Abstract
A hybrid white organic light-emitting diode (WOLED) with an external quantum efficiency above 20% was developed using a new blue thermally activated delayed fluorescent material, 4,6-di(9H-carbazol-9-yl)isophthalonitrile (DCzIPN), both as a blue emitter and a host for a yellow phosphorescent emitter. DCzIPN showed high quantum efficiency of 16.4% as a blue emitter and 24.9% as a host for a yellow phosphorescent emitter. The hybrid WOLEDs with the DCzIPN host based yellow emitting layer sandwiched between DCzIPN emitter based blue emitting layers exhibited high external quantum efficiency of 22.9% with a warm white color coordinate of (0.39, 0.43) and quantum efficiency of 21.0% with a cool white color coordinate of (0.31, 0.33) by managing the thickness of the yellow emitting layer.
Similar content being viewed by others
Introduction
White organic light-emitting diodes (WOLEDs) have been studied for application in display and lightings and are being used in large size TV and specialty lightings. However, current WOLEDs still have some problems to overcome and one of critical issues of WOLEDs is power consumption which is determined by driving voltage and efficiency of the devices. Low driving voltage and high efficiency are required to reduce the power consumption of WOLEDs. In particular, high efficiency is a key parameter to the low power consumption because of low efficiency of fluorescent blue material currently used in WOLEDs for display and lighting devices.
There are two approaches to improve the efficiency of the WOLEDs. One method is to adopt phosphorescent emitting materials in all red, green and blue colors1,2,3,4,5,6,7,8. High external quantum efficiency above 20% can be realized by high quantum efficiency of the phosphorescent emitting materials, but the all phosphorescent WOLEDs suffer from limited availability and poor stability of blue triplet emitters. The other method is to combine high triplet energy blue fluorescent emitting materials with phosphorescent red and green emitting materials9,10,11,12,13. The blue emitting materials are designed to have high triplet energy for energy transfer to red and green phosphorescent emitting materials and harvest triplet excitons for red and green emission. Theoretically, the internal quantum efficiency of the WOLEDs can reach 100% by this approach because both singlet and triplet excitons of blue fluorescent materials can be utilized for light emission13. However, the external quantum efficiency of the WOLEDs with the fluorescent blue emitter is still below 20% due to loss processes such as triplet-triplet fusion and reverse energy transfer from phosphorescent emitters to the blue fluorescent emitter.
The drawback of the two approaches described above can be avoided by thermally activated delayed fluorescent (TADF) emitter which can harvest both singlet and triplet excitons for fluorescent light-emission14,15,16,17,18,19,20,21,22. A blue TADF emitter can show better quantum efficiency than blue fluorescent emitter and can effectively harvest triplet excitons of red and green phosphorescent emitters because of theoretical internal quantum efficiency of 100% and high triplet energy for energy transfer to red and green phosphorescent emitters. Therefore, the TADF emitter based WOLEDs can potentially give similar external quantum efficiency to all phosphorescent WOLEDs. Although a simple WOLED with the TADF emitter was reported23,24, the development of high efficiency WOLED derived from the TADF emitter is required.
In this work, a new hybrid WOLED combining a blue TADF emitter and a yellow phosphorescent emitter was newly developed as a high efficiency WOLED. A new blue TADF emitter, 4,6-di(9H-carbazol-9-yl)isophthalonitrile (DCzIPN), was synthesized both as a TADF emitter and a triplet host for the yellow triplet emitter to develop the new hybrid WOLED. DCzIPN showed external quantum efficiency of 16.4% as a blue emitter in the blue device and external quantum efficiency of 24.9% as a triplet host for the yellow triplet emitter in the yellow device. It was demonstrated that the hybrid WOLEDs with the DCzIPN host based yellow emitting layer sandwiched between the DCzIPN doped blue TADF emitting layers exhibited high external quantum efficiency of 22.9% with a warm white color coordinate of (0.39, 0.43) and quantum efficiency of 21.0% with a cool white color coordinate of (0.31, 0.33) by managing the thickness of the yellow emitting layer. This work is the first demonstration of a hybrid WOLED with a blue TADF emitting material combined with a yellow triplet emitter and above 20% external quantum efficiency in the hybrid WOLEDs showing warm and cool white color. This work proved that the TADF emitter based hybrid WOLEDs can be comparable to all phosphorescent WOLEDs.
Results
Synthesis of a blue TADF emitter
In general, blue TADF emitters should possess high singlet energy about 2.7 eV and triplet energy between 2.4 eV and 2.7 eV for efficient blue TADF emission. Triplet excitons can be converted into singlet excitons by up-conversion process from triplet excited state to singlet excited state and the singlet energy should be around 2.7 eV for blue emission. The triplet energy of blue TADF emitter is higher than that of yellow triplet emitters, which may enable the application of the blue TADF emitter as the host material for phosphorescent organic light-emitting diodes (OLEDs). Therefore, the TADF blue emitter can give high quantum efficiency as an emitter in the blue device and as a host in the yellow phosphorescent OLEDs and the combination of the TADF emitter doped blue emitting layer with a yellow phosphorescent emitting layer with the TADF emitter as a host can produce high efficiency hybrid WOLEDs without any interlayer between the blue and yellow emitting layer.
To develop high efficiency blue TADF device, yellow phosphorescent device and hybrid WOLEDs, a new TADF blue emitter, DCzIPN, was synthesized. Synthetic scheme of DCzIPN is shown in Figure 1 The DCzIPN TADF emitter was synthesized from 1,5-dibromo-2,4-difluorobenzene which was prepared by bromination of 1-bromo-2,4-difluorobenzene. The 1,5-dibromo-2,4-difluorobenzene was cyanated using CuCN to produce 4,6-difluoroisophthaonitrile, which was modified with carbazole using sodium hydride. Synthetic yield of DCzIPN was 82% and the final product was purified by vacuum train sublimation. Chemical structure of DCzIPN was confirmed by 1H and 13C nuclear magnetic resonance spectrometer, mass analysis and elemental analysis. Purity of DCzIPN was above 99% from high performance liquid chromatography analysis.
Photophysical properties of DCzIPN were analyzed using ultraviolet-visible (UV-Vis) and PL spectrometers. Figure 2 shows UV-Vis absorption, solid PL in polystyrene (PS) and low temperature PL spectra of DCzIPN. Strong π-π* absorption of DCzIPN was observed at 229 nm and weak n-π* absorption was detected up to 410 nm. Peak positions of solid PL emission of DCzIPN in PS (1 wt%) and low temperature PL emission were 447 nm and 455 nm, which corresponded to a singlet energy of 2.77 eV and triplet energy of 2.72 eV, respectively. There was 0.05 eV difference between the singlet and triplet energy of DCzIPN, which may activate TADF emission of DCzIPN. Absolute PL quantum efficiency of DCzIPN was 0.35 from integrating sphere measurement in toluene and was 0.87 in N,N'-dicarbazolyl-3,5-benzene matrix.
The TADF emission of DCzIPN was confirmed by transient and time resolved PL measurement. Transient PL spectrum of DCzIPN is shown in Figure 3. Excited state lifetime of delayed light emission was 1.2 μs from the fitting of the decay curve. Prompt and delayed PL spectra of DCzIPN are plotted in Figure 4. Delay time for the delayed PL measurement was 10 μs. The prompt PL and delayed PL spectra were overlapped, suggesting that delayed PL emission is originated by delayed fluorescence emission.
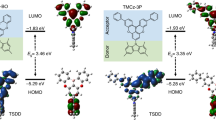
Molecular simulation result of DCzIPN calculated using B3LYP/6-31G* basis sets of Gaussian 09 program is presented in Figure 5 to study the molecular orbital distribution of DCzIPN. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of DCzIPN were dispersed over electron donating carbazole unit and electron withdrawing CN unit, respectively. Although the HOMO and LUMO were separated due to donor-acceptor character of DCzIPN, there was orbital overlap between the HOMO and LUMO in the central phenyl ring, which implies that the light-emitting properties of DCzIPN would be improved by radiative transition process.
Ionization potential (IP) and electron affinity (EA) of DCzIPN were analyzed using cyclic voltammetry (CV) measurements. Oxidation potential and reduction potential of DCzIPN were 1.46 V and -1.34 V, respectively. The IP and EA were -6.26 eV and -3.56 eV from the oxidation potential and reduction potential, respectively. Deep IP and EA were observed in the DCzIPN host due to strong electron withdrawing character of two CN units. The IP and EA gap of DCzIPN was 2.80 eV because of strong electron donor-acceptor structure.
Device performances of the single color devices
As DCzIPN showed high singlet energy of 2.77 eV and delayed fluorescent emission with a lifetime of 1.2 μs, it can be used as a blue TADF emitter. In addition, DCzIPN has high triplet energy of 2.72 eV for energy transfer to yellow triplet emitter and narrow IP-EA gap of 2.80 eV for facile hole and electron injection from hole and electron transport layers, which enables the use of DCzIPN as a host material for yellow triplet emitter. The DCzIPN emitter was doped in mCP host at an optimum doping concentration of 15% to develop high efficiency blue TADF device and it was doped with iridium(III) bis(4-phenylthieno[3,2-c]pyridinato-N,C2’)acetylacetonate (PO-01) at a doping concentration of 5% to fabricate high efficiency yellow phosphorescent OLEDs. Device structure of blue and yellow devices was indium tin oxide (ITO, 150 nm)/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS, 60 nm)/4,4'-(cyclohexane-1,1-diyl)bis(N-phenyl-N-p-tolylaniline) (TAPC, 20 nm)/mCP (10 nm)/mCP:DCzIPN or DCzIPN: PO-01 (25 nm)/diphenylphosphine oxide-4-(triphenylsilyl)phenyl (TSPO1, 35 nm)/LiF (1 nm)/Al (200 nm). Chemical structures of the organic materials used in the device fabrication are displayed in Figure 6. Figure 7 shows current density-voltage-luminance curves of the blue TADF and yellow phosphorescent OLEDs. The current density of the yellow phosphorescent OLED was higher than that of the blue device due to narrow gap of DCzIPN for good charge injection properties of DCzIPN. The turn-on voltage of the device was 2.0 V in the yellow device, while it was 3.5 V in the blue device due to energy barrier for charge injection. The luminance was also high in the yellow device and the driving voltage at 1,000 cd/m2 was 5.5 V.
Quantum efficiency-luminance curves of the blue and yellow devices are shown in Figure 8. Maximum quantum efficiency of the DCzIPN TADF OLEDs was 16.4% although the efficiency was reduced at high luminance. The PL quantum efficiency of the DCzIPN TADF emitter in mCP matrix at a doping concentration of 15% was 0.87, which was comparable or superior to that of other TADF emitters reported in the literature14 and contributed to the high quantum efficiency of the blue DCzIPN device. Additionally, high quantum efficiency was obtained due to exciton and charge confinement inside the emitting layer. The yellow phosphorescent OLED with DCzIPN as a host material exhibited a maximum quantum efficiency of 24.9%, which is better than the quantum efficiency reported in other work23. The high quantum efficiency of the yellow device is related with charge balance, exciton confinement and efficient energy transfer. DCzIPN has bipolar charge transport properties as can be confirmed by the hole and electron only device data (Figure 9), which contributed to balance holes and electrons in the emitting layer. Exciton confinement inside the emitting layer by high triplet energy mCP hole transport material (2.90 eV) and TSPO1 electron transport material (3.39 eV) and DCzIPN host material (2.72 eV) increased the quantum efficiency of the yellow device due to suppression of non-radiative decay of the yellow triplet emitter. Additionally, efficient energy transfer with an energy transfer efficiency of 97% at 3% doping concentration also played an important role of increasing the quantum efficiency. From this result, it can be concluded that DCzIPN effectively harvested triplet emission of yellow triplet emitter without triplet exciton quenching.
Electroluminescence (EL) spectra of the blue and yellow OLEDs are shown in Figure 10. EL peak maximum of the DCzIPN blue device was 462 nm with a color coordinate of (0.17, 0.19) and single emission peak with little vibrational emission peak was observed by DCzIPN. The yellow phosphorescent device showed an emission peak at 558 nm with a shoulder at 585 nm and the color coordinate of the device was (0.48, 0.51). Both blue and yellow devices showed pure emission of dopant materials without any emission from host materials.
Device performances of the hybrid white OLEDs
As DCzIPN showed high quantum efficiency in blue TADF OLEDs and yellow phosphorescent OLEDs, hybrid WOLEDs were developed by stacking the blue TADF OLED and the yellow phosphorescent OLEDs without any interlayer which is commonly used for the hybrid WOLEDs. The interlayer can be omitted because DCzIPN was proven to be effective as both a blue emitter and a host for a yellow emitter. There would be little non-radiative quenching process between the blue and yellow emitting layers as the emission energy of DCzIPN is effectively transferred to the yellow triplet emitter. Two devices with three emitting layers (Device I and II) with the yellow emitting layer embedded in the blue emitting layer were fabricated to manage the emission color of the WOLEDs. Basic device structure of WOLEDs was ITO (150 nm)/PEDOT:PSS (60 nm)/TAPC (20 nm)/mCP (10 nm)/mCP:DCzIPN (15-x/2 nm)/DCzIPN:PO-01 (x nm)/mCP:DCzIPN (15-x/2 nm)/TSPO1 (35 nm)/LiF (1 nm)/Al (200 nm). The thickness of the whole emitting layer was 30 nm and the thicknesses of the yellow emitting layer (x) were 1.5 nm and 3.0 nm in the device I and II, respectively.
Current density-voltage-luminance curves of the hybrid WOLEDs are shown in Figure 11. The current density of the device II was higher than that of device I and the high current density of device II is caused by higher current density of the yellow emitting layer than that of the blue emitting layer as shown in Figure 7 because thick yellow emitting layer was used in the device II. The luminance also followed the same tendency as the current density.
Quantum efficiency-luminance curves and EL spectra of the hybrid WOLEDs at 1,000 cd/m2 are shown in Figure 12 and 13. Maximum quantum efficiencies of device I and device II were 21.0% and 22.9%, respectively and the color coordinates of device I and device II were (0.31,0.33) and (0.39,0.43). Both device I and device II showed high quantum efficiency because singlet and triplet excitons of the DCzIPN blue emitter were not non-radiatively quenched by the yellow phosphorescent emitting layer. The relatively high quantum efficiency of device II compared to device I is due to strong yellow emission in the device II. As shown in the blue and yellow device data, the quantum efficiency of the yellow phosphorescent OLED was higher than that of blue TADF OLED. Therefore, more charge recombination in the yellow emitting layer would lead to high quantum efficiency, resulting in high quantum efficiency in the device II with strong yellow emission. Although the quantum efficiency of device I was lower than that of device II, high quantum efficiency of 21.0% was obtained in the device I in spite of strong blue intensity, which is better than the quantum efficiency of other WOLEDs with cool white color25. In the case of other cool white color WOLEDs, the quantum efficiency was rather low because of low quantum efficiency of blue devices. The problem of the low quantum efficiency could be solved by using the DCzIPN TADF emitter with a quantum efficiency of 16.4%.
Luminance dependent EL spectra of device I and device II are shown in Figure 14. The device I showed strong blue emission at 1,000 cd/m2, but it was reduced at 100 cd/m2 and 5,000 cd/m2 by the recombination zone shift at different luminances. In the case of device II, weak blue emission was observed at 1,000 cd/m2 and strong blue emission was detected at 100 cd/m2 and 5,000 cd/m2 due to recombination zone change.
Discussion
The DCzIPN TADF emitter was designed to have donor-acceptor structure to reduce the singlet-triplet energy gap for efficient reverse intersystem crossing and to emit blue color by attaching two carbazoles to isophthalonitrile. The donor-acceptor structure of DCzIPN enabled the fabrication of blue TADF emitter with high quantum efficiency. Although blue emitters with similar molecular structures with two CN and two carbazole units were reported, the quantum efficiency was low and pure blue emission was not observed14. However, current molecular design with two CN units and two carbazole units at meta position of benzene resulted in high quantum efficiency of 16.4% and deep blue color coordinate of (0.17, 0.19) with a peak emission wavelength of 462 nm.
The DCzIPN TADF emitter could also function as a host material for PO-01 triplet emitter by harvesting triplet excitons due to high triplet energy and increasing recombination efficiency owing to donor-acceptor structure. The quantum efficiency of the DCzIPN:PO-01 device could approach the highest external quantum efficiency of the PO-01 device. Therefore, the DCzIPN was effective both as a TADF emitter and triplet host material for phosphorescent OLEDs.
The dual function of DCzIPN as a TADF emitter and a triplet host is useful to develop white OLEDs because the device structure can be simplified by decreasing the number of materials used in the device structure. In the two color white OLED structure, DCzIPN can play a role of deep blue emitter and a host for a yellow phosphorescent emitter. Therefore, a novel white OLED device architecture with DCzIPN both in the blue and yellow emitting layers could be developed without any interlayer.
The quantum efficiency value obtained in the new white OLED structure is much better than that of other hybrid WOLEDs25 and is even better than that of all phosphorescent WOLEDs26,27. The simple change of the emitting layer thickness in the WOLEDs with the yellow phosphorescent OLED sandwiched between the DCzIPN TADF blue OLEDs could manage the emission spectrum of WOLEDs from cool white to warm while achieving high quantum efficiency in the hybrid OLEDs Therefore, the combination of the blue TADF device and yellow phosphorescent OLED device was effective to improve the quantum efficiency and manage the color coordinate of WOLEDs. This is the first report achieving above 20% external quantum efficiency in the hybrid white organic light-emitting diodes with both cool and white color.
In conclusion, high efficiency hybrid WOLEDs with a new DCzIPN blue emitter both as a blue TADF emitter and a host for yellow phosphorescent triplet emitter were developed by embedding the yellow phosphorescent emitting layer inside the blue TADF emitting layer. The change of the thickness of the yellow emitting layer could manage the EL emission spectra of the WOLEDs from cool white to warm white. High quantum efficiency of 21.0% with a color coordinate of (0.31, 0.33) was achieved in the cool white color WOLED and high quantum efficiency of 22.9% with a color coordinate of (0.39, 0.43) was obtained in the warm white color WOLED. The quantum efficiency of the hybrid WOLEDs realized in this work was better than that of other hybrid WOLEDs fabricated using fluorescent blue emitting materials and was comparable to that of all phosphorescent WOLEDs. Therefore, the hybrid WOLED with the TADF and phosphorescent emitters can be an alternative for the conventional hybrid WOLEDs and all phosphorescent WOLEDs to enhance the quantum efficiency of the WOLEDs.
Methods
Synthesis of 1,5-Dibromo-2,4-difluorobenzene
A solution of bromine (22.6 g, 142 mmol) in dichloromethane (30 mL) was added dropwisely to a mixture of 1-bromo-2,4-difluorobenzene (25.0 g, 130 mmol), iron power for electrolytic grade (2.0 g) and dichloromethane (30 mL). The reaction mixture was boiled overnight with stirring and then cooled to room temperature. The mixture was poured into a 10% solution of Na2S2O5 (150 mL). The mixture was washed with distilled water and then extracted using dichloromethane (100 ml). The mixture was purified by column chromatography on silica gel using n-hexane as an eluent. The white needle form product was obtained (35.21 g, 94% yield).
1H NMR (200 MHz, CDCl3): δ 7.76 (t, J = 7.3 Hz, 1H), 7.00 (t, J = 8.3 Hz, 1H). MS (FI) m/z 271 [M+].
Synthesis of 4,6-difluoroisophthaonitrile
To a solution of 1,5-dibromo-2,4-difluorobenzene (10.5 g, 38 mmol) in dimethylformamide (80 mL) was added copper(I) cyanide (8.0 g, 88 mmol). The solution was refluxed overnight. The reaction mixture was cooled to room temperature and passed through a filter and then concentrated under reduced pressure. The mixture was extracted with dichloromethane and then purified by column chromatography on silica gel using n-hexane/dichloromethane as an eluent. A white powdery product was obtained (3.2 g, 50% yield).
1H NMR (200 MHz, CDCl3): 8.04 (t, J = 6.8 Hz, 1H), 7.24 (t, J = 8.5Hz, 1H), MS (FI) m/z 164 [M+].
Synthesis of DCzIPN
Sodium hydride (60% in oil, 1.2 g, 24 mmol) was washed with hexane three times and the washed sodium hydride was added to a stirred solution of 9H-carbazole (3.1 g, 18 mmol) in dry tetrahydrofuran (50 mL) under a nitrogen atmosphere at room temperature. After stirring for 30 min, 4,6-difluoroisophalonitrile (1.0 g, 6.1 mmol) was added. The reaction mixture was stirred at room temperature overnight. The reaction was quenched with methanol and water and the solution was washed with distilled water three times. After extraction using dichloromethane, the mixture was purified by column chromatography on silica gel using chloroform/n-hexane as an eluent. The greenish yellow product was purified again by sublimation. A greenish yellow product was obtained (2.3 g, 82% yield).
1H NMR (400 MHz, CDCl3): δ 8.46 (s, 1H), 8.14 (d, J = 7.2 Hz, 3H), 7.94 (s, 1H), 7.50-7.46 (m, 3H), 7.40-7.35 (m, 10H) 13C NMR (100 MHz, CDCl3): δ 145.88, 140.84, 139.73, 129.49, 126.81, 124.78, 122.28, 121.05, 114.30, 111.43, 109.86, MS (FAB) m/z 459 [(M+H)+]. Analysis (calculated for C32H18N4) : C, 83.82; H, 3.96; N, 12.22. Found : C, 83.69; H, 3.96; N, 12.11.
Device fabrication
Device structure of the blue and yellow devices was ITO (150 nm)/PEDOT:PSS (60 nm)/TAPC (20 nm)/mCP (10 nm)/mCP:DCzIPN or DCzIPN:PO-01 (25 nm)/TSPO1 (35 nm)/LiF (1 nm)/Al (200 nm). The doping concentrations of DCzIPN and PO-01 were 5 wt% and 15 wt%, respectively. Hybrid WOLEDs had device structures of ITO (150 nm)/PEDOT:PSS (60 nm)/TAPC (20 nm)/mCP (10 nm)/mCP:DCzIPN (5 nm)/DCzIPN:PO-01 (1.5 nm)/mCP:DCzIPN (23.5 nm)/TSPO1 (35 nm)/LiF (1 nm)/Al (200 nm) (device I) and ITO (150 nm)/PEDOT:PSS (60 nm)/TAPC (20 nm)/mCP (10 nm)/mCP:DCzIPN (13.5 nm)/DCzIPN:PO-01 (3.0 nm)/mCP:DCzIPN (13.5 nm)/TSPO1 (35 nm)/LiF (1 nm)/Al (200 nm) (device II). The doping concentrations of DCzIPN and PO-01 were 5 wt% and 15 wt%, respectively. All devices were prepared by vacuum thermal evaporation process except for the PEDOT:PSS layer which was formed by spin coating process. Doping concentration of the dopant materials was controlled by changing the relative deposition rate of host and dopant materials. After deposition of LiF and Al, the OLEDs were encapsulated inside glove box using a glass cover with a CaO getter attached. The encapsulation glass and the substrate were sealed using epoxy adhesive by ultraviolet curing process.
Measurements
All device performances were measured in ambient condition using the encapsulated devices. Keithley 2400 source measurement unit and CS 1000 spectroradiometer were used for the measurement of current density-voltage-luminance characteristics of the fabricated devices. Quantum efficiency of the devices was calculated by assuming Lambertian distribution of light emission. Transient PL and time resolved PL data were obtained using an optical measurement system equipped with a pulsed Nd-YAG laser (355 nm) and an intensified charge-coupled device (ICCD) detector. Chemical characterization method of the synthesized compound was the same as that reported in our previous work28.
References
Reineke, S. et al. White organic light-emitting diodes with fluorescent tube efficiency. Nature 459, 234–238 (2009).
Sun, Y. R. & Forrest, S. R. High-efficiency white organic light emitting device with three separate phosphorescent emission layers. Appl. Phys. Lett. 91, 263503 (2007).
Schwartz, G. et al. Highly efficient white organic light emitting diodes comprising an interlayer to separate fluorescent and phosphorescent regions. Appl. Phys. Lett. 89, 083509 (2006).
Eom, S. H. et al. White phosphorescent organic light-emitting devices with dual triple-doped emissive layers. Appl, Phys. Lett., 94, 153303 (2009).
Ho, C. L. et al. High-efficiency and color-stable white organic light-emitting devices based on sky blue electrofluorescence and orange electrophosphorescence. Appl. Phys. Lett. 92, 083301 (2008).
Han, C. et al. A Single Phosphine Oxide Host for High-Efficiency White Organic Light-Emitting Diodes with Extremely Low Operating Voltages and Reduced Efficiency Roll-Off. Adv. Mater. 23, 2491-2495 (2011).
Seo, C. W. & Lee, J. Y. High efficiency in two color and three color phosphorescent white organic light-emitting diodes using a 2,7-substituted 9-phenylcarbazole derivative as the host material. Org. Electron. 12, 1459–1464 (2011).
Wang, Q. et al. Highly efficient single-emitting-layer white organic light-emitting diodes with reduced efficiency roll-off. Appl. Phys. Lett. 94, 103503 (2009).
Schwartz, G. et al. Triplet Harvesting in Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 19, 1319–1333 (2009).
Schwartz, G. et al. Harvesting Triplet Excitons from Fluorescent Blue Emitters in White Organic Light-Emitting Diodes. Adv. Mater. 19, 3672–3676 (2007).
Kondakova, M. E. et al. Highly efficient fluorescent-phosphorescent triplet-harvesting hybrid organic light-emitting diodes. J. Appl.Phys. 107, 014515 (2010).
Zheng, C. et al. Novel Efficient Blue Fluorophors with Small Singlet-Triplet Splitting: Hosts for Highly Efficient Fluorescence and Phosphorescence Hybrid WOLEDs with Simplified Structure. Adv. Mater. 25, 2205–2211 (2013).
Sun, Y. R. et al. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature, 440, 908–912 (2006).
Uoyama, H. et al. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 236–240 (2012).
Dias, F. B. et al. Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Adv. Mater. 25, 3707–3714 (2013).
Zhang, Q. et al. Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J. Am. Chem. Soc. 134, 14706–14709 (2012).
Li, J. et al. Highly Efficient Organic Light-Emitting Diode Based on a Hidden Thermally Activated Delayed Fluorescence Channel in a Heptazine Derivative. Adv. Mater. 25, 3319–3323 (2013).
Nakagawa, T., Ku, S.-Y., Wong, K.-T. & Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem. Commun. 48, 9580–9582 (2012).
Tanaka, H., Shizu, K., Miyazaki, H. & Adachi, C. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative. Chem. Commun. 48, 11392–11394 (2012).
Czerwieniec, R., Kowalski, K. & Yersin, H. Highly efficient thermally activated fluorescence of a new rigid Cu(I) complex [Cu(dmp)(phanephos)]+. Dalton Trans. 42, 9826–9830 (2013).
Yersin, H. et al. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 255, 2622–2652 (2011).
Czerwieniec, R., Yu, J. & Yersin, H. Blue-Light Emission of Cu(I) Complexes and Singlet Harvesting. Inorg. Chem. 50, 8293–8301 (2011).
Jou, J. et al. Using light-emitting dyes as a co-host to markedly improve efficiency roll-off in phosphorescent yellow organic light emitting diodes. J. Mater. Chem. 1, 394–400 (2013).
Kim, B. S., Yook, K. S. & Lee, J. Y. Above 20% external quantum efficiency in novel hybrid white organic light-emitting diodes having green thermally activated delayed fluorescent emitter. Sci. Rep. 4, 6019 (2014).
Sun, N. et al. High-Performance Hybrid White Organic Light-Emitting Devices without Interlayer between Fluorescent and Phosphorescent Emissive Regions. Adv. Mater. 26, 1617–1621 (2013).
Wang, Q. et al. Harvesting Excitons Via Two Parallel Channels for Efficient White Organic LEDs with Nearly 100% Internal Quantum Efficiency: Fabrication and Emission-Mechanism Analysis. Adv. Funct. Mater. 19, 84–95 (2009).
Sasabe, H. et al. High-Efficiency Blue and White Organic Light-Emitting Devices Incorporating a Blue Iridium Carbene Complex. Adv. Mater. 22, 5003–5007 (2010).
Cho, Y. J., Yook, K. S. & Lee, J. Y. A Universal Host Material for High External Quantum Efficiency Close to 25% and Long Lifetime in Green Fluorescent and Phosphorescent OLEDs. Adv. Mater. 26, 4050–4055 (2014).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology(2013R1A1A2007991) and Ministry of Science, ICT and future Planning (2013R1A2A2A010674) and development of red and blue OLEDs with external quantum efficiency over 20% using delayed fluorescent materials funded by MOTIE.
Author information
Authors and Affiliations
Contributions
Y.C. synthesized the organic materials used in this work. K.Y. fabricated devices, analyzed the device data of the hybrid devices and designed the device structure of the hybrid devices. J.L. supervised all experiments and prepared the manuscript for submission. All authors reviewed the manuscript and contributed to this work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cho, Y., Yook, K. & Lee, J. Cool and warm hybrid white organic light-emitting diode with blue delayed fluorescent emitter both as blue emitter and triplet host. Sci Rep 5, 7859 (2015). https://doi.org/10.1038/srep07859
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07859
This article is cited by
-
Tuning of the Singlet–Triplet Energy Gap of Donor-Linker-Acceptor Based Thermally Activated Delayed Fluorescent Emitters
Journal of Fluorescence (2024)
-
Recent advances in thermally activated delayed fluorescence for white OLEDs applications
Journal of Materials Science: Materials in Electronics (2020)
-
Strategic-tuning of radiative excitons for efficient and stable fluorescent white organic light-emitting diodes
Nature Communications (2019)
-
Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors
Nature Materials (2019)
-
Highly efficient single-stack hybrid cool white OLED utilizing blue thermally activated delayed fluorescent and yellow phosphorescent emitters
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.