Abstract
Among the four groups of HIV-1 (M, N, O and P), HIV-1M alone is pandemic and has rapidly expanded across the world. However, why HIV-1M has caused a devastating pandemic while the other groups remain contained is unclear. Interestingly, only HIV-1M Vpu, a viral protein, can robustly counteract human tetherin, which tethers budding virions. Therefore, we hypothesize that this property of HIV-1M Vpu facilitates human-to-human viral transmission. Adopting a multilayered experimental-mathematical approach, we demonstrate that HIV-1M Vpu confers a 2.38-fold increase in the prevalence of HIV-1 transmission. When Vpu activity is lost, protected human populations emerge (i.e., intrinsic herd immunity develops) through the anti-viral effect of tetherin. We also reveal that all Vpus of transmitted/founder HIV-1M viruses maintain anti-tetherin activity. These findings indicate that tetherin plays the role of a host restriction factor, providing ‘intrinsic herd immunity’, whereas Vpu has evolved in HIV-1M as a tetherin antagonist.
Similar content being viewed by others
Introduction
Human immunodeficiency virus type 1 (HIV-1), the causative agent of acquired immunodeficiency syndrome (AIDS), emerged around 100 years ago1 through zoonotic transmission of simian immunodeficiency viruses (SIVs) in chimpanzees (SIVcpz)2,3 and gorillas (SIVgor)4. According to molecular phylogenetic analyses, zoonotic transmission has occurred at least 4 times, leading to diversification of the virus into 4 different groups; namely, HIV-1 M (“major” or “main”), N (“new” or “non-M-non-O”), O (“outlier”) and P5. Among these 4 HIV-1 groups, HIV-1M alone is pandemic and currently infects more than 30 million people worldwide. However, why HIV-1M rapidly created a worldwide pandemic while the other groups remained endemic is unclear.
In 2009, Neil et al.6 and Van Damme et al.7 identified a cellular anti-HIV-1 protein called tetherin (also known as bone marrow stromal antigen 2 [BST2], CD317 and HM1.24). Tetherin impairs viral release by tethering bud virions to the surfaces of HIV-1-producing cells. Conversely, tetherin is antagonized by viral protein U (Vpu), an accessory protein encoded by HIV-16,7. In addition, Vpu potently induces the degradation of CD4 molecules in infected cells8. Interestingly, HIV-1M Vpu alone possesses both of these abilities, while the Vpus of the other HIV-1 groups (i.e., N, O and P) possess neither or only one ability9,10,11. Regarding anti-tetherin activity, HIV-1N Vpu is a much weaker tetherin antagonist than HIV-1M Vpu11,12 and the Vpus of HIV-1O and HIV-1P exert no adverse effect on tetherin10,11. From these observations, Kirchhoff proposed that the ability of Vpu to counteract tetherin has promoted human-to-human viral transmission13. However, because human-to-human spread cannot be experimentally replicated, this hypothesis remains speculative. Therefore, we conducted an experimental-mathematical investigation and concluded that human tetherin is a potent inducer of intrinsic herd immunity in humans and that HIV-1M Vpu has acquired the ability to overcome this hurdle.
Methods
A structured epidemiological model based on the distribution of set-point viral loads
To investigate the relationship between the distributed set-point viral loads in HIV-1 infected patients and human-to-human viral transmission, we developed a novel mathematical model that accounts for host heterogeneity (i.e., the different set-point viral loads). The novel mathematical model is given by (Fig. 1):
Construct of the mathematical model.
The variables S(t, V) and I(t, V) denote the number of susceptible and infected individuals, respectively and N(t) is the total number of host individuals. All variables are specified at time t. The parameters b(V) and d denote the birth and removal rates of susceptible individuals respectively and μ(V) is the death rate of infected individuals. The force of infection is assumed as  where c and β(V) denote the partner exchange rate and the transmission probability, respectively. Therefore, the rate of change in the number of susceptible and infected individuals at time t (i.e., the de novo transmission) is
where c and β(V) denote the partner exchange rate and the transmission probability, respectively. Therefore, the rate of change in the number of susceptible and infected individuals at time t (i.e., the de novo transmission) is  . Figure 1 was drawn by KS using Illustrator CS5.1 (Adobe).
. Figure 1 was drawn by KS using Illustrator CS5.1 (Adobe).


where S(t, V) represents the number of susceptible individuals who will acquire the set-point viral load V if infected by HIV-1 in the future and I(t, V) is the number of infected individuals with the set-point viral load V at time t. N(t) is the total host population size (i.e.,  ). Here we assume that the set-point viral load V of each infected individual is determined by host properties such as the genetic background and immune state14,15,16,17. Host populations are born at rate b(V) and removed at rate d. Because most of the HIV infection occurs during the asymptomatic period and the transmission probability per partnership is correlated with the patients’ set-point viral load V, we model this relationship by an increasing Hill function as described elsewhere18,19; That is we write
). Here we assume that the set-point viral load V of each infected individual is determined by host properties such as the genetic background and immune state14,15,16,17. Host populations are born at rate b(V) and removed at rate d. Because most of the HIV infection occurs during the asymptomatic period and the transmission probability per partnership is correlated with the patients’ set-point viral load V, we model this relationship by an increasing Hill function as described elsewhere18,19; That is we write  where βmax is the maximum transmission probability and Vβ50 is the viral load at which the probability is half its maximum. The parameter kβ is the steepness of the transmission probability versus the set-point viral load. Therefore, the force of infection at time t is given by
where βmax is the maximum transmission probability and Vβ50 is the viral load at which the probability is half its maximum. The parameter kβ is the steepness of the transmission probability versus the set-point viral load. Therefore, the force of infection at time t is given by  where c is the contact rate. Finally, we assume that the asymptomatic period endures as a decreasing Hill function of the viral load as described elsewhere18,19; that is,
where c is the contact rate. Finally, we assume that the asymptomatic period endures as a decreasing Hill function of the viral load as described elsewhere18,19; that is,  where Dmax is the maximum duration of the asymptomatic period in years, VD50 is the viral load at which the duration is half its maximum and kD is the steepness of the (decreasing) duration versus the set-point viral load. The initial condition is S(0,V) = b(V)/d and
where Dmax is the maximum duration of the asymptomatic period in years, VD50 is the viral load at which the duration is half its maximum and kD is the steepness of the (decreasing) duration versus the set-point viral load. The initial condition is S(0,V) = b(V)/d and  . Similar mathematical models have been proposed in previous studies18,19,20.
. Similar mathematical models have been proposed in previous studies18,19,20.
Cell culture, infection, transfection, Western blotting, TZM-bl assay, flow cytometry
293T cells, HeLa cells and TZM-bl cells (obtained through NIH AIDS Research and Reference Reagent Program) were maintained in DMEM (Sigma) medium containing FCS and antibiotics. Human PBMCs were maintained in RPMI1640 (Sigma) medium containing FCS and antibiotics. For in vitro HIV-1 infection assay (Figure S1), PHA-activated human PBMCs were infected with WT or vpu-deficient HIV-1 (strain NL4-3) at multiplicity of infection 0.1 (i.e., 10,000 TCID50 [50% tissue culture infectious dose] of virus solution was inoculated into 100,000 PHA-activated human PBMCs). The expression plasmids of HA-tagged Vpus of T/F viruses were obtained from GeneArt Gene Synthesis service (Life Technologies). Transfection was performed using Lipofectamine 2000 (Life Technologies). Western blotting, TZM-bl assay and flow cytometry were performed as previously described21,22,23.
Results
HIV-1M Vpu increases the set-point viral load of HIV-1-infected humans
To examine whether or not the anti-tetherin ability of Vpu is linked to effective human-to-human HIV-1 transmission13, we performed a virus replication assay using primary human CD4+ T lymphocytes. The experimental data were analyzed by a previously proposed mathematical model24,25, which verified that Vpu increases the virus production rate (p) (see Text S1). We also analyzed 15 datasets provided in previous studies7,26,27,28,29,30,31 and found that the average production rate of wild-type HIV-1 was 1.54-fold higher than that of vpu-deficient HIV-1. The estimated parameter values are listed in Table S1. Because the virus production rate is known to affect the set-point viral load (V) in infected individuals32,33, we expected that a typical set-point of 1.0 × 105 copies/ml (for example) would decrease to 0.4 × 105 copies/ml in HIV-1M lacking vpu (see Text S2). These findings strongly suggest an important connection between Vpu and the within-host dynamics of HIV-1.
Epidemiological impact of set-point viral loads
Because HIV-1-infected patients with higher viral loads are more infectious34,35 and have reduced lifespans34,36,37, the efficacy of human-to-human HIV-1 transmission is determined by the patients’ set-point viral load. In addition, the distribution of the set-point plays a critical role in viral spread35. We describe the human-to-human spread of HIV-1M by the structured epidemiological model described in Methods (Fig. 1). This model includes the transmission rate (β(V)) and the death rate of the patient (μ(V)) as functions of viral load34. Because patients’ set-point values are highly variable34,35, by adopting the set-point distribution approach in our mathematical model we can quantitatively detail the spread of HIV-1. Here we used the distribution of set-point viral loads in 311 untreated heterosexual HIV-1M infected patients in the Zambian transmission study35 and calculated the basic reproduction number (R0W), the expected number of people infected throughout their infectious lifespan38,39 (see Text S3). The estimated value of R0W (4.67;see Text S4) is consistent with previous estimates39,40.
Tetherin-mediated intrinsic herd immunity and its counteraction by Vpu
To investigate the impact of Vpu on the human-to-human spread of HIV-1M, we assumed that HIV-1 has lost its Vpu function (i.e., we consider vpu-deficient HIV-1) and compared several quantities concerning the epidemiological contribution of Vpu. As explained above, viruses lacking Vpu reproduce more slowly and yield a lower set-point viral load in patients (see Text S2). Interestingly, vpu-deficient HIV-1 did not establish infection in some patients with lower set-point viral loads (<3.19 × 104 RNA copies/ml) (see Text S5). In other words, the anti-viral effect of tetherin confers potential protection against HIV-1 infection among some human populations41. Because the distribution of viral loads in the Zambian transmission study is well described by a weighted skew-normal distribution of the logarithm of the viral load (see Text S4), we can estimate that 20% of human populations are protected from vpu-deficient HIV-1 by tetherin (Fig. 2A) and are prevented from transmitting HIV-1 (this situation is known as herd immunity). In addition, several observed distributions of the set-point are negatively skewed34,35, suggesting that tetherin-mediated herd immunity plays a critical role in the human-to-human spread of HIV-1M.
Prediction of tetherin-mediated intrinsic herd immunity.
(A) (left) The distribution of set-point viral load in untreated individuals in the Zambian Transmission Study (black bars). The black curve is the distribution predicted by the structured epidemiological model. (right) The predicted distribution of set-point viral load in untreated individuals in the Zambian Transmission Study, assuming non-functional Vpu (orange bars). The bar at “0” shows the fraction of populations protected from vpu-deficient HIV-1 infection. (B) Simulated spread of wild-type and vpu-deficient HIV-1 among a population of 1 million individuals. The black and orange curves describe the number of individuals infected by wild-type and mutant virus, respectively. (C) Prevalence of wild-type (black curve) and vpu-deficient (orange curve) HIV-1 infection in (B).
In vpu-deficient HIV-1, the calculated R0M reduced to 3.90, suggesting that human-to-human transmission is 1.17-fold more efficient in wild-type HIV-1 than in vpu-deficient HIV-1. However, comparing the basic reproduction numbers may be of limited applicability when infected individuals are rare in a population. To further assess the impact of Vpu on the spread of HIV-1M, we simulated wild-type and vpu-deficient HIV-1 transmission among 1 million individuals (Fig. 2B). The parameter values used in these simulations are summarized in Table S2. Our simulations show that Vpu shortens the time between initial and peak HIV-1 infection and increases the steady-state number of infected individuals (Fig. 2B). Interestingly, the prevalence (i.e., the number of infected individuals divided by the number of total individuals) of wild-type and vpu-deficient HIV-1 infection were calculated as 78.2% and 32.8%, respectively (Fig. 2C). Thus, the Vpu of HIV-1M increases the viral prevalence by 2.38-fold, indicating that Vpu effectively overcomes tetherin mediated herd immunity.
Conserved anti-tetherin ability of Vpu in transmitted/founder (T/F) viruses
Reportedly, the antagonistic effect of Vpu against tetherin is conferred by 3 amino acids in the transmembrane domain of Vpu (14AxxxAxxxW22 in strain NL4-3). In addition, 2 amino acids in the cytoplasmic domain of Vpu (52SxxxS56 in strain NL4-3) are essential for down-regulating CD442. To further validate the importance of Vpu on human-to-human viral transmission, we analyzed the vpu sequences of transmitted/founder (T/F) viruses, the viruses that are transferred and efficiently established in new individuals43,44 and recovered from patients at each Fiebig stage (III–V). As shown in Fig. 3A, both of the motifs important for CD4 down-regulation and tetherin antagonism are highly conserved, suggesting that the ability of Vpu to down-regulate tetherin and CD4 is important for establishing HIV-1 infection in new patients.
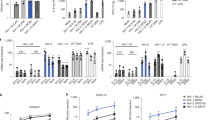
Conservation of Vpu motifs and the ability of T/F virus Vpus.
(A) Conservation of the motifs in HIV-1M Vpus that counteract tetherin and down-regulate CD4 at each stage of viral infection. (left) Logo plot of the Vpu amino acid sequences at Fiebig stages III (n = 183), IV (n = 29) and V (n = 119). The crucial Vpu motifs for tetherin counteraction (AxxxAxxxA motif) and CD4 down-regulation (SxxxS motif) are indicated in black. (right) The percentage conservation of these motifs at each Fiebig stage. (B–E) Infection ability of the Vpus T/F viruses. The HA-tagged Vpu-expressing plasmids were co-transfected with pNL4-3ΔvpuΔnef (B, D, E) or without pNL4-3ΔvpuΔnef (C) into HeLa cells (B, D, E) or TZM-bl cells (C). Labels denote: –, empty vector; +, NL4-3 Vpu; 1-10, T/F virus Vpus: 1, THRO_TF1 (Genbank accession no. JN944930); 2, SUMA_TF1, (JN944928); 3, RHPA_TF1 (JN944917); 4, TRJO_TF1 (JN944936); 5, CH040_TF1 (JN944905); 6, REJO_TF1 (JN944911); 7, CH077_TF1 (JN944909); 8, CH058_TF1 (JN944907); 9, WITO_TF1 (JN944938); 10, CH106_TF1 (JN944897). (B) Representative result of Western blotting (kDa = kilodalton). (C) Surface expression levels of tetherin (top) and CD4 (bottom) on cells transfected with Vpu-expressing plasmids. (D) The infectivity of released virus was determined by TZM-bl assay. (E) The correlation between the level of surface tetherin (shown in panel c, top) and virus infectivity (shown in panel d). The data are expressed as averages and the standard deviations of triplicate assays. In Panels C and D, statistical significance was determined by Student’s t test (P < 0.05). In Panel E, the correlation between the level of surface tetherin (x-axis) and virus infectivity (y-axis) was determined by Spearman’s rank test.
To demonstrate the link between Vpu activity and establishment of HIV-1 infection, we constructed expression plasmids for the Vpus of T/F viruses and evaluated their ability to infect cell lines. Intriguingly, most of the transfected Vpus down-regulated tetherin but did not efficiently down-regulate CD4 (Fig. 3B–D). Furthermore, the surface expression level of tetherin was significantly negatively correlated with the level of virus release (Fig. 3E; r = −0.678, P = 0.0245 by Spearman rank correlation coefficient), suggesting that the ability of Vpu to antagonize tetherin is well correlated with efficient virus release. Collectively, these findings suggest that the potential of Vpu to antagonize tetherin is more important than its anti-CD4 activity and is well conserved in T/F viruses.
Discussion
Tetherin suppresses viral release in single-round replication assays in cultured cells such as HeLa cells and is a recognized inhibitory factor of HIV-1 replication6,7. However, the anti-viral effect of tetherin seems enigmatic because vpu-deficient HIV-1, which cannot counteract tetherin, can replicate in cultures of tetherin-expressing cells such as Jurkat cells and human primary peripheral mononuclear cells (PBMCs). Thus, whether tetherin exerts a lesser effect on HIV-1 replication in vivo is a pertinent question. In fact, previous studies by our group and others have demonstrated that vpu-deficient HIV-1 efficiently replicates in humanized mouse models45,46, indicating that Vpu is dispensable for viral propagation in vivo. However, Vpu significantly enhances the efficiency of HIV-1 infection in a humanized mouse model46. Especially interesting is that only the Vpus of the pandemic virus HIV-1M counteract both tetherin and CD4. The Vpus of the other endemic HIV-1 groups (i.e., N, O, P) antagonize at most one of these protective agents9,10,11. From these observations, we hypothesize that the ability of Vpu to antagonize both tetherin and CD4 increases the infective efficacy of HIV-1M, enabling extensive human-to-human viral transmission. Furthermore, there had been only a dozen of HIV-1N-infected individuals in Cameroon47,48,49 and the antagonistic activity of HIV-1N against tetherin has been shown to be intrinsically weak11. Sauter et al. revealed that the Vpu of N1Fr201150, a recently designated HIV-1N strain, has acquired anti-tetherin activity after the virus was introduced to Europe from Cameroon12. These findings further support a close association between anti-tetherin ability of Vpu and viral epidemics. However, because the efficacy of human-to-human virus transmission cannot be evaluated from experimental studies alone, we supplemented our experimental techniques with a mathematical investigation. Comparing the infectivity of wild-type and vpu-deficient HIV-1, we demonstrated that active Vpu confers a 2.38-fold advantage in prevalence of infection (Fig. 2C). This suggests that tetherin plays a “herd immunity” role against HIV-1 in human populations and that HIV-1M has retaliated by expressing Vpu. Indeed, tetherin impairs the release of a broad spectrum of enveloped viruses51. Therefore, the tetherin-mediated herd immunity in human populations may affect the spread of diverse viruses.
Several molecular clones of non-vpu encoding HIV-1M strains have been isolated from chronically infected patients. In these strains, designated HXB218, BH852, MAL53 and Zr654, the initiation triplet AUG of vpu has been deleted18,52,53,54 or a frameshift mutation has occurred in the vpu ORF55. Conversely, all documented T/F viruses that have invaded new individuals encode vpu44,56. Therefore, vpu may be essential for infecting new individuals but may be dispensable or less important in viremia maintenance and disease progression. In fact, we have previously revealed that Vpu boosts the number of cell-free viruses and promotes the establishment of viruses in a humanized mouse model46. We also found that in earlier pathogenic stages (particularly Fiebig stages III–IV), the motif for tetherin down-regulation (AxxxAxxxW) is conserved and that tetherin is effectively counteracted by the Vpus of T/F viruses (Fig. 3). In contrast, although the motif for CD4 down-regulation (SxxxS) is similarly conserved, CD4 levels may be maintained in the presence of Vpu-producing T/F viruses (Fig. 3). These findings suggest that anti-tetherin activity is more important than CD4 down-regulation, particularly in T/F viruses. However, according to a recent study by Pickering et al.57, Vpu preserves the ability to downregulate CD4 in infected individuals and also counteracts tetherin despite the extensive sequence variation of Vpu. They attributed both tetherin antagonism and CD4 down-regulation of strain NL4-3 to at least 4 amino acids, 19A, 23W, 53S and 56S. Therefore, Vpu may facilitate viral propagation during the human-to-human transmission phase.
The experimental-mathematical approach adopted here has quantitatively revealed the replication dynamics of retroviruses24,58,59 and enteroviruses25 in cell culture systems. To our knowledge, we present the first estimate of the anti-viral effect of tetherin in human populations by combining experimental and epidemiological data with a structured mathematical model. The data-driven mathematical approach can elucidate viral infection dynamics in ways that are impossible by conventional experimental strategies alone. Especially, the mathematical model incorporates virological information of infected individuals such as the viral load, a crucial parameter for investigating infectious disease spread. As discussed herein, tetherin-mediated herd immunity is revealed only when the set-point viral load is considered as a distribution. If modeled only by the average viral load, the herd immunity effect might be obscured in the population dynamics and the anti-viral effect of tetherin might be underestimated. Thus, quantitative epidemiological details can only be revealed in a multi-scaled modeling approach.
Additional Information
How to cite this article: Iwami, S. et al. Pandemic HIV-1 Vpu overcomes intrinsic herd immunity mediated by tetherin. Sci. Rep. 5, 12256; doi: 10.1038/srep12256 (2015).
Change history
05 November 2015
The PDF version of this Article previously published contained a typographical error in the spelling of the author Yoshio Koyanagi. This has now been corrected; the HTML version of the paper was correct from the time of publication
References
Worobey, M. et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature 455, 661–664, 10.1038/nature07390 (2008).
Gao, F. et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441, 10.1038/17130 (1999).
Keele, B. F. et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313, 523–526, 10.1126/science.1126531 (2006).
Plantier, J. C. et al. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15, 871–872, 10.1038/nm.2016 (2009).
Heeney, J. L., Dalgleish, A. G. & Weiss, R. A. Origins of HIV and the evolution of resistance to AIDS. Science 313, 462–466, 10.1126/science.1123016 (2006).
Neil, S. J., Zang, T. & Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252 (2008).
Willey, R. L., Maldarelli, F., Martin, M. A. & Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66, 7193–7200 (1992).
Sauter, D. et al. HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env or Nef. Retrovirology 8, 103, 10.1186/1742-4690-8-103 (2011).
Yang, S. J., Lopez, L. A., Exline, C. M., Haworth, K. G. & Cannon, P. M. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology 8, 78, 10.1186/1742-4690-8-78 (2011).
Sauter, D. et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6, 409–421, 10.1016/j.chom.2009.10.004 (2009).
Sauter, D. et al. Human tetherin exerts strong selection pressure on the HIV-1 group N Vpu protein. PLoS Pathog. 8, e1003093, 10.1371/journal.ppat.1003093 (2012).
Kirchhoff, F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8, 55–67, 10.1016/j.chom.2010.06.004 (2010).
Loomis-Price, L. D. et al. Correlation between humoral responses to human immunodeficiency virus type 1 envelope and disease progression in early-stage infection. J. Infect. Dis. 178, 1306–1316 (1998).
Musey, L. et al. Cytotoxic-T-cell responses, viral load and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337, 1267–1274, 10.1056/NEJM199710303371803 (1997).
Goldstein, S., Brown, C. R., Dehghani, H., Lifson, J. D. & Hirsch, V. M. Intrinsic susceptibility of rhesus macaque peripheral CD4(+) T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J. Virol. 74, 9388–9395 (2000).
Carrington, M. et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283, 1748–1752 (1999).
Ratner, L. et al. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res. Hum. Retroviruses 3, 57–69 (1987).
Metzger, V. T., Lloyd-Smith, J. O. & Weinberger, L. S. Autonomous targeting of infectious superspreaders using engineered transmissible therapies. PLoS Comput. Biol. 7, e1002015, 10.1371/journal.pcbi.1002015 (2011).
Hyman, J. M., Li, J. & Stanley, E. A. The differential infectivity and staged progression models for the transmission of HIV. Math. Biosci. 155, 77–109 (1999).
Kobayashi, T. et al. Identification of amino acids in the human tetherin transmembrane domain responsible for HIV-1 Vpu interaction and susceptibility. J. Virol. 85, 932–945, 10.1128/JVI.01668-10 (2011).
Sato, K. et al. Comparative study on the effect of human BST-2/Tetherin on HIV-1 release in cells of various species. Retrovirology 6, 53, 10.1186/1742-4690-6-53 (2009).
Sato, K. et al. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J. Virol. 82, 1021–1033, 10.1128/JVI.01044-07 (2008).
Iwami, S. et al. Quantification system for the viral dynamics of a highly pathogenic simian/human immunodeficiency virus based on an in vitro experiment and a mathematical model. Retrovirology 9, 18, 10.1186/1742-4690-9-18 (2012).
Fukuhara, M. et al. Quantification of the dynamics of enterovirus 71 infection by experimental-mathematical investigation. J. Virol. 87, 701–705, 10.1128/JVI.01453-12 (2013).
Neil, S. J., Sandrin, V., Sundquist, W. I. & Bieniasz, P. D. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2, 193–203, 10.1016/j.chom.2007.08.001 (2007).
Schubert, U. et al. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 70, 809–819 (1996).
Schubert, U., Clouse, K. A. & Strebel, K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69, 7699–7711 (1995).
Schubert, U., Bour, S., Willey, R. L. & Strebel, K. Regulation of virus release by the macrophage-tropic human immunodeficiency virus type 1 AD8 isolate is redundant and can be controlled by either Vpu or Env. J. Virol. 73, 887–896 (1999).
Theodore, T. S. et al. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retroviruses 12, 191–194 (1996).
Schindler, M. et al. Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue. Retrovirology 7, 1, 10.1186/1742-4690-7-1 (2010).
Perelson, A. S. & Ribeiro, R. M. Modeling the within-host dynamics of HIV infection. BMC Biol. 11, 96, 10.1186/1741-7007-11-96 (2013).
Perelson, A. S. Modelling viral and immune system dynamics. Nat. Rev. Immunol. 2, 28–36, 10.1038/nri700 (2002).
Fraser, C., Hollingsworth, T. D., Chapman, R., de Wolf, F. & Hanage, W. P. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc. Natl. Acad. Sci. USA 104, 17441–17446, 10.1073/pnas.0708559104 (2007).
Fideli, U. S. et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res. Hum. Retroviruses 17, 901–910, 10.1089/088922201750290023 (2001).
Geskus, R. B. et al. The H. I. V. RNA setpoint theory revisited. Retrovirology 4, 65, 10.1186/1742-4690-4-65 (2007).
Mellors, J. W. et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272, 1167–1170 (1996).
Anderson, R. M. & May, R. M. Immunisation and herd immunity. Lancet 335, 641–645 (1990).
Anderson, R. M. & May, R. M. Epidemiological parameters of HIV transmission. Nature 333, 514–519, 10.1038/333514a0 (1988).
Velasco-Hernandez, J. X., Gershengorn, H. B. & Blower, S. M. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect. Dis. 2, 487–493 (2002).
Rashid, H., Khandaker, G. & Booy, R. Vaccination and herd immunity: what more do we know? Curr. Opin. Infect. Dis. 25, 243–249, 10.1097/QCO.0b013e328352f727 (2012).
Vigan, R. & Neil, S. J. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J. Virol. 84, 12958–12970 (2010).
Keele, B. F. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 105, 7552–7557, 10.1073/pnas.0802203105 (2008).
Salazar-Gonzalez, J. F. et al. Genetic identity, biological phenotype and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206, 1273–1289, 10.1084/jem.20090378 (2009).
Dave, V. P., Hajjar, F., Dieng, M. M., Haddad, E. & Cohen, E. A. Efficient BST2 antagonism by Vpu is critical for early HIV-1 dissemination in humanized mice. Retrovirology 10, 128, 10.1186/1742-4690-10-128 (2013).
Sato, K. et al. Vpu augments the initial burst phase of HIV-1 propagation and downregulates BST2 and CD4 in humanized mice. J. Virol. 86, 5000–5013, 10.1128/JVI.07062-11 (2012).
Simon, F. et al. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 4, 1032–1037, 10.1038/2017 (1998).
Vallari, A. et al. Four new HIV-1 group N isolates from Cameroon: Prevalence continues to be low. AIDS Res. Hum. Retroviruses 26, 109–115, 10.1089/aid.2009.0178 (2010).
Yamaguchi, J. et al. Identification of HIV type 1 group N infections in a husband and wife in Cameroon: viral genome sequences provide evidence for horizontal transmission. AIDS Res. Hum. Retroviruses 22, 83–92, 10.1089/aid.2006.22.83 (2006).
Delaugerre, C., De Oliveira, F., Lascoux-Combe, C., Plantier, J. C. & Simon, F. HIV-1 group N: travelling beyond Cameroon. Lancet 378, 1894, 10.1016/S0140-6736(11)61457-8 (2011).
Sato, K., Gee, P. & Koyanagi, Y. Vpu and BST2: Still Not There Yet? Front. Microbiol. 3, 131, 10.3389/fmicb.2012.00131 (2012).
Ratner, L. et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313, 277–284 (1985).
Alizon, M., Wain-Hobson, S., Montagnier, L. & Sonigo, P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell 46, 63–74 (1986).
Srinivasan, A. et al. Molecular characterization of human immunodeficiency virus from Zaire: nucleotide sequence analysis identifies conserved and variable domains in the envelope gene. Gene 52, 71–82 (1987).
Sanchez-Pescador, R. et al. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science 227, 484–492 (1985).
Parrish, N. F. et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. U. S. A. 110, 6626–6633, 10.1073/pnas.1304288110 (2013).
Pickering, S. et al. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 10, e1003895, 10.1371/journal.ppat.1003895 (2014).
Iwami, S., Koizumi, Y., Ikeda, H. & Kakizoe, Y. Quantification of viral infection dynamics in animal experiments. Front. Microbiol. 4, 264, 10.3389/fmicb.2013.00264 (2013).
Kobayashi, T. et al. Quantification of deaminase activity-dependent and -independent restriction of HIV-1 replication mediated by APOBEC3F and APOBEC3G through experimental-mathematical investigation. J. Virol. 88, 5881–5887, 10.1128/JVI.00062-14 (2014).
Acknowledgements
This work was supported in part by JST PRESTO program (to S.I.); JST CREST program (to S.I. and K.S.); a Grant-in-Aid for Young Scientists B25800092 (to S.I.) from the Japan Society for the Promotion of Science (JSPS); Inamori Foundation (to S.I.); the Aihara Innovative Mathematical Modeling Project, JSPS, through the “Funding Program for World-Leading Innovative R & D on Science and Technology (FIRST Program)”, initiated by Council for Science and Technology Policy (to S.I., K.A. and K.S.); Kyushu University Interdisciplinary Programs in Education and Projects in Research Development (to S.I.); Takeda Science Foundation (to K.S.); Sumitomo Foundation Research Grant (to K.S.); Senshin Medical Research Foundation (to K.S.); Imai Memorial Trust for AIDS Research (to K.S.); Ichiro Kanehara Foundation (to K.S.); Kanae Foundation for the Promotion of Medical Science (to K.S.); Suzuken Memorial Foundation (to K.S.).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: S.I., K.S., Y.I., K.A. and Y.K. Analyzed the data: S.I., S.M., H.I., Y.K. and F.R. Carried out the experiments: K.S., T.K., J.S.T., Y.K., N.M. and Y.K. Wrote the paper: S.I., K.S., S.M., H.I., Y.I., K.A. and Y.K. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Iwami, S., Sato, K., Morita, S. et al. Pandemic HIV-1 Vpu overcomes intrinsic herd immunity mediated by tetherin. Sci Rep 5, 12256 (2015). https://doi.org/10.1038/srep12256
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12256
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.