Abstract
A variety of fluorescent proteins have been identified that undergo shifts in spectral emission properties over time or once they are irradiated by ultraviolet or blue light. Such proteins are finding application in following the dynamics of particular proteins or labelled organelles within the cell. However, before genes encoding these fluorescent proteins were available, many proteins have already been labelled with GFP in transgenic cells; a number of model organisms feature collections of GFP-tagged lines and organisms. Here we describe a fast, localized and non-invasive method for GFP photoconversion from green to red. We demonstrate its use in transgenic plant, Drosophila and mammalian cells in vivo. While genes encoding fluorescent proteins specifically designed for photoconversion will usually be advantageous when creating new transgenic lines, our method for photoconversion of GFP allows the use of existing GFP-tagged transgenic lines for studies of dynamic processes in living cells.
Similar content being viewed by others
Introduction
Fusions of Green Fluorescent Protein (GFP) and its derivatives are extremely valuable tools for examining gene expression, protein and RNA localization, protein-protein interactions, protein synthesis and degradation and organelle movement. The development of photoconvertible fluorescent proteins such as mEosFP, tdEosFP, Dendra, Dronpa, Kaede, KikGR, mOrange allows new questions to be asked concerning dynamic processes in living cells1,2,3,4,5,6. However, a number of collections of GFP-tagged organisms had been made before these proteins became available for use. For example, a collection of strains (http://yeastgfp.yeastgenome.org/) expressing full-length GFP-tagged proteins is available for yeast7,8. In Drosophila, there is a collection (http://cooley.medicine.yale.edu/flytrap/) in which GFP has tagged full-length endogenous proteins expressed from their endogenous loci or used in an enhancer trap9,10. In Arabidopsis, lines are available with GFP tags on transcription factors11, with GFP as promoter-reporters11 and with GFP labels on subcellular structures12. In addition to specific collections, many investigators with interests in particular proteins or subcellular locations have produced cell lines or organisms with GFP tags. For example, various transgenic mouse lines (http://www.jaxmice.jax.org/) have been produced with GFP labels on proteins13; retransforming such lines with genes encoding new photoconvertible proteins would require considerable time and expense. Because many proteins have already been tagged with EGFP or GFP modified by an S65T mutation, being able to photoconvert these common forms of GFP would allow the use of existing transgenic lines for studies of dynamic changes.
EGFP has been known to change color after 488 nm illumination under low oxygen conditions14, low oxygen with added FAD15 or by added oxidizing agents such as potassium ferricyanide, benzylquinone (BQ) or MTT16. The fluorescence spectra of the photoconverted species varied depending on the conditions used to catalyze the conversion. The “red” emission maximum was at 560 nm in the FAD-mediated conversion15, while three peaks were reported (560, 590 and 600 nm) under anaerobic conditions (no added FAD)14. The addition of electron acceptors such as BQ resulted in 600 nm emissions with the exception of one report of a 675 nm peak17. Performing GFP photoconversion in low oxygen requires placing the tissue in special conditions and use of oxidizers obviates one of the advantages of GFP—the ability to image without concerns about uptake of additional chemicals. We report here a method that can be used to convert GFP from green to red without a special environment or exogenous chemicals. We show its effectiveness in plant, mammalian and Drosophila cells.
Results
Several common variants of GFP can be photoconverted
While performing photobleaching studies on plant cells labeled with GFP, we made the unexpected observation that EGFP, mGFP4-T and GFP modified by an S65T mutation can be photoconverted by 405 nm light in vivo. The sequences of the variant GFPs we have analyzed are shown in Fig. 1a.
GFP variants used for photoconversion.
(a) Alignment of the coding region of the GFP variants. The alignment was created using GeneDoc (http://www.nrbsc.org/gfx/genedoc/). (b) Schematic representation of GFP transgenes used in photoconversion experiments. Pr1b-SP, secretory signal peptide from the tobacco pathogenesis-related protein 1; EGFP, enhanced green fluorescent protein; L, linker (GGGS)3; KDEL, endoplasmic reticulum retrieval signal peptide; HttQ103, exon 1 of human HttQ103 gene; Cytosolic mGFP4-T in tobacco cell culture and cytosolic S65T-GFP in Drosophila gut cells. In cytosolic mGFP4-T, V (valine) was replaced by A (alanine) and Q (glutamine) was replaced by R (arginine) relative to EGFP. In cytosolic S65T-GFP, L (leucine) was replaced by F (phenylalanine) relative to EGFP.
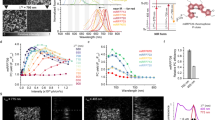
To verify whether EGFP could be photoconverted in vitro from its normal green state to a red state, we investigated irradiation of purified EGFP. By using the Zeiss 710 bleaching mode with a 405 nm laser for photoconversion, rather than 488 nm as used in the previous reports, purified EGFP was imaged before and after application of the 405 nm light. The green-state of purified EGFP was imaged with excitation at 488 nm and the emission was acquired between 505–525 nm. The photoconverted EGFP (red state) was monitored using 561 nm excitation and detected between 580–670 nm. Photoconversion occurred, as demonstrated by the decrease in green fluorescence and the simultaneous increase in red fluorescence (Fig. 2a). We observed a maximum emission peak around 612 nm when using 561 nm excitation (Fig. 2b). The red species of EGFP is not excited by 488 nm light and we could detect no red species formed using 488 nm light alone for photoconversion.
Photoconversion of purified EGFP in vitro.
(a) Typical pre- and post-photoconversion images of a thin (~10 μm) layer of 30 μM EGFP using 405 nm excitation delivered through a 1.2 NA objective to the region within the circle using the Zen software bleaching mode (Green channel: 505–525 nm; Red channel: 580–670 nm; scale bar: 50 μm). See also Supplementary Movie S1. 10 iterations of 257 ms each were performed. (b) Emission spectra of photoconverted red state EGFP acquired using the Zeiss 710 spectral detector (5 nm bandwidth intervals). (c) Absorption spectra of EGFP (~2 μM) at pH 5.0 and pH 8.0. (d) Time course of the increase in red fluorescence with successive 405 nm irradiations within the region of interest (ROI). EGFP (30 mM) was subjected to a sequence of 10 “bleach” iterations over the ROI (1.8 seconds) followed by image acquisition using 488 and 561 nm illumination. The 405 nm power at the sample was at 2.5 mW, corresponding to 6.8 MW/cm2 at the focus of the 1.2 NA objective lens. Plotted data points are the average pixel values across the entire field of view which estimates the total photo-product produced. Error bars represent the SEM of three trials at pH 5.0 and two at pH 8.0. Black lines are fits to exponential increase for the red channel (1-exp(-αt)) and decrease (exp(-αt)) for the green. Returned values of α were ~0.14 s−1 for all data sets except the decay of the pH 8.0 green channel (which was 0.24 s−1).
The need for 405 nm excitation indicated that we were most likely photoconverting the protonated or “A” form of the chromophore18 as indicated by the increased yield of red photo-product at pH 5.0 compared to pH 8.0 under identical illumination conditions and total EGFP concentration (Fig. 2d). At pH 5.0, the A405 of EGFP is ~2.6 times higher than at pH 8.0 (Fig. 2c). This approximately accounts for the increase in red photo-product as shown in the time course data (Fig. 2d), where a 2.3 fold difference in red fluorescence was found between pH 5.0 and pH 8.0. The rate of red species formation was the same at both pH values (~0.14 s−1 under our conditions) indicating that the protonated form is being converted and it is the concentration of the “A” form that matters. This finding also indicates that cellular compartments with higher pH may be less suitable for imaging of photoconverted GFP unless concentrations are relatively high. The photoconversion appears to be irreversible and red-GFP was still visible several hours after photoconversion (data not shown) in our in vitro experiments.
GFP will be present in a variety of concentrations in transgenic cells and organisms, depending on levels of protein expression and stability. We investigated the intensity of the fluorescent signal relative to concentration of purified GFP. Sufficient GFP must be present to observe the photoconverted form and the signal appears to saturate at approximately 30 μM (Fig. 3).
Correlation between EGFP concentration and red fluorescence intensity following irradiation.
EGFP was diluted in PBS buffer at the following concentrations: 0.875, 1.7, 3.4, 6.8, 13.75, 27.5, 55 μM. All photoconversion and imaging parameters were kept similar for all tested EGFP concentrations. Data are from 3 independent experiments.
GFP variants can be photoconverted in vivo
To determine whether GFP could be converted from the green to the red state in vivo, we performed photoconversion experiments in transgenic tobacco cells in which GFP was localized in the cytosol19 (Fig. 4a-c). Converting GFP from green to red state resulted in rapid diffusion of the red form within the plant cytosol. In less than 3 seconds after irradiation, red-GFP could be detected in the red channel. The photoconverted red-GFP then diffused through the whole cell and was readily visible throughout the cell within 2 minutes (Fig. 4a and Supplementary Movie S2). Fluorescence intensity was measured in the area indicated by a white circle in the irradiated area. There is a noticeable decrease in intensity of the green signal and a concomitant increase in intensity of the red signal, which is an indication of photoconversion from green to red state (Fig. 4c). We detected no red signal when plant cells not expressing GFP were irradiated and imaged under the same conditions.
Photoconversion of GFP from green to red state in vivo.
Single snapshots from the pre-photoconversion and post-photoconversion are shown. (a) Tobacco suspension cell culture in which GFP is targeted to cytosol. Photoconverted GFP spreads through the cytosol via cytoplasmic strands. Photoconversion was performed at 50% laser power and 30 iterations of 111 milliseconds (ms). (b) Schematic representation of a tobacco suspension culture cell. The cell wall (brown) surrounds the plasma membrane (red). A large vacuole (blue) occupies the majority of the space inside the cell. The endoplasmic reticulum network (gray) surrounds the vacuole and the nucleus (white) and extends through the rest of the cytosol (white). Cytoplasmic strands exist as extensions of the cytosol between organelles. (c) Changes in fluorescence intensity in the irradiated area in (a) over time indicate increasing red and decreasing green fluorescence. (d) Drosophila gut cells from 3rd instar larvae expressing S65T-GFP. (e) Rat PC12 cells expressing EGFP fusion to exon 1 of human HttQ103 gene. White arrowheads highlight cytosolic inclusions. Images acquired by 30 iterations with the duration of 190 ms each. Laser power was adjusted at 70%. Bars 10 μm.
We could also observe the photoconversion of EGFP in other eukaryotic model organisms, including Drosophila and rat cells. We obtained a Drosophila line in which S65T-GFP was expressed in gut cells from 3rd instar larvae20 (Fig. 4d) and a rat PC12 cell line expressing EGFP fused to exon 1 of the human Huntington poly Q (HttQ103) gene21 (Fig. 4e). When Drosophila or rat cells were irradiated using conditions similar to those that were effective for plant cells, we observed photoconversion from the green to red state (Fig. 4d,e).
Transient expression methods are also frequently used to study the subcellular localization of GFP-tagged proteins. In plants, several methods exist for transient expression, including particle bombardment, infiltration of Agrobacterium tumefaciens (Agroinfiltration), or protoplast transfection. In addition to photoconversion of stably transformed tobacco cells, we were able to photoconvert EGFP that was transiently expressed in Nicotiana benthamiana epidermal leaf cells by Agroinfiltration (Fig. 5, Supplementary Movies S3-4). We were able to label the endoplasmic reticulum (ER) with GFP targeted to the ER and observe movement through the ER (Fig. 5a). Also, GFP located in the cytosol could be photoconverted and we could follow its movement through the cell (Fig. 5b).
EGFP photoconversion occurs in N. benthamiana cells transiently expressing EGFP.
(a) ER-targeted EGFP photoconverts from green to red upon excitation with 405-nm laser at 60% of laser power and 100 iterations with duration of 145 milliseconds each. The photoconverted red protein travels within the ER network of the cell. (b) Cytosolic EGFP photoconverts and spreads within the cytosolic space of the cell. Photoconversion was performed at 70% of laser power and 30 iterations with duration of 190 milliseconds each. White circles represent the irradiated area. Bar 20 μm. (c) Schematic representation of a Nicotiana benthamiana epidermal leaf cell. Puzzle-shaped epidermal cells are surrounded with the cell wall (brown). The space between the cell walls of two neighboring cells, apoplast, is shown in green. The plasma membrane (red) surrounds the cell. A large vacuole (blue) occupies the majority of the inner space of the cell. Endoplasmic reticulum (black) is localized around the nucleus and spreads throughout the cytosol (white), which is pushed against the plasma membrane by the vacuole. Chloroplasts are drawn in black as oval structures in the cytosol.
Discussion
Variant fluorescent proteins that have been designed for photoconversion applications will usually be superior to GFP in terms of rapidity of shift in emission and magnitude and may be brighter at low concentration. Thus, the primary advantage of using GFP in photoconversion is the ability to use existing transgenic lines.
Nevertheless, GFP has other advantages over the use of some other fluorescent proteins. Non-GFP photoconvertible fluorescent proteins sometimes exhibit unexpected subcellular localization behavior. One example is a study performed by Schattat et al.22, in which mEOS2 was used to assess movement of protein into plastid extensions termed stromules23. In contrast to prior and subsequent studies using GFP24,25,26, following photoconversion, mEOS2 did not move between plastid bodies and appeared to become “stuck” within the plastid body or partly into the stromule22. Another example of abnormal localization and loss of movement was observed in experiments with photoconvertible fluorescent protein fusions to the SHORT-ROOT (SHR) protein, which normally moves between different cell types in the root27. When tdEosFP was fused to SHR, the fusion protein exhibited abnormal intracellular localization and did not move into the endodermis of the root, unlike GFP fusions28.
Another benefit of GFP-based photoconversion is the absence of any random or auto-photoconversion in our tested samples. For some of the non-GFP-based photoconverting proteins, auto-photoconversion has been reported. For example, in mEosFP-cytosolic plants grown in bright fluorescent white light, up to a quarter of hypocotyl epidermal cells contained red nuclei and in cells transiently expressing cytosolic mEosFP, auto-conversion was observed without any intentional photoconversion8. Another concern involved with the use of mEosFP is the partial photoconversion under short exposure times. Photoconversion of mEosFP happens in a concentric manner, therefore producing variability in shades, which is problematic since both partial photoconversion and co-localization of the green and red will produce yellow hues29.
Photoconversion of GFP also has an advantage in material labelled with multiple fluorophores. GFP photoconversion would avoid crosstalk between multiple fluorophores since its maximum emission peak at 612 nm is different from that of the most commonly used photoconvertible proteins such as mEOS2 (red) at 584 nm and Dendra2 (red) at 573 nm, or with other fluorescent proteins such as EBFP, ECFP, EYFP with maximum emission peaks at 445, 476, 527 respectively and many other fluorescent proteins.
In cells with high expression levels of GFP, lower laser powers (20–30% corresponding to ~500 μW) and shorter exposure times can be used for photoconversion experiments. In cells with lower GFP expression, sequential and simultaneous imaging of cells during the photoconversion is recommended in order to prevent photodamage in the cells under irradiation. To track the in vivo movement of proteins, multiple irradiations with time intervals (from seconds to minutes) were found to be useful to follow trafficking of the photoconverted protein and photoconversion of newly synthesized/trafficked proteins in the irradiated area (Supplementary Movies 3-4).
In summary, we have demonstrated that GFP photoconversion can be added to the cell biologist’s toolkit for monitoring protein dynamics in transgenic cells, provided adequate amounts of GFP are expressed for sufficient sensitivity. Using GFP photoconversion is particularly advantageous when transgenic cells and lines have already been produced with GFP labelling, as re-transformation with a different gene encoding a new photoconvertible protein may not be necessary. The method we describe could find applications in a broad range of biological systems.
Methods
Constructs and transgenes
ER-EGFP and cytosolic-EGFP plant expression vectors and PC12 cell line expressing HttQ103-EGFP were previously published21,30. The genotype of the Drosophila larvae is FM7i, P{w[+mC] = ActGFP}JMR320.
Strains used and culture conditions
For transient expression experiments, we used 7-week-old N. benthamiana plants grown at 22 °C with a 16 hour photoperiod at 110 μmol m−2 s−1. Plants were fertilized with the water soluble fertilizer (N:P:K = 20:8:20) (Plant Products, Brampton, ON, Canada) at 0.25 g/L. A. tumefaciens strain EHA105 containing the cytosolic-EGFP and ER-EGFP were grown to an optical density at 600 nm (OD600) of 0.5–0.8 and collected by centrifugation at 1000 g for 30 minutes. The collected pellets were resuspended in Agroinfiltration solution (3.2 g/L Gamborg’s B5 medium and vitamins, 20 g/L sucrose, 10 mM MES pH 5.6, 200 μM 4′-Hydroxy-3′, 5′-dimethoxyacetophenone) to a final OD600 of 0.30 and incubated at room temperature (21 °C) with gentle agitation for 1 hour. The suspension was then used for infiltration of the abaxial leaf epidermis of N. benthamiana with a 1 ml syringe (Conley et al.30)
Rat PC12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2. To induce the expression of HttQ103-EGFP, cells were treated with 2.5 μM tebufenocide (Sigma, St. Louis, MI) for 24 to 48 hours. Tobacco cell suspensions were grown in NT1 medium23 with regular shaking at 22 °C. Drosophila larvae were grown at room temperature in standard corn meal agar9.
Purified EGFP was obtained by HisPur Ni NTA bead-based chromatography of the 6xHis-EGFP plasmid (Addgene, Cambridge, MA, USA) expressed in E. coli.
Microscopy and imaging
A Zeiss LSM 710 confocal microscope equipped with a 10X dry, 40X or 63X water immersion objective was used for the imaging and photoconversion of GFPs. Green fluorescence was detected with a 505–530 nm band pass filter and red fluorescent emission was detected with a spectral detector between 580–640 nm. EGFP fluorescence and absorption at pH 5.0 and pH 8.0 was measured in a MOPS-citrate buffer (120 mM KCl, 5 mM NaCl, 1 mM MgCl, 10 mM MES, 10 mM MOPS and 10 mM citrate) adjusted to the desired pH.
Additional Information
How to cite this article: Sattarzadeh, A. et al. Green to red photoconversion of GFP for protein tracking in vivo. Sci. Rep. 5, 11771; doi: 10.1038/srep11771 (2015).
References
Baker, S. M., Buckheit, R. W., 3rd & Falk, M. M. Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes. BMC Cell Biol. 11, 15 (2010).
Terskikh, A. et al. “Fluorescent timer”: protein that changes color with time. Science 290, 1585–1588 (2000).
Pletnev, S. et al. Orange fluorescent proteins: structural studies of LSSmOrange, PSmOrange and PSmOrange2. PLoS One 9, e99136 (2014).
Wachter, R. M., Watkins, J. L. & Kim, H. Mechanistic diversity of red fluorescence acquisition by GFP-like proteins. Biochemistry 49, 7417–7427 (2010).
Adam, V., Berardozzi, R., Byrdin, M. & Bourgeois, D. Phototransformable fluorescent proteins: Future challenges. Current Opin. Chem. Biol. 20C, 92–102 (2014).
Wiedenmann, J. et al. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 101, 15905–15910 (2004).
Howson, R. et al. Construction, verification and experimental use of two epitope-tagged collections of budding yeast strains. Comp. Funct. Genomics 6, 2–16 (2005).
Huh, W. K. et al. Global analysis of protein localization in budding yeast. Nature 425, 686–691 (2003).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050–15055 (2001).
Quinones-Coello, A. T. et al. Exploring strategies for protein trapping in Drosophila. Genetics 175, 1089–1104 (2007).
Xiao, Y. L. et al. High throughput generation of promoter reporter (GFP) transgenic lines of low expressing genes in Arabidopsis and analysis of their expression patterns. Plant Meth. 6, 18 (2010).
Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97, 3718–3723 (2000).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Elowitz, M. B., Surette, M. G., Wolf, P. E., Stock, J. & Leibler, S. Photoactivation turns green fluorescent protein red. Current Biol. 7, 809–812 (1997).
Matsuda, A. et al. Condensed mitotic chromosome structure at nanometer resolution using PALM and EGFP- histones. Plos One 5, e12768 (2010).
Bogdanov, A. M. et al. Green fluorescent proteins are light-induced electron donors. Nature Chem. Biol. 5, 459–461 (2009).
Saha, R. et al. Light driven ultrafast electron transfer in oxidative redding of Green Fluorescent Proteins. Sci. Reports 3, 1580 (2013).
Brejc, K. et al. Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc. Natl. Acad. Sci. USA 94, 2306–2311 (1997).
Kohler, R. H., Zipfel, W. R., Webb, W. W. & Hanson, M. R. The green fluorescent protein as a marker to visualize plant mitochondria in vivo. Plant J. 11, 613–621 (1997).
Reichhart, J. M. & Ferrandon, D. Green balancers. Drosophila Information Service 81, 201–202 (1998).
Aiken, C. T., Tobin, A. J. & Schweitzer, E. S. A cell-based screen for drugs to treat Huntington’s disease. Neurobiol. Disease 16, 546–555 (2004).
Schattat, M. H. et al. Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24, 1465–1477 (2012).
Kohler, R. H. & Hanson, M. R. Plastid tubules of higher plants are tissue-specific and developmentally regulated. J. Cell Sci. 113 (Pt 1), 81–89 (2000).
Hanson, M. R. & Sattarzadeh, A. Stromules: recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 155, 1486–1492 (2011).
Hanson, M. R. & Sattarzadeh, A. Trafficking of proteins through plastid stromules. Plant Cell 25, 2774–2782 (2013).
Kohler, R. H., Cao, J., Zipfel, W. R., Webb, W. W. & Hanson, M. R. Exchange of protein molecules through connections between higher plant plastids. Science 276, 2039–2042 (1997).
Nakajima, K., Sena, G., Nawy, T. & Benfey, P. N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311 (2001).
Wu, S., Koizumi, K., Macrae-Crerar, A. & Gallagher, K. L. Assessing the utility of photoswitchable fluorescent proteins for tracking intercellular protein movement in the Arabidopsis root. PLoS One 6, e27536 (2011).
Mathur, J. et al. mEosFP-based green-to-red photoconvertible subcellular probes for plants. Plant Physiol. 154, 1573–1587 (2010).
Conley, A. J., Joensuu, J. J., Menassa, R. & Brandle, J. E. Induction of protein body formation in plant leaves by elastin-like polypeptide fusions. BMC Biol. 7, 48 (2009).
Acknowledgements
We thank Dr. Robert Cumming (University of Western Ontario) for providing the EGFP-labelled rat PC12 cell line and Dr. Daniel Barbash (Cornell) for the S65T-GFP-labelled Drosophila cells. We are grateful to Alex Molnar (Agriculture and Agri-Food Canada) for assistance with the preparation of the figures and Karen Nygard at the Biotron Imaging Facility (University of Western Ontario) for providing help with microscopy. This work was supported by grant DE-FG02-09ER16070 the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy to M.R.H. and by Agriculture and Agri-Food Canada A-base project 1725 to R.M.
Author information
Authors and Affiliations
Contributions
A.S., R.S., R.M., W.R.Z. and M.R.H. designed research, A.S. and R.S. performed all experiments. A.S., R.S., R.M., W.R.Z. and M.R.H. analyzed data and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sattarzadeh, A., Saberianfar, R., Zipfel, W. et al. Green to red photoconversion of GFP for protein tracking in vivo. Sci Rep 5, 11771 (2015). https://doi.org/10.1038/srep11771
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11771
This article is cited by
-
A general strategy to red-shift green fluorescent protein-based biosensors
Nature Chemical Biology (2020)
-
Role of Hydrogen Bonding in Green Fluorescent Protein-like Chromophore Emission
Scientific Reports (2019)
-
A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells
Nature Plants (2018)
-
Role of green fluorescent proteins and their variants in development of FRET-based sensors
Journal of Biosciences (2018)
-
Genetically encoded indicators of neuronal activity
Nature Neuroscience (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.