Abstract
During Drosophila oogenesis, follicle cells sequentially undergo three distinct cell-cycle programs: the mitotic cycle, endocycle and gene amplification. Notch signaling plays a central role in regulating follicle-cell differentiation and cell-cycle switches; its activation is essential for the mitotic cycle/endocycle (M/E) switch. Cut, a linker between Notch signaling and cell-cycle regulators, is specifically downregulated by Notch during the endocycle stage. To determine how signaling pathways coordinate during the M/E switch and to identify novel genes involved in follicle cell differentiation, we performed an in vivo RNAi screen through induced knockdown of gene expression and examination of Cut expression in follicle cells. We screened 2205 RNAi lines and found 33 genes regulating Cut expression during the M/E switch. These genes were confirmed with the staining of two other Notch signaling downstream factors, Hindsight and Broad and validated with multiple independent RNAi lines. We applied gene ontology software to find enriched biological meaning and compared our results with other publications to find conserved genes across tissues. Specifically, we found earlier endocycle entry in anterior follicle cells than those in the posterior, identified that the insulin-PI3K pathway participates in the precise M/E switch and suggested Nejire as a cofactor of Notch signaling during oogenesis.
Similar content being viewed by others
Introduction
The Drosophila egg chamber, the developmental unit of oogenesis, consists of sixteen germ-line cells: one oocyte and fifteen nurse cells, which are covered by a single layer of somatic follicle cells. The follicular epithelium is an excellent model system for the study of cell cycle regulation and cell differentiation in development. During oogenesis, follicle cells sequentially transition between three distinct cell cycle programs: the mitotic cycle (early oogenesis, stages 1–6), endocycle (midoogenesis, stages 7–10A) and gene amplification (late oogenesis, stages 10B–13). Previous studies have identified multiple signaling pathways, such as Notch, Hedgehog, EGFR, Wingless, JAK/STAT, Hippo and JNK, as being involved in spatiotemporal regulation of follicle cell differentiation during different stages of oogenesis1. Among these pathways, Notch signaling is crucial. Its activation and inactivation in follicle cells is essential for the mitotic cycle/endocycle (M/E) and the endocycle/gene amplification switches, respectively1.
Just before the onset of the endocycle, at stage 5 of Drosophila oogenesis, ligand Delta is highly upregulated in the germline. Germline Delta then binds to the Notch receptor in the somatic follicle cells to initiate signaling. Notch is a single transmembrane protein, which undergoes cleavage after its extracellular domain interacts with ligand Delta. The Notch intercellular domain (NICD) migrates into the nucleus upon being cleaved by the gamma-secretase complex2,3. Inside the nucleus, NICD, together with co-activator Mastermind (Mam), interacts with the CBF1/Suppressor of Hairless/LAG-1 (CSL) transcription repressor (Suppressor of Hairless [Su(H)] in Drosophila), changing Su(H) from a repressor to an activator by forming the NICD/Mam/Su(H) trimeric complex and thus inducing transcription of downstream genes4. After Notch signaling activation, the follicle cells undergo the M/E switch and cell differentiation2,3.
Cut is a homeodomain-containing transcription factor that was previously identified as a downstream target negatively regulated by Notch signaling during the M/E switch, In this process, Cut serves as a linker between Notch signaling and cell cycle regulators. The expression of Cut is observed in the mitotic cycle (Fig. 1A), which is then specifically downregulated by Notch signaling during the endocycle in the mainbody follicle cells5. This downregulation of Cut by Notch is critical for proper follicle cell differentiation from immature cell fate to mature cell fate and its continued expression beyond stage 6 in mainbody follicle cells can be used as a marker for failed M/E transition5.
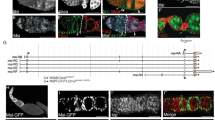
The strategy of our in vivo RNAi screen to identify genes involved in follicle cell differentiation and cell cycle switches.
(A-A”) In wild-type follicle cells, Cut expression is present during early oogenesis, but disappears in mainbody follicle cells during mid-oogenesis. PH3 staining in follicle cells was used to indicate early-stage (stages 1–6) egg chambers. (B) Crossing scheme for the in vivo RNAi screen. (C-C”’) Follicle cells expressing Notch RNAi in a stage-7 egg chamber showed upregulated Cut expression and small nuclei. (D-D”’) Follicle cells expressing mam RNAi in a stage-8 egg chamber showed upregulated Cut expression and small nuclei. DAPI staining marks cell nuclei. Anterior is to the left. Clone region marked by presence of RFP and outlined with dotted lines. Bars, 10 μm.
RNA interference (RNAi) utilizes short double-stranded RNA (dsRNA) molecules and acts through the RNA-induced silencing complex (RISC) for gene silencing. The RNAi machinery recognizes its RNA targets through dsRNA in a sequence-specific manner and can efficiently knock down endogenous or exogenously introduced dsRNAs6. The RNAi technique has been well developed and widely used to study gene function and has become a popular and reliable tool to greatly enhance understanding of molecular and genetic mechanisms of human diseases, leading to promising therapeutic application7,8,9. By using genome-wide RNAi libraries, almost any gene can be selectively silenced and the process can be accomplished in a high throughput and unbiased manner. In the past several years, a number of genome-scale RNAi high-throughput screens have been done in cultured cells and tissues of both Drosophila and mammals to study different biological processes including signal transduction10, cancer biology11, epithelial development12, stem cell identity13 and host cell responses to infection14,15. From these screens, scientists have identified many new components of these biological processes and have gained more insight into the complexity of biological systems.
In this study, we applied RNAi-induced gene-specific silencing to perform a large-scale in vivo RNAi screen to identify genes involved in follicle cell differentiation and cell cycle switches by analyzing Cut expression during Drosophila oogenesis. Our screen identified 33 genes out of 2205 RNAi lines, 9 of which had heretofore not been linked to Notch signaling. In addition, we found the anterior follicle cells enter endocycle earlier than their posterior counterparts, identified that the Insulin-PI3K pathway participates in the precise M/E switch and suggested the involvement of a cofactor Nej in Notch signaling during Drosophila oogenesis.
Results
A large-scale in vivo RNAi screen to identify genes involved in follicle cell differentiation and cell cycle switching by analyzing Cut
To understand how the Notch signaling pathway actively participates during the M/E switch and to identify novel genes involved in follicle cell differentiation, we planned a genome-wide in vivo RNAi screen through induced knockdown of gene expression by flip-out GAL4-UAS RNAi system and examination of Cut expression in follicle cells (Fig. 1B). RNAi lines were obtained from collection of the Transgenic RNAi Project (TRiP) in the Bloomington Drosophila Stock Center.
Before executing a large scale in vivo RNAi screen, we tested the efficacy of the flip-out GAL4-UAS RNAi screening strategy. Several RNAi lines targeting Notch pathway components were applied and defects in the M/E switch as well as prolonged Cut expression in follicle cells were observed after induced RNAi expression. For example, during midoogenesis (stages 7–10A), knockdown of Notch induced Cut upregulation and the small nuclei phenotype (Fig. 1C). As a co-activator of Notch and key component of NICD/Mam/Su(H) trimeric complex4, Mam knockdown showed a similar phenotype as Notch knockdown (Fig. 1D). These results indicate the flip-out GAL4-UAS RNAi system could efficiently reduce Notch components and inhibit proper endocycle entry in follicle cells, thus demonstrating the large-scale in vivo screen strategy was technically feasible and that it could help us identify additional genes involved in follicle cell differentiation and cell cycle switches. We then performed a double-blind screen, during which 2205 RNAi lines (Supplemental Table 1) were randomly chosen and only Bloomington Stock Numbers (BL#) were labeled during the screen to avoid any bias and found 33 lines showing Cut expression defects during M/E switch (Table 1).
Genes validated via Hindsight (Hnt) and Broad (Br) expression
In addition to Cut, Hnt (also known as Pebbled), a C2H2 Zinc finger transcription factor, is involved in Notch signaling during Drosophila oogenesis. Its expression is specifically induced by Notch during the endocycle stage to mediate Notch-dependent downregulation of Cut, String and Hedgehog signaling in follicle cells during midoogenesis16. Both Cut and Hnt have been implied to be regulated directly by Notch via Su(H)17. Br, another C2H2 Zinc finger transcription factor, is upregulated in the mainbody follicle cells during the M/E switch directly by Notch signaling via Su(H) binding to its brE enhancer region18. Both Hnt and Br expression were observed in order to validate all genes in Table 1. For example, knockdown of Pten led to downregulation of Cut in early oogenesis (Fig. 2A), concomitant with upregulation of Hnt and Br (Fig. 2B,C). This effect of Pten knockdown on the M/E switch was not very strong; a phenotype was only detectable around stages 5/6.
Validation of identified genes from the screen.
(A-A”) Candidate genes were identified by Cut expression. Downregulation of Cut (green in A, white in A”) was observed in follicle cells expressing Pten RNAi (BL#25967). (B-C) Hnt and Cut stainings were applied to validate identified genes. (B-B”) Upregulation of Hnt (green in B, white in B”) was observed in Pten RNAi (BL#25967) follicle cells. (C-C”) Upregulation of Br (green in C, white in C”) was observed in Pten RNAi (BL#25967) follicle cells. (D-F”) Different RNAi lines were examined to validate identified genes. Downregulation of Cut (green in D, white in D”), upregulation of Hnt (green in E, white in E”) and Br (green in F, white in F”) were observed in follicle cells expressing Pten RNAi (BL#25841). Anterior is to the left. Clone region marked by presence of RFP and outlined with dotted lines. Bars, 10 μm.
Multiple lines were examined to validate genes
To reduce the chance of off-target effects, we either tested multiple RNAi lines for the same gene, from Bloomington Stock Center or NIG-FLY, or mutant lines in order to confirm the phenotype (Table 1). For example, knockdown of Pten by one RNAi line (BL#25967) induced early entry into the endocycle, as indicated by precocious downregulation of Cut and upregulation of Hnt and Br in early oogenesis (Fig. 2A–C). We further tested another Pten RNAi line (BL#25841), which showed consistent phenotypes (Fig. 2D–F).
Analysis of identified genes from the screen
In order to investigate potential general features, we analyzed the frequency of gene ontology (GO) annotations of the 33 identified genes against the Drosophila genome background. GO annotations include three categories: biological process, molecular function and cellular component. The top 20 enriched terms were selected in each category (Supplemental Table 2). The top four enriched terms of biological process are general terms, including regulation of cellular progress, regulation of biological progress, biological regulation and regulation of gene expression. Regulation of metabolic process and cell cycle comes next, suggesting metabolic pathways and cell cycle regulators are utilized by Notch signaling to regulate follicle cell differentiation and cell cycle switches. The top five enriched terms of molecular function all involve binding, including protein binding, binding, ion binding, translation initiation factor binding and nucleotide binding, indicating the majority of the identified Notch signaling components (25/33) either form protein-protein complexes to initiate/regulate translation, or directly/indirectly bind to nucleotides to control gene expression, or act through ion channels to regulate signaling transduction. The top four enriched terms of cellular component are cell, cell part, intracellular part and intracellular, confirming Notch signaling activities mainly happen within cells. The fifth enriched term is cytoskeleton, implying Notch relies on cytoskeleton proteins for proper signaling transduction. Due to the small sample size (33 genes), GO annotations give us very broad description. By extracting the detailed information of the 33 identified genes from FlyBase, we assigned most of these genes into six categories, including Notch and associated factors, trafficking components, genes associated with protein degradation, the Hippo pathway components, cytoskeleton associated genes and genes related to transcription or translation (Fig. 3A, Table 1).
The Merdes Lab also carried out an extensive RNAi screen by treating Drosophila S2 cells with dsRNA and identified 900 Notch regulator candidates19. Further in vivo experiments in the wing and eye confirmed 333 of 501 tested candidates as Notch regulators. We extracted and organized their raw data, including the 900 candidate genes tested in S2 cells, 268 in wing disc and adult wing, 175 in eye disc and adult eye (Supplemental Table 3). Venn diagrams (Fig. 3B) show 11 of 33 genes from our screen were shared by follicle cells and S2 cells, including br, Prosα7, nej, Uba1, Not1, Ca-P60A, N, Ssrp, drk, mam and mop. We further compared candidate genes between the follicle cells, wing and eye (Fig. 3C). Only two genes, Ssrp and mop,were shared by all three tissues (Fig. 3C). The only shared genes between the follicle cells and wing were N, mam, Not1 and Ca-P60A (Fig. 3C). It should be noted that the raw data in the eye added a few extra positive genes and only tested ectopic upregulation of Notch19, suggesting we might be able to consider Ssrp, mop, N, mam, Not1 and Ca-P60A as common Notch signaling associated genes across tissues. The two conserved genes in the list, N and mam, further justified our speculation. There were also two genes shared by the follicle cells and eye, Rab11 and shi (Fig. 3C). shi has been known to control vesicular trafficking via Rab11-positive endosomes20, indicating the endocytic trafficking pathway is critical for Notch signaling in both follicular epithelium and eye development.
In the DeDecker Lab, another independent genome-wide RNAi screen was done to identify Notch modulators21. Drosophila Kc167 cells were treated with dsRNA and 399 putative Notch modifiers were identified. After removing two redundant genes, we organized their raw data and extracted 397 candidate genes tested in Kc167 cells (Supplemental Table 3). Mourikis et al. tested N, mam and Su(H) as positive controls, though they did not include them in the list of candidate genes. This partially explains the poor overlap percentage between our results, Kc167 cell data and S2 cell data. Only Ssrp overlaps in the three sets of data. Jub and Rpt4 were shared by the follicle cells and Kc167 cells (Fig. 3D).
In addition, the Knoblich Lab applied in vivo genome-wide RNAi screen combined with a tissue-specific Gal4 driver to knock down genes and examined the effects in external sensory organ morphology22. From their results and others, 177 candidate genes involved in Notch signaling were proposed to regulate external sensory organ development (Supplemental Table 3). We compared our 33 identified genes with the 177 candidate genes and found 6 genes in common, including N, mam,α-Adaptin, cdc2, sec6, and Smr.
In most in vivo genome-wide RNAi screen projects, scientists always use tissue-specific Gal4 driver to knock down the expression of genes and examine the effect in adult flies. The advantages of this strategy include fast screening and easy identification. However, this process is limited in that knockdown of some essential genes might cause lethality of adult flies, or that subtle effects might go unnoticed. In our study, we applied flip-out system to control the timing of induction of RNAi effect and compare RNAi knockdown clonal cells with neighboring wildtype cells to identify these subtle changes. For instance, the genes Ca-P60A, br, eIF3-S9, exba, MED15, mop, nej, Not1, Rab11, Rop, Rpt4, shi, Spt6, Ssrp, T-cp1 that we found regulate Notch signaling could not be analyzed by Mummery-Widmer et al. studies because of lethality at the pupal stage22. This example emphasizes the importance of spatiotemporal inactivation of genes and studies like ours could complement previous in vivo genome-wide RNAi screen projects to identify novel genes involved in Notch signaling.
Anterior follicle cells undergo the M/E switch earlier than posterior follicle cells
Throughout the screen project, we observed some other interesting phenomena, such as earlier endocycling in anterior follicle cells as opposed to that of posterior follicle cells. During the M/E switch, wild-type anterior follicle cells showed decreased Cut expression, while the same posterior follicle cells still possessed strong expression (Fig. 2D”). Furthermore, during the change to endocycle and in accordance with Cut patterns, anterior wild-type follicle cells showed weak Hnt and Br expression first, while posterior follicle cells had no expression (Fig. 2B”). Consistent with our findings, further analysis of wild-type egg chambers showed early Cut downregulation, Hnt and Br upregulation in the anterior follicle cells (Supplemental Fig. 1), suggesting anterior follicle cells undergo the M/E switch earlier.
The insulin-PI3K pathway interacts with Notch signaling during the M/E switch
From the screen, we identified Pten, which encodes Drosophila PTEN (phosphatase and tensin homolog). Pten, along with exba, were the only two identified genes to display premature endocycle entry when their gene expression was knocked down. Pten is well known as a negative regulator of the insulin-PI3K pathway23. The insulin-PI3K pathway is highly conserved across species and is important for nutrition-dependent growth and cell size regulation24. In Drosophila, when the insulin receptor (InR) is bound by insulin-like peptides, its substrate, Chico, will be phosphorylated and activated, thus stimulating phosphoinositide 3-kinase (PI3K) to convert phosphatidylinositol-3,4-bis-phosphate (PIP2) lipids into the phosphatidylinositol-3,4,5-triphosphate (PIP3). This PIP2-PIP3 conversion is reversible by the lipid PTEN25. We wondered whether the whole insulin-PI3K pathway participates in regulating Cut expression during the M/E switch. As such, we tested two important genes in the insulin-PI3K pathway, InR and PI3K. In contrast to Pten, both InR and PI3K are positive regulators of the insulin-PI3K pathway. During the M/E switch, knockdown of InR led to upregulated Cut, while overexpression of the constitutively active form of InR suppressed Cut (Fig. 4A,B). Similarly, PI3K reduction led to upregulated Cut, whereas increased wild-type PI3K resulted in lower levels of Cut (Fig. 4C,D). These phenotypes (Figs 2) were only observed near the M/E switch, indicating the insulin-PI3K pathway is important for the precise transition into the endocycle and follicle cell differentiation.
The insulin-PI3K pathway interacts with Notch signaling.
(A-A”) InR RNAi follicle cells (marked by presence of RFP and outlined with dotted lines) showed upregulated Cut expression (green in A, white in A”). (B-B”) Overexpressing the constitutively active form of InR (InR CA) in follicle cells suppressed Cut expression (green in B, white in B”). (C-C”) PI3K RNAi expressing follicle cells showed upregulated Cut expression (green in C, white in C”). (D-D”) Overexpressing wild-type PI3K in follicle cells reduced Cut expression (green in D, white in D”). (E-E”) Pten RNAi expressing follicle cells showed reduced NRE-EGFP level (green in E, white in E”). Anterior is to the left. Clone regions marked by presence of RFP and outlined with dotted lines. Bars, 10 μm.
A recent report describes how Cut, Hnt and the Notch activity reporter, Notch response element (NRE), briefly overlap between stage 6 and 7, which is termed the M/E switch (MES) stage26. During this short but special MES stage, Cut may act in a feedback loop to positively regulate Notch. Starvation was able to pause the MES stage. Knockdown of InR had been shown to elevate NRE-lacZ expression26. Here we further report that knockdown of the negative regulator Pten repressed the NRE-EGFP reporter19 (Fig. 4E), suggesting the insulin-PI3K pathway is essential for the transient MES through Cut-maintained Notch activity. We studied multiple key components of insulin-PI3K signaling and linked the signaling with Notch during the normal M/E transitional stages.
Nejire is involved in Notch signaling to regulate the M/E switch
Cofactors affect signal transduction cascades through modulation of transcriptional repression/activation. Many cofactors are involved in remodeling of chromatin through histone modification, which involves packing/unpacking DNA into higher-order chromatin structure. Cofactors also play critical roles in maintaining or altering the chromatin structure around the enhancer/promoter regions to regulate gene expression27. Here, we focused on the coactivator called Nej, also known as dCBP, which was identified from the screen. In Drosophila, Nej contains multiple domains as a member of the CBP/p300 family, one of which is the histone acetyltransferase domain. CBP/p300 family members can serve as a protein scaffold to recruit different sequence-specific transcription factors to form a multicomponent transcriptional regulatory complex. They can acetylate nucleosomal histones to remodel chromatin structure and nonhistone proteins, such as p53 tumor suppressor28. Nej has been identified as a transcriptional coactivator involved in a number of signaling pathways, such as the Wingless, Hedgehog and Dpp pathways29,30,31,32,33, although its involvement in the Notch pathway is not well understood. An in vitro assay showed MAML1, a mammalian Mam homolog, can potentiate NICD-mediated transcription on naked DNA templates, but not on chromatin templates. With the help of p300, a mammalian Nej homolog that can interact with NICD, MAML1 can potentiate transcription on chromatin templates34, suggesting a potential role of Nej in the Notch pathway. Nej was identified from our screen, indicating a role as a coactivator functioning in the follicle cells to regulate the M/E switch.
In this study, we used Drosophila follicle cells as a model system to explore the relationship between Nej and Notch signaling in vivo. Nej was detected ubiquitously in follicle cells during oogenesis and its expression was still present in N55e11 (loss-of-function allele of N) follicle cell clones (Fig. 5A), further suggesting Nej is not a Notch downstream target, rather a ubiquitously present coactivator for proper developmental events. nej RNAi successfully abolishes Nej expression (Fig. 5B) and thus serves as a reliable tool to study loss-of-function during Drosophila oogenesis. Because nej RNAi lines (BL#27724, #31728, #37489) showed consistent phenotypes, one nej RNAi line (BL#37489) was randomly selected for use in future experiments. As described in Table 1, when nej RNAi was introduced, the expression of Cut was upregulated during midoogenesis (Fig. 5C), while Hnt and Br were downregulated during midoogenesis (Fig. 5D, E). Phospho-histone 3 (PH3), a mitotic marker, is present in the wild-type follicle cells in an oscillating pattern during the mitotic cycle, then disappears after the follicle cells enter the endocycle2. In Nej knockdown egg chambers, we were still able to detect that 14% of stage 7 mosaic egg chambers showed PH3-positive follicle cells (n = 43; Fig. 5F), indicating nej RNAi expressing follicle cells fail to properly transition from the mitotic cycle into the endocycle. In addition, mainbody follicle cells are not fully differentiated in early oogenesis and can be labeled by immature cell fate marker Fasciclin III (FasIII). FasIII is undetectable in endocycling (“mature”) mainbody follicle cells beyond stage 75,16, however, nej RNAi expressing follicle cells still retained FasIII expression during midoogenesis (Fig. 5G), implying these cells maintain an undifferentiated cell fate. All these results (Fig. 5C–G) confirmed Nej is required for proper follicle cell differentiation and M/E switching. We further tested whether nej is involved in Notch signaling and intriguingly, knockdown of nej does not lead to suppressed Notch activity reporters NRE-EGFP. Instead, NRE-EGFP was upregulated (Fig. 5H), whereby we speculate that the upregulation of NRE-EGFP reporter is due to the upregulated Cut at the MES stage. Meanwhile, we did not rule out other possibilities. For example, nej might be required by other genes or signaling pathways, which indirectly regulate the M/E switch. Our results for the first time link Notch and nej in vivo in Drosophila.
Nejire interacts with Notch signaling to regulate the ME switch and maintain MES.
(A-A”) Nej was ubiquitously expressed in follicle cells during oogenesis. N55e11 follicle-cell FLP/FRT clones (marked by the absence of RFP, red in A, white in A’; outlined) still retained expression of Nej (green in A, white in A”). (B-H) Follicle cells expressing nej RNAi. Clone region marked by presence of RFP and outlined with dotted lines. (B-B”) nej RNAi follicle cells in a stage-7 egg chamber successfully abolished Nej expression (green in B, white in B”). (C-E”) Upregulation of Cut (green in C, white in C”) and downregulation of Hnt (green in D, white in D”) and Br (green in E, white in E”) were also observed in nej RNAi follicle cells. (F-F”) Follicle cells expressing nej RNAi showed sporadic PH3 staining (green in F, white in F”). (G-G”) nej RNAi expressing follicle cells retained FasIII expression (green in G, white in G”) during midoogenesis. (H-H”) nej RNAi expressing follicle cells showed increased NRE-EGFP level (green in H, white in H”). Anterior is to the left. Bars, 10 μm.
Discussion
Notch signaling in humans has a variety of important roles in cell differentiation and stem cell formation and has been associated with genetic diseases and cancers35,36. To better understand the contribution of Notch signaling to normal development and to be able to treat the diseases caused by aberrant Notch activity, a systematic identification and characterization of its components and connections to other pathways is necessary and informative.
From this large-scale in vivo RNAi screen, we were able to identify genes involved in follicle cell differentiation and cell cycle switches mediated by Notch signaling. Identifying each of these genes is tremendously important to gain insight into the proliferation and differentiation of cells and further understand how they are related to diseases, including cancers. Among the 33 identified genes, N and mam are well known as conserved components of the Notch pathway37,38. Our analysis (Fig. 3C) highlights the genes Ssrp, mop, Not1 and Ca-P60A as potential candidates associated with Notch signaling across tissues. Ssrp as yet has not been linked to Notch signaling, however, it is implicated in two previously mentioned RNAi screen papers and by our results as well (Fig. 3B–D). Ssrp shows nucleosomal DNA binding ability39,40, indicating that it could be a critical component of chromatin remodeling complexes regulating Notch signaling. Our finding that Ssrp regulates epithelial follicle cell differentiation and cell cycle transition during Drosophila oogenesis is consistent with a previous report showing that Ssrp1a, the zebrafish Ssrp homolog, controls development of tissues like the liver and eye by promoting cell proliferation and differentiation41. mop is a very interesting gene, encoding a protein-tyrosine phosphatase that contains BRO1 domain and ALIX V-shaped domain. Mop physically interacts with multiple signaling pathway components, including the endocytic pathway components Hrs42, Rab4, Rab543 and Vps2844, the Notch pathway component Su(dx)44, the Hippo pathway component Yki45 and Drk from our screen44. Not1 is a key component of the CCR4-NOT complex, which is responsible for catalyzing mRNA deadenylation46. Ca-P60A positively regulates calcium-transporting ATPase activity for proper intracellular trafficking of the Notch receptor47. The commonality of these genes across tissues suggests that chromatin remodeling, the endocytic pathway and gene silencing all play important roles in regulating Notch signaling.
We reference and summarize detailed information of all the identified genes in Table 1. In general, most of these genes fall into six categories (Fig. 3A). Group I includes Notch and associated factors, containing Notch signaling conserved components N and mam, NICD-Su(H)-Mam complex-associated factor e(y)148, cofactors nej34 and Smr49 and the Notch downstream target br18. We also speculate zinc finger protein CG9797 might be a downstream target of Notch, similar to Cut, Hnt and Br. Mop is physically involved with Su(dx), acting as a potential Notch-associated factor. Group II contains trafficking components. The endocytic pathway includes α-Adaptin50, Ca-P60A47, Hsc70-451, rab11 and shi20. Binding of α-Adaptin by Numb is required for targeting Notch for degradation50. Ca-P60A and Hsc70-4 regulate proper intracellular trafficking of the Notch receptor47,51. rab11 and shi control Notch trafficking via endosomes20. Exocytosis requires rop52and sec653. Specifically, sec6 is a key component of exocysts53. Group III are genes associated with protein degradation, composed of proteasome subunit Prosα7, proteasome-associated regulatory complex subunit Rpt454 and E1 ubiquitin-activating enzyme Uba155. Group IV is related to the Hippo pathway, which promotes Notch signaling in the posterior follicle cells during the M/E switch56 and contains ex, mats and jub57. The cytoskeleton and its structure has profound impact on signaling transduction58 and its associated genes are in Group V, including drk59, me31B, Rpt4 and T-cp160. Group VI generally includes genes related to transcription or translation, such as eIF3-S961, fs(1)K1062, exba63, Not146,Spt664 and MED1565. eIF3-S9 and exba are eukaryotic translation initiation factor subunits61,63. fs(1)K10 negatively regulates translation62. Not1 is responsible for catalyzing mRNA deadenylation46. Spt6 is a transcription elongation factor64. MED15 is a subunit of the Mediator complex, which is required for regulating RNA polymerase II (pol II) transcripts65. Other subunits, including MED6, MED7, MED8, MED11, MED14, MED17, MED20, MED23, MED25, MED26, MED27, MED30, MED31, have been identified as Notch modulators in previous screen projects, highlighting the importance of the Mediator complex in regulating Notch signaling66. While Ssrp and cdc2 are not grouped into the six categories, they are equally important. Ssrp and cdc2 indicate the critical roles of chromatin remodeling and cell cycle regulation in modulating Notch, respectively39,40.
This screen advances our knowledge of the M/E switch and Notch signaling network. Previously, we generally considered follicle cells to be uniform until the M/E switch67. However, our findings suggest the anterior follicle cells enter endocycle earlier, indicating that a symmetry-breaking process happens before the M/E switch. Through the gene validation process, we discovered that both the insulin-PI3K pathway and nej participate in the proper M/E switch and are especially important for the MES. During the MES, Cut, Hnt and Notch activity reporters all coexist, with Cut enhancing Notch signaling instead of suppressing it. However, the exact method whereby this transient stage is maintained and its biological importance are still open questions and await further investigation. Our screen findings connect the whole insulin-PI3K pathway and a cofactor nej with the MES stage, opening the gate to further elucidation of its molecular mechanisms and biological implications.
Materials and Methods
Fly Stocks and Genetics
The following fly lines were used: RNAi flies used for the screen were from the DrosophilaRNAi Screening Center, distributed by Bloomington Drosophila Stock Center (Supplemental 1), UAS-InRRNAi(BL#31594), UAS-InR CA (BL#8248), UAS-PI3K RNAi(BL#27690), UAS- PI3K (BL#8286), NRE-EGFP(BL#3072719), N55e11 (amorphic allele5,16), brnpr3(amorphic allele18), PBac{SAstopDsRed}LL08100 (Kyoto DGRC#142194), uba1s3484 (Kyoto DGRC#114337), exe1 (amorphic allele56)and w1118 was used as a wild-type control.
To generate mosaic egg chambers expressing UAS constructs, the flip-out Gal468 stock hsFLP; actin<CD2<Gal4,UAS-RFP/TM3,Sb was applied. Occasionally, hsFLP; actin<CD2<Gal4,UAS-GFP was used as well. hsFLP; actin<CD2<Gal4,UAS-RFP/TM3,Sb virgin female flies were selected to cross with UAS-RNAi males. After two weeks culture under 25 °C, F1 generation adult female flies were collected to undergo 30 minutes heat shock at 37 °C for two consecutive days, in order to create follicle cell clones. After heat shock, all flies were maintained in fresh food vials with wet yeast paste for two days before dissection. For FLP/FRT clone induction69,70, previously described procedures were followed18.
Immunohistochemistry and Image Analysis
Immunohistochemistry and image acquisition were carried out as previously described (Sun and Deng, 2005). The following primary antibodies were used: mouse anti-Cut (2B10) 1:15, mouse anti-Br-Core (25E9) 1:30, mouse anti-Hnt (1G9) 1:15, mouse anti-FasIII (7G10) 1:15 (Development Studies Hybridoma Bank, USA), rabbit anti-PH3 1:200 (Upstate Biotechnology, NY, USA), guinea pig anti-dCBP (Nej) 1:1000 (provided by Mattias Mannervik, Stockholm University, Stockholm, Sweden)31. Corresponding Alexa Fluor secondary antibodies (1:400; Invitrogen) were selected according to primary antibodies. DAPI (Invitrogen) was applied for nuclei staining. Images were acquired with a Zeiss LSM 510 confocal microscope and processed in Photoshop and Image J.
Gene Ontology (GO) term enrichment analysis and Venn diagram generation
All identified genes from the RNAi screen were analyzed for an enrichment of GO terms, including biological process, molecular function and cellular component, via the GO tool AmiGO2 (http://amigo.geneontology.org/amigo). Venn diagrams were initially created by the tool Venn diagram generator (http://www.bioinformatics.lu/venn.php), then reproduced and processed in Microsoft Paint and Photoshop. Detailed gene information was extracted from FlyBase (http://flybase.org).
Additional Information
How to cite this article: Jia, D. et al. A large-scale in vivo RNAi screen to identify genes involved in Notch-mediated follicle cell differentiation and cell cycle switches. Sci. Rep. 5, 12328; doi: 10.1038/srep12328 (2015).
References
Klusza, S. & Deng, W. M. At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. Bioessays 33, 124–134 (2011).
Deng, W. M., Althauser, C. & Ruohola-Baker, H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737–4746 (2001).
Lopez-Schier, H. & St Johnston, D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev 15, 1393–1405 (2001).
Fortini, M. E. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16, 633–647 (2009).
Sun, J. & Deng, W. M. Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299–308 (2005).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Deng, Y. et al. Therapeutic potentials of gene silencing by RNA interference: principles, challenges and new strategies. Gene 538, 217–227 (2014).
Li, T., Wu, M., Zhu, Y. Y., Chen, J. & Chen, L. Development of RNA interference-based therapeutics and application of multi-target small interfering RNAs. Nucleic Acid Ther 24, 302–312 (2014).
Mansoori, B., Sandoghchian Shotorbani, S. & Baradaran, B. RNA interference and its role in cancer therapy. Adv Pharm Bull 4, 313–321 (2014).
Lindquist, R. A. et al. Genome-scale RNAi on living-cell microarrays identifies novel regulators of Drosophila melanogaster TORC1-S6K pathway signaling. Genome Res 21, 433–446 (2011).
Su, B. et al. A genome-wide RNAi screen identifies FOXO4 as a metastasis-suppressor through counteracting PI3K/AKT signal pathway in prostate cancer. PLoS One 9, e101411 (2014).
Berns, N., Woichansky, I., Friedrichsen, S., Kraft, N. & Riechmann, V. A genome-scale in vivo RNAi analysis of epithelial development in Drosophila identifies new proliferation domains outside of the stem cell niche. J Cell Sci 127, 2736–2748 (2014).
Ding, L. et al. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell 4, 403–415 (2009).
Pache, L., Konig, R. & Chanda, S. K. Identifying HIV-1 host cell factors by genome-scale RNAi screening. Methods 53, 3–12 (2011).
Zhou, H. et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4, 495–504 (2008).
Sun, J. & Deng, W. M. Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev Cell 12, 431–442 (2007).
Krejcí, A. et al. Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci Signal 2, ra1 (2009).
Jia, D., Tamori, Y., Pyrowolakis, G. & Deng, W. M. Regulation of broad by the Notch pathway affects timing of follicle cell development. Dev Biol 392, 52–61 (2014).
Saj, A. et al. A combined ex vivo and in vivo RNAi screen for notch regulators in Drosophila reveals an extensive notch interaction network. Dev Cell 18, 862–876 (2010).
Pelissier, A., Chauvin, J. P. & Lecuit, T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol 13, 1848–1857 (2003).
Mourikis, P., Lake, R. J., Firnhaber, C. B. & DeDecker, B. S. Modifiers of notch transcriptional activity identified by genome-wide RNAi. BMC Dev Biol 10, 107 (2010).
Mummery-Widmer, J. L. et al. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458, 987–992 (2009).
Gupta, A. & Dey, C. S. PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell 23, 3882–3898 (2012).
Britton, J. S., Lockwood, W. K., Li, L., Cohen, S. M. & Edgar, B. A. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell 2, 239–249 (2002).
Grewal, S. S. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol 41, 1006–1010 (2009).
Jouandin, P., Ghiglione, C. & Noselli, S. Starvation induces FoxO-dependent mitotic-to-endocycle switch pausing during Drosophila oogenesis. Development 141, 3013–3021 (2014).
Huang, Z. Q., Li, J., Sachs, L. M., Cole, P. A. & Wong, J. A role for cofactor-cofactor and cofactor-histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBO J 22, 2146–2155 (2003).
Chan, H. M. & La Thangue, N. B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114, 2363–2373 (2001).
Akimaru, H. et al. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature 386, 735–738 (1997).
Anderson, J., Bhandari, R. & Kumar, J. P. A genetic screen identifies putative targets and binding partners of CREB-binding protein in the developing Drosophila eye. Genetics 171, 1655–1672 (2005).
Lilja, T., Aihara, H., Stabell, M., Nibu, Y. & Mannervik, M. The acetyltransferase activity of Drosophila CBP is dispensable for regulation of the Dpp pathway in the early embryo. Dev Biol 305, 650–658 (2007).
Ludlam, W. H. et al. The acetyltransferase activity of CBP is required for wingless activation and H4 acetylation in Drosophila melanogaster. Mol Cell Biol 22, 3832–3841 (2002).
Takaesu, N. T., Johnson, A. N., Sultani, O. H. & Newfeld, S. J. Combinatorial signaling by an unconventional Wg pathway and the Dpp pathway requires Nejire (CBP/p300) to regulate dpp expression in posterior tracheal branches. Dev Biol 247, 225–236 (2002).
Wallberg, A. E., Pedersen, K., Lendahl, U. & Roeder, R. G. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol Cell Biol 22, 7812–7819 (2002).
Lathia, J. D., Mattson, M. P. & Cheng, A. Notch: from neural development to neurological disorders. J Neurochem 107, 1471–1481 (2008).
Wang, Z., Li, Y., Banerjee, S. & Sarkar, F. H. Emerging role of Notch in stem cells and cancer. Cancer Lett 279, 8–12 (2009).
Artavanis-Tsakonas, S., Matsuno, K. & Fortini, M. E. Notch signaling. Science 268, 225–232 (1995).
Wilson, J. J. & Kovall, R. A. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell 124, 985–996 (2006).
Shimojima, T. et al. Drosophila FACT contributes to Hox gene expression through physical and functional interactions with GAGA factor. Genes Dev 17, 1605–1616 (2003).
Tsunaka, Y. et al. Phosphorylated intrinsically disordered region of FACT masks its nucleosomal DNA binding elements. J Biol Chem 284, 24610–24621 (2009).
Koltowska, K. et al. Ssrp1a controls organogenesis by promoting cell cycle progression and RNA synthesis. Development 140, 1912–1918 (2013).
Huang, H. R., Chen, Z. J., Kunes, S., Chang, G. D. & Maniatis, T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc Natl Acad Sci USA 107, 8322–8327 (2010).
Chen, D. Y. et al. The Bro1-domain-containing protein Myopic/HDPTP coordinates with Rab4 to regulate cell adhesion and migration. J Cell Sci 125, 4841–4852 (2012).
Guruharsha, K. G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011).
Kwon, Y. et al. The Hippo signaling pathway interactome. Science 342, 737–740 (2013).
Temme, C., Zaessinger, S., Meyer, S., Simonelig, M. & Wahle, E. A complex containing the CCR4 and CAF1 proteins is involved in mRNA deadenylation in Drosophila. EMBO J 23, 2862–2871 (2004).
Periz, G. & Fortini, M. E. Ca(2+)-ATPase function is required for intracellular trafficking of the Notch receptor in Drosophila. EMBO J 18, 5983–5993 (1999).
Xie, G., Yu, Z., Jia, D., Jiao, R. & Deng, W. M. E(y)1/TAF9 mediates the transcriptional output of Notch signaling in Drosophila. J Cell Sci 127, 3830–3839 (2014).
Heck, B. W. et al. The transcriptional corepressor SMRTER influences both Notch and ecdysone signaling during Drosophila development. Biol Open 1, 182–196 (2012).
Berdnik, D., Torok, T., Gonzalez-Gaitan, M. & Knoblich, J. A. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell 3, 221–231 (2002).
Hing, H. K., Bangalore, L., Sun, X. & Artavanis-Tsakonas, S. Mutations in the heatshock cognate 70 protein (hsc4) modulate Notch signaling. Eur J Cell Biol 78, 690–697 (1999).
Toonen, R. F. & Verhage, M. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol 13, 177–186 (2003).
Le Bras, S., Rondanino, C., Kriegel-Taki, G., Dussert, A. & Le Borgne, R. Genetic identification of intracellular trafficking regulators involved in Notch-dependent binary cell fate acquisition following asymmetric cell division. J Cell Sci 125, 4886–4901 (2012).
Sumegi, M., Hunyadi-Gulyas, E., Medzihradszky, K. F. & Udvardy, A. 26S proteasome subunits are O-linked N-acetylglucosamine-modified in Drosophila melanogaster. Biochem Biophys Res Commun 312, 1284–1289 (2003).
Lee, T. V. et al. The E1 ubiquitin-activating enzyme Uba1 in Drosophila controls apoptosis autonomously and tissue growth non-autonomously. Development 135, 43–52 (2008).
Yu, J., Poulton, J., Huang, Y. C. & Deng, W. M. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation and oocyte polarity. PLoS One 3, e1761 (2008).
Rauskolb, C., Pan, G., Reddy, B. V., Oh, H. & Irvine, K. D. Zyxin links fat signaling to the hippo pathway. PLoS Biol 9, e1000624 (2011).
Forgacs, G., Yook, S. H., Janmey, P. A., Jeong, H. & Burd, C. G. Role of the cytoskeleton in signaling networks. J Cell Sci 117, 2769–2775 (2004).
Kiger, A. A. et al. A functional genomic analysis of cell morphology using RNA interference. J Biol 2, 27 (2003).
Hughes, J. R. et al. A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol 6, e98 (2008).
Lasko, P. The drosophila melanogaster genome: translation factors and RNA binding proteins. J Cell Biol 150, F51–56 (2000).
Cooperstock, R. L. & Lipshitz, H. D. Control of mRNA stability and translation during Drosophila development. Semin Cell Dev Biol 8, 541–549 (1997).
Lee, S. et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development 134, 1767–1777 (2007).
Petruk, S. et al. Transcription of bxd noncoding RNAs promoted by trithorax represses Ubx in cis by transcriptional interference. Cell 127, 1209–1221 (2006).
Boube, M. et al. Drosophila homologs of transcriptional mediator complex subunits are required for adult cell and segment identity specification. Genes Dev 14, 2906–2917 (2000).
Guruharsha, K. G., Kankel, M. W. & Artavanis-Tsakonas, S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 13, 654–666 (2012).
Spradling, A. Developmental genetics of oogenesis. In The development of Drosophila melanogaster. Cold Spring Harbor Lab. Press, NY (1993).
Pignoni, F. & Zipursky, S. L. Induction of Drosophila eye development by decapentaplegic. Development 124, 271–278 (1997).
Golic, K. G. & Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499–509 (1989).
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993).
Jo, H. S., Kang, K. H., Joe, C. O. & Kim, J. W. Pten coordinates retinal neurogenesis by regulating Notch signalling. EMBO J 31, 817–828 (2012).
Mahoney, M. B. et al. Presenilin-based genetic screens in Drosophila melanogaster identify novel notch pathway modifiers. Genetics 172, 2309–2324 (2006).
Acknowledgements
We would like to thank Mattias Mannervik, Claudia Temme and Elmar Wahle for kindly providing antibodies. We thank the Biological Science Imaging Facility at Florida State University, AmiGO2 and Venn diagram generator tool providers for technical help. The DSHB, the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) , the Bloomington, Kyoto and NIG stock centers for providing us antibodies and fly stocks. Special thanks to other members of the Deng lab for technical help and discussions on this project; W.-M.D. is supported by NIH grant R01GM072562 and National Science Foundation IOS-1052333.
Author information
Authors and Affiliations
Contributions
D.J. and W.M.D. designed the experiments; D.J. performed the experiments; M.S., R.B., J.B., C.H. and Y.C.H. participated in the screen process; D.J., M.S. and W.M.D. analyzed the data; D.J. made the figures and wrote the paper; G.C., M.S., Y.C.H. and W.M.D. revised the paper. All authors approved the manuscript for submission.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jia, D., Soylemez, M., Calvin, G. et al. A large-scale in vivo RNAi screen to identify genes involved in Notch-mediated follicle cell differentiation and cell cycle switches. Sci Rep 5, 12328 (2015). https://doi.org/10.1038/srep12328
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12328
This article is cited by
-
Identification and expression analysis of heat shock protein family genes of gall fly (Procecidochares utilis) under temperature stress
Cell Stress and Chaperones (2023)
-
Analysis of the Temporal Patterning of Notch Downstream Targets during Drosophila melanogaster Egg Chamber Development
Scientific Reports (2020)
-
Automatic stage identification of Drosophila egg chamber based on DAPI images
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.