. J. & Zaman, M. J. Exporting ‘failure’: why research from rich countries may not benefit the developing world. Rev Saude Publica. 44, 185–189 (2010).
Chinnock, P., Siegfried, N. & Clarke, M. Is evidence-based medicine relevant to the developing world? PLoS Med. 2, e107, 10.1371/journal.pmed.0020107 (2005).
Panagiotou, O. A., Contopoulos-Ioannidis, D. G. & Ioannidis, J. P. Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment. BMJ 346, f707, 10.1136/bmj.f707 (2013).
Higgins, J. T., Green, S. S. Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration & Wiley-Blackwell, 2008).
THE WORLD BANK. Country and Lending Groups. (2014) Available at: http://data.worldbank.org/about/country-and-lending-groups (Accessed: 9th March 2015).
STATA. Multilevel mixed-effects reference manual release 13. (StataCorp LP, Texas, 2013).
Amin, F., AI-Hajeri, A., Civelek, B., Fedorowicz, Z. & Manzer, B. M. Enhanced external counterpulsation for chronic angina pectoris. Cochrane Database Syst Rev. 2, CD007219, 10.1002/14651858.CD007219.pub2 (2010).
Arguedas, J. A., Leiva, V. & Wright, J. M. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 10, CD008277, 10.1002/14651858.CD008277.pub2 (2013).
Arsenault, K. A. et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 1, CD003611, 10.1002/14651858.CD003611.pub3 (2013).
Baharoglu, M. I. et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 8, CD001245, 10.1002/14651858.CD001245.pub2 (2013).
Bath, P. M., Sprigg, N. & England, T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. 6, CD005207, 10.1002/14651858.CD005207.pub4 (2013).
Batterink, J., Stabler, S. N., Tejani, A. M. & Fowkes, C. T. Spironolactone for hypertension. Cochrane Database Syst Rev. 8, CD008169, 10.1002/14651858.CD008169.pub2 (2010).
Baumeister, H., Hutter, N. & Bengel, J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 9, CD008012, 10.1002/14651858.CD008012.pub3 (2011).
Brady, M. C., Kelly, H., Godwin, J. & Enderby, P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 5, CD000425, 10.1002/14651858.CD000425.pub3 (2012).
Bruins-Slot, K. M. & Berge, E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 8, CD008980, 10.1002/14651858.CD008980.pub2 (2013).
Buchleitner, A. M., Martínez-Alonso, M., Hernández, M., Solà, I. & Mauricio, D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev. 9, CD007315, 10.1002/14651858.CD007315.pub2 (2012).
Campbell-Burton, C. A. et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 12, CD008860, 10.1002/14651858.CD008860.pub2 (2011).
Ciccone, A., Celani, M. G., Chiaramonte, R., Rossi, C. & Righetti, E. Continuous versus intermittent physiological monitoring for acute stroke. Cochrane Database Syst Rev. 5, CD008444, 10.1002/14651858.CD008444.pub2 (2013).
Clarkesmith, D. E., Pattison, H. M. & Lane, D. A. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 6, CD008600, 10.1002/14651858.CD008600.pub2 (2013).
Clifford, D. M. et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2, CD006536, 10.1002/14651858.CD006536.pub3 (2012).
Coupar, F., Pollock, A., Legg, L. A., Sackley, C. & Van-Vliet, P. Home-based therapy programmes for upper limb functional recovery following stroke. Cochrane Database Syst Rev. 5, CD006755, 10.1002/14651858.CD006755.pub2 (2012).
De-Lima, L. G., Soares, B. G. O., Saconato, H., Atallah, A. N. & Da- Silva, E.M. Beta-blockers for preventing stroke recurrence. Cochrane Database Syst Rev. 10, CD007890, 10.1002/14651858.CD007890.pub3 (2013).
Demetrios, M., Khan, F., Turner-Stokes, L., Brand, C. & Mc-Sweeney, S. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst Rev. 6, CD009689, 10.1002/14651858.CD009689.pub2 (2013).
Diao, D., Wright, J. M., Cundiff, D. K. & Gueyffier, F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev. 8, CD006742, 10.1002/14651858.CD006742.pub2 (2012).
Elsner, B., Kugler, J., Pohl, M. & Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 11, CD009645, 10.1002/14651858.CD009645.pub2 (2013).
Fearon, P. & Langhorne, P. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev. 9, CD000443, 10.1002/14651858.CD000443.pub3 (2012).
Fisher, S. A. et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 4, CD007888, 10.1002/14651858.CD007888.pub2 (2014).
Fletcher-Smith, J. C., Walker, M. F., Cobley, C. S., Steultjens, E. M. & Sackley, C. M. Occupational therapy for care home residents with stroke. Cochrane Database Syst Rev. 6, CD010116, 10.1002/14651858.CD010116.pub2 (2013).
Forster, A. et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev. 11, CD001919, 10.1002/14651858.CD001919.pub3 (2012).
Geeganage, C., Beavan, J., Ellender, S. & Bath, P. M. W. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 10, CD000323, 10.1002/14651858.CD000323.pub2 (2012).
George, S., Crotty, M., Gelinas, I. & Devos, H. Rehabilitation for improving automobile driving after stroke. Cochrane Database Syst Rev. 2, CD008357, 10.1002/14651858.CD008357.pub2 (2014).
Gois, P. H. F. & Souza, E. R. M. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev. 1, CD008652, 10.1002/14651858.CD008652.pub2. Review (2013).
Guo, J., Shi, Z., Yang, K., Tian, J. H. & Jiang, L. Endothelin receptor antagonists for subarachnoid hemorrhage. Cochrane Database Syst Rev. 9, CD008354, 10.1002/14651858.CD008354.pub2 (2012).
Hao, Z. et al. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst Rev. 3, CD000091, 10.1002/14651858.CD000091.pub2 (2012).
Hao, Z., Wang, D., Zeng, Y. & Liu, M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 5, CD008862, 10.1002/14651858.CD008862.pub2 (2013).
Hemmingsen, B. et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 11, CD008143, 10.1002/14651858.CD008143.pub3 (2013).
Hemmingsen, B. et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane Database Syst Rev. 4, CD009008, 10.1002/14651858.CD009008.pub2 (2013).
Heran, B. S. et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 7, CD001800, 10.1002/14651858.CD001800.pub2 (2011).
Heran, B. S., Chen, J. M., Wang, J. J. & Wright, J. M. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database Syst Rev. 11, CD008167, 10.1002/14651858.CD008167.pub3 (2012).
Heran, B. S., Galm, B. P. & Wright, J. M. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst Rev. 8, CD004643, 10.1002/14651858.CD004643.pub3 (2012).
Horjus, D. L., Oudman, I., Van-Montfrans, G. A. & Brewster, L. M. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst Rev. 11, CD005184, 10.1002/14651858.CD005184.pub2. (2011).
Laver, K. E., George, S., Thomas, S., Deutsch, J. E. & Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2, CD008349, 10.1002/14651858.CD008349.pub3. (2011).
Leach, M. J. & Kumar, S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 9, CD007170, 10.1002/14651858.CD007170.pub2 (2012).
Liakopoulos, O. J., Kuhn, E. W., Slottosch, I., Wassmer, G. & Wahlers, T. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev. 4, CD008493, 10.1002/14651858.CD008493.pub2 (2012).
Lin, S. et al. External counterpulsation for acute ischaemic stroke. Cochrane Database Syst Rev. 1, CD009264, 10.1002/14651858.CD009264.pub2 (2012).
Lip, G. Y. H., Felmeden, D. C. & Dwivedi, G. Antiplatelet agents and anticoagulants for hypertension. Cochrane Database Syst Rev. 12, CD003186. 10.1002/14651858.CD003186.pub3 (2011).
Liu, J. & Wang, L. N. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database Syst Rev. 2, CD009622, 10.1002/14651858.CD009622.pub2 (2013).
Liu, Z., Liu, L., Zhang, Z., Chen, Z. & Zhao, B. Cholesterol-reducing agents for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 4, CD008184, 10.1002/14651858.CD008184.pub2 (2013).
Liu, J. & Wang, L. N. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 1, CD010693, 10.1002/14651858.CD010693.pub2 (2014).
Liu, C., Chen, J., Gao, Y., Deng, B. & Liu, K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2, CD004434, 10.1002/14651858.CD004434.pub5 (2013).
Loetscher, T. & Lincoln, N. B. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev. 5, CD002842, 10.1002/14651858.CD002842.pub2 (2013).
Lyrer, P. & Engelter, S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 10, CD000255, 10.1002/14651858.CD000255.pub2 (2010).
MacKay-Lyons, M., Thornton, M., Ruggles, T. & Che, M. Non-pharmacological interventions for preventing secondary vascular events after stroke or transient ischemic attack. Cochrane Database Syst Rev. 3, CD008656, 10.1002/14651858.CD008656.pub2 (2013).
Malanda, U. L. et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 1, CD005060, 10.1002/14651858.CD005060.pub3 (2012).
Mead, G. E. et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 11, CD009286, 10.1002/14651858.CD009286.pub2 (2012).
Musini, V. M., Rezapour, P., Wright, J. M., Bassett, K. & Jauca, C. D. Blood pressure lowering efficacy of loop diuretics for primary hypertension. Cochrane Database Syst Rev. 8, CD003825, 10.1002/14651858.CD003825.pub3 (2012).
Onady, G. M. & Stolfi, A. Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev. 7, CD004730, 10.1002/14651858.CD004730.pub3 (2013).
Ooi, C. P. & Loke, S. C. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev. 12, CD009361, 10.1002/14651858.CD009361.pub2 (2012).
Ooi, C. P. & Loke, S. C. Sweet potato for type 2 diabetes mellitus. Cochrane Database Syst Rev. 9, CD009128, 10.1002/14651858.CD009128.pub3 (2013).
O’Rourke, K., Berge, E., Walsh, C. D. & Kelly, P. J. Percutaneous vascular interventions for acute ischaemic stroke. Cochrane Database Syst Rev. 10, CD007574, 10.1002/14651858.CD007574.pub2 (2010).
Pal, K. et al. Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 3, CD008776, 10.1002/14651858.CD008776.pub2 (2013).
Rees, K. et al. Anti-hypertensive drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies and clinical trials. Cochrane Database Syst Rev. 11, CD008535, 10.1002/14651858.CD008535.pub2 (2011).
Salazar, C. A., del-Aguila, D. & Cordova, E. G. Direct thrombin inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in people with non-valvular atrial fibrillation. Cochrane Database Syst Rev. 3, CD009893, 10.1002/14651858.CD009893.pub2 (2014).
Sandercock, P. A. & Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev. 9, CD000064, 10.1002/14651858.CD000064.pub2 (2011).
Saunders, D. H., Sanderson, M., Brazzelli, M., Greig, C. A. & Mead, G. E. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 10, CD003316, 10.1002/14651858.CD003316.pub5 (2013).
Shyangdan, D. S. et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 10, CD006423, 10.1002/14651858.CD006423.pub2 (2011).
Siebenhofer, A. et al. Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database Syst Rev. 9, CD008274, 10.1002/14651858. CD008274.pub2 (2011).
Siebenhofer, A. et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 3, CD007654, 10.1002/14651858.CD007654.pub3 (2013).
Sridharan, K., Mohan, R., Ramaratnam, S. & Panneerselvam, D. Ayurvedic treatments for diabetes mellitus. Cochrane Database Syst Rev. 12, CD008288, 10.1002/14651858.CD008288.pub2 (2011).
Stabler, S. N., Tejani, A. M., Huynh, F. & Fowkes, C. Garlic for the prevention of cardiovascular morbidity and mortality in hypertensive patients. Cochrane Database Syst Rev. 8, CD007653, 10.1002/14651858.CD007653.pub2 (2012).
Svircevic, V. et al. Epidural analgesia for cardiac surgery. Cochrane Database Syst Rev. 6, CD006715, 10.1002/14651858.CD006715.pub2 (2013).
Swinnen, S. G., Simon, A. C., Holleman, F., Hoekstra, J. B. & DeVries, J. H. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 7, CD006383, 10.1002/14651858.CD006383.pub2 (2011).
Stroke Unit Trialists’ Collaboration1. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 9, CD000197, 10.1002/14651858.CD000197.pub3 (2013).
Sykes, L., Wood, E. & Kwan, J. Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev. 1, CD005398, 10.1002/14651858.CD005398.pub3 (2014).
Usinger, L., Reimer, C. & Ibsen, H. Fermented milk for hypertension. Cochrane Database Syst Rev. 4, CD008118, 10.1002/14651858.CD008118.pub2 (2012).
Vale, N. et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev. 9, CD006870, 10.1002/14651858.CD006870.pub3 (2011).
Valentine, N., Van de laar, F. A. & Van-Driel, M. L. Adenosine-diphosphate (ADP) receptor antagonists for the prevention of cardiovascular disease in type 2 diabetes mellitus. Cochrane Database Syst Rev. 11, CD005449, 10.1002/14651858.CD005449.pub2 (2012).
Verheyden, G. S. et al. Interventions for preventing falls in people after stroke. Cochrane Database Syst Rev. 5, CD008728, 10.1002/14651858.CD008728.pub2 (2013).
Wei, I., Pappas, Y., Car, J., Sheikh, A. & Majeed, A. Computer-assisted versus oral-and-written dietary history taking for diabetes mellitus. Cochrane Database Syst Rev. 12, CD008488, 10.1002/14651858.CD008488.pub2 (2011).
Westendorp, W. F. et al. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database Syst Rev. 1, CD008530, 10.1002/14651858.CD008530.pub2 (2012).
Wiysonge, C. S. et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 11, CD002003, 10.1002/14651858.CD002003.pub4 (2012).
Wong, G. W. & Wright, J. M. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. Cochrane Database Syst Rev. 2, CD007452, 10.1002/14651858.CD007452.pub2 (2014).
Xiao, Y., Luo, M., Wang, J. & Luo, H. Inspiratory muscle training for the recovery of function after stroke. Cochrane Database Syst Rev. 5, CD009360, 10.1002/14651858.CD009360.pub2 (2012).
Zhao, P., Xu, P., Wan, C. & Wang, Z. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 10, CD004184, 10.1002/14651858.CD004184.pub2 (2011).
Zheng, G. H., Liu, J. P., Chu, J. F., Mei, L. & Chen, H. Y. Xiongshao for restenosis after percutaneous coronary intervention in patients with coronary heart disease. Cochrane Database Syst Rev. 5, CD009581, 10.1002/14651858.CD009581.pub2 (2013).
Zhou, Q. et al. Shengmai (a traditional Chinese herbal medicine) for heart failure. Cochrane Database Syst Rev. 4, CD005052, 10.1002/14651858.CD005052.pub5 (2014).
Zhu, F., Lee, A. & Chee, Y. E. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. 10, CD003587, 10.1002/14651858.CD003587.pub2 (2012).
Ziganshina, L. E., Abakumova, T. & Kuchaeva, A. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 4, CD007026, 10.1002/14651858.CD007026.pub2 (2010).
Zhang, D., Freemantle, N. & Cheng, K. K. Are randomized trials conducted in China or India biased? A comparative empirical analysis. J Clin Epidemiol. 64, 90–95, 10.1016/j.jclinepi.2010.02.010 (2011).
Morrison, A. et al. The effect of english-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 28, 138–144, 10.1017/s0266462312000086 (2012).
Ioannidis, J. P. et al. Increasing value and reducing waste in research design, conduct and analysis. Lancet 383, 166–175, 10.1016/s0140-6736(13)62227-8 (2014).
Chan, A. W. et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 383, 257–266, 10.1016/s0140-6736(13)62296-5 (2014).
Jones, A. C. & Geneau, R. Assessing research activity on priority interventions for non-communicable disease prevention in low- and middle-income countries: a bibliometric analysis. Glob Health Action 5, 1–13, 10.3402/gha.v5i0.18847 (2012).
Lawrence, J., Bai, S., Hung, H. M. & O’Neill, R. Regional treatment effects in studies of cardiorenal drugs: a summary of recent clinical trials. J Am Coll Cardiol. 60, 1117–1118, 10.1016/j.jacc.2012.04.051 (2012).
Lu, Z. et al. Effectiveness of interventions for hypertension care in the community—a meta-analysis of controlled studies in China. BMC Health Serv Res. 12, 216, 10.1186/1472-6963-12-216 (2012).
Acknowledgements
This project was funded by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
F.S. conceived and designed the study. H.F. and F.S. involved in identifying and selecting relevant Cochrane Systematic reviews (CSRs). H.F. extracted data from the included CSRs and F.S. checked the data. Both authors contributed to data analyses and manuscript writing.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fan, H., Song, F. An assessment of randomized controlled trials (RCTs) for non-communicable diseases (NCDs): more and higher quality research is required in less developed countries. Sci Rep 5, 13221 (2015). https://doi.org/10.1038/srep13221
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13221
This article is cited by
-
Addressing disparities in the global epidemiology of stroke
Nature Reviews Neurology (2024)
-
Challenges and opportunities for cancer clinical trials in low- and middle-income countries
Nature Cancer (2020)
-
Patient and miniscrew implant factors influence the success of orthodontic miniscrew implants
Evidence-Based Dentistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.