Abstract
Oviposition site-selection in insects is mediated through innate recognition templates (IRTs) tuned to specific chemical cues. These cues aid gravid insects in choosing suitable oviposition sites and may even enhance the fitness of their offspring by warding off predators and parasitoids. However, studies on the evolution of oviposition site-selection and cues instigating oviposition in domesticated insects remain elusive. Using the interaction between the silkmoth, Bombyx mori and its host plant mulberry, Morus alba, as a model system, we demonstrate that centuries of domestication of silkmoth has not impaired its oviposition site-selection function. Silkmoths significantly preferred mulberry leaves to filter paper as oviposition sites. Oviposition assays with filter paper, filter paper treated with leaf volatiles and leaf alone proved that surface texture was not a significant criterion for oviposition site-selection, but volatile cues were. Oviposition assays with electrophysiologically active compounds from mulberry revealed that two of the volatiles, valencene and α-humulene, aided moths in choosing suitable oviposition sites and enhanced egg-laying significantly. Moreover, we show that generalist egg-parasitoids are strongly repelled by valencene and α-humulene. Our results demonstrate that IRTs tuned to cues that aid crucial functions like oviposition site-selection are less likely to be impaired even after centuries of domestication.
Similar content being viewed by others
Introduction
In insects, selecting an appropriate oviposition site is a complex process that involves multiple modalities and is crucial to the fitness of their offspring1,2. As insect eggs are easy targets for predators and egg parasitoids, gravid females must make decisions on the suitability of an oviposition site in order to keep their offspring safe3,4. During egg-laying, insects assess a plethora of factors such as color5, nutrition6, temperature7 and microbial composition1,3. Furthermore, recent studies have shown that insects tend to select oviposition sites that are repulsive to parasitoids and other threats that may harm eggs or neonate larvae3,4. However, it was unclear whether such oviposition site-selection function is still preserved in the highly domesticated insect, Bombyx mori and the current study was designed to test if it was maintained after a long history of domestication. A recent study has shown that domestication has impaired the olfactory system of B. mori so that it is less sensitive to environmental cues8. However, oviposition site selection is an ‘innate behavior’ and usually passed on to offspring from parents over generations9,10.
Humans have domesticated B. mori for nearly 5000 years. This domestication process has degraded many phenotypic and olfactory characters and has made it easier for humans to handle the insect for silk production and breeding processes8. It has also made the silkmoth totally dependent on humans for survival. Although domesticated animals differ from their ancestors, they can still exhibit “intuitive or innate” behaviors. Such innate behaviors may be seen when animals are exposed to specific cues (e.g. olfactory, visual or physical cues) or attain physiological changes (e.g. when an insect becomes gravid)11,12. As adult silkmoths have degenerate mouthparts, the aim of the adults in case of male is finding a mate and in case of female is to find a favorable oviposition site. Although B. mori is highly domesticated, we hypothesised that certain innate behaviors allowing location of suitable oviposition sites may be preserved.
Results
Gravid B. mori prefer mulberry leaves as oviposition site
We first assessed the egg-laying preference of B. mori towards mulberry leaves or its traditional oviposition site, paper, using a binary choice assay. The moths had no restrictions and were allowed to move around freely in the choice arena. In ten replicates, moths consistently preferred and laid most of the eggs on mulberry leaves. Moths (n = 1 per trial, 10 trials, each lasting 24 h) deposited 93.97 ± 3.7% (mean ± s.e.m) of eggs on mulberry leaves, compared to 6.03 ± 0.14% on filter paper (Figure 1a). Silkmoths clearly showed preference for mulberry leaves (Figure 1b) as oviposition sites (Paired t-test; t = 11.82; df = 9; P < 0.0001; n = 10). Presumably, moths may have used volatile cues to navigate towards and oviposit on mulberry leaves. Therefore, we conclude that silkmoth, although domesticated for centuries without access to mulberry plants as oviposition sites, still retain their oviposition site preference. Since the tested moths had no prior experience with leaves, except in their larval stages, we further conclude that the preference for mulberry leaves is innate.
Silkmoths prefer mulberry leaves as oviposition sites.
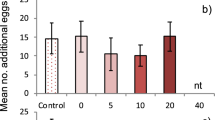
(a) Binary-choice assays revealed that silkmoths preferred to lay most of their eggs on to mulberry leaves than on filter paper. Eggs deposited on mulberry leaves or paper were counted and converted into percentages as represented in the graph. Significant difference was analyzed by paired t-test (P < 0.0001). (b) Set-up of the preliminary binary choice oviposition bioassay and section of eggs laid on leaf is zoomed for better view. (c) Representative EAD response from silkmoth antenna towards volatiles of mulberry leaf Porapak Q elute separated by GC-FID. Seven volatile compounds elicited consistent response (n = 6). (d) Oviposition assay conducted with EAD active fractions pinpointed the efficacy of individual EAD active volatile compounds in eliciting and enhancing oviposition and was determined by oviposition index. Significant differences are denoted by different letters (ANOVA followed by Tukey's test; P < 0.0001). All photos were by V.K.
Specific mulberry leaf volatile compounds induce and enhance oviposition
Moths depend on olfaction to locate mates, food and oviposition sites. As shown in binary choice assays, silkmoths clearly preferred leaves as oviposition sites, suggesting that chemicals present in mulberry leaves are important oviposition cues. Electrophysiology studies revealed that the silkmoth's antenna responded to specific volatile compounds of mulberry leaves. Therefore, we asked which volatile compounds from mulberry might be mediating the oviposition preference in the silkmoth, B. mori. Although intact leaves are virtually odorless to humans, they release volatile plumes that can be detected by insects. GC-EAD results revealed that silkmoths can detect 7 volatile compounds from mulberry leaves (Figure 1c). Individual electrophysiologically active volatiles were further subjected to oviposition assays. Interestingly, the moths showed oviposition preference for certain EAD-active volatiles by laying most of the eggs onto filter papers treated with valencene (OI = 0.57 ± 0.06; mean ± s.e.m) and α-humulene (OI = 0.62 ± 0.01) compared to other cues (figure 1d). ANOVA followed by Tukey's multiple comparison test showed that there was no significant difference between eggs laid on valencene and α-humulene treated filter papers (n = 10; q = 0.8649; P = 0.45). We infer that the presence of valencene and α-humulene is necessary for the increased rate of oviposition as seen on mulberry leaves.
Leaf texture is not a criterion for oviposition site selection
Plant physical cues (leaf texture) can play an important role during oviposition site selection and host acceptance13. Therefore, we conducted an experiment to find whether leaf texture was involved in oviposition site selection. Moths were provided with empty filter paper, filter paper treated with leaf volatiles or mulberry leaf and were allowed to oviposit for a period of 24 h after which the eggs were enumerated. The moths laid a similar number of eggs on filter paper treated with leaf volatiles (662.4 ± 46.96 eggs; mean ± s.e.m) and mulberry leaf (721.7 ± 49.99 eggs) compared to filter paper alone (226.3 ± 33.64 eggs). One-way ANOVA followed by Tukey's multiple comparison test between the number of eggs in different treatments showed that eggs laid on filter paper treated with leaf volatiles and mulberry leaf were significantly not different (Figure 2; n = 10; q = 1.345; P = 0.38). This demonstrates that volatiles from mulberry leaves were sufficient for the oviposition site selection process. We presume that due to many decades of domestication where gravid moths are given only paper as an oviposition substrate, the silkmoth may have lost the ability of detecting physical cues of mulberry leaves. This also proves that insects retain IRTs of olfactory cues (host volatiles) that remain unaltered for generations.
Leaf texture is not a criterion for oviposition site selection.
Silkmoths were presented with either filter paper, filter paper treated with leaf volatiles or leaf itself as oviposition sites to find if a physical cue (leaf texture) is a criterion for oviposition site selection. Error bars represent SEM. Significant differences are denoted by similar letters (ANOVA followed by Tukey's test; P < 0.0001; n = 10).
Specific mulberry volatile protects eggs from parasitoids
Why do silkmoths prefer to lay eggs on mulberry? In evolutionary time, the mulberry silkmoth, B. mori, has specialized on mulberry as its host. Mulberry is detrimental to other insects and mammals that feed on it because they contain polyhydroxylated alkaloids that inhibit alpha-glucosidase14. Alkaloids are sequestered in the body of insect larvae and presumably help them to ward off potential predators15. But, what is the fate of the eggs in the wild? In the wild, egg parasitoids are the major cause of insect egg destruction16. A female insect must find a suitable oviposition site that if free from egg or larval parasitoids17. There are no studies that show mulberry plants as a barrier against egg parasitoids. To investigate if there was any possible barrier in mulberry leaves against egg-parasitoids, we next examined the olfactory behavior of Trichogrammachilonis, a general parasitoid that infests most insects' eggs, using a 4-arm olfactometer assay. First, we provided wasps with mulberry leaf volatiles; surprisingly, wasps were repelled by the leaf volatiles. Wasps spent 0.7 ± 0.2 (mean ± s.e.m) mins and 3.8 ± 0.4 mins in treatment and control arms respectively. Paired t-tests revealed that time spent by wasps in treatment and control was significantly different (t = 5.173, df = 5, P = 0.0035, n = 6). The olfactometer assay results indicated that mulberry leaf volatiles contain compounds that are repulsive to parasitoid wasps (Figure 3). We next wondered whether the repellent activity caused by leaf volatiles was mediated through valencene and α-humulene that triggered oviposition in silkmoths. Provided with a choice between control and the selected volatiles (valencene or α-humulene), wasps spent more time in the control than the treatment arm of the olfactometer. In the olfactometer assay with valencene as the treatment, wasps spent 0.3 ± 0.1 min (mean ± s.e.m) and 2.9 ± 0.1 min in treatment and control arm respectively. A paired t-test showed that the mean time spent was significantly different (t = 8.995, df = 5, P = 0.0003, n = 6). A similar trend was observed in assays with α-humulene as treatment. Wasps spent 0.6 ± 0.4 min in treatment and 2.9 ± 0.1 min in control arms and this difference was significant (t = 3.492, df = 5, P = 0.0174, n = 6). Together the results of the olfactometer assay indicate that wasps clearly avoided valencene and α-humulene (Figure 3). Although these volatiles decrease the probability that T. chilonis parasitises silkmoth eggs when laid in the presence of these volatiles it is possible that other egg parasitoids are less repelled. Using a binary choice assay, we tested this notion. We placed silkmoth eggs (n = 450 for each treatment) on filter paper treated with valencene (100 μl of 0.21 mg/ml), α-humulene (100 μl of 1.73 mg/ml) or control (100 μl of redistilled diethyl ether). The solvent was allowed to evaporate and the eggs were placed on the treated or control filter paper and were immediately exposed to gravid wasps (n = 1000) for 24 h, after which we transferred the exposed eggs into plastic vials. Parasitized eggs turned reddish in color, whereas non-parasitized eggs turned black. Eggs were separated based on the coloration and were held in plastic vials for either parasitoids or silkmoth larvae to emerge. As expected eggs placed on filter paper treated with valencene were significantly less parasitized (Paired t-test, t = 26.97, df = 9, P < 0.0001, n = 10) (8.70 ± 1.82% eggs; mean ± s.e.m) as compared to the control (88.0 ± 2.77% eggs). A similar trend was observed in assays with α-humulene where 9.80 ± 0.74% eggs in treatment and 82.80 ± 2.48% eggs (Paired t-test, t = 25.63, df = 9, P < 0.0001, n = 10) in control were parasitized (Figure 4a & 4b). This experiment demonstrated that parasitization (Figure 4c) in treatments was significantly different from the parasitization in control. A heatmap of EAG response of silkmoths to electrophysiologically active compounds at different concentrations proved that valencene and α-humulene triggered more response than other compounds even at lower concentrations (Figure 4d). Previously, we showed consistent EAG response towards oviposition stimulants at varied concentration in Bactrocera dorsalis9. It seems silkmoths also show a similar response towards crucial oviposition site selection cues. In short, the silkmoth's preference for mulberry leaves as oviposition site is supposedly a repercussion of lowered parasitization risk conferred by specific volatiles present in mulberry leaf volatiles.
Specific mulberry leaf volatiles repel egg parasitoids.
Two oviposition stimulants (α-humulene and valencene) repelled T. chilonis. Mulberry leaf volatiles and two oviposition stimulants (α-humulene and valencene) were presented to egg parasitoids in a 4-arm olfactometer assay. Egg parasitoids were repelled by these oviposition stimulants. Significant difference was analyzed by paired t-test (see Results). Leaf image was taken by V.K.
Oviposition stimulants confer protection against parasitization of silkmoth eggs by parasitoids.
(a) Percentage of eggs parasitized by T. chilonis (egg parasitoid) in the presence of α-humulene in the oviposition substrate (filter paper) was lower compared to control. (b) A similar trend was observed in the presence of valencene (filter paper). Significant difference was analyzed by paired t-test (P < 0.0001). Error bars represent s.e.m (c) A pictorial representation of the general egg parasitoid, T. chilonis, ovipositing on a silkmoth egg (Photo by V.K.) (d) Each EAD active volatiles were puffed over silkmoth antenna at different concentrations and the response was recorded using EAG. The EAG response were recorded and converted into a heatmap. Figure 4d represent heatmap based on dose-response profile of gravid female silkmoth's antenna towards 7 EAD-active volatile compounds (n = 6 per compound).
Discussion
We demonstrated that although domestication for thousands of years has impaired the perception of volatile odors in female silkmoth8, the oviposition site selection behavior mediated by specific host volatile cues is still conserved. The oviposition strategy of an insect is an intricate trade-off between many factors and predation risks to her offspring can have a strong influence. Any wrong move may lower fitness, thus, the female's key goal is to choose an oviposition site that maximizes offspring survival. Discriminating host species is challenging and costly18,19,20,21, it is therefore important for an insect to detect specific chemical information to distinguish suitable from unsuitable oviposition sites22. Our study illustrates that through co-evolution, silkmoths have developed an innate recognition template to specific host volatiles. These volatiles not only instigate oviposition but also aid gravid female silkmoths in selecting suitable oviposition sites. Here, we show that silkmoths prefer mulberry leaves to paper as oviposition sites. This preference is innate and may be mediated through IRTs tuned to specific terpenes present in the headspace volatiles of mulberry leaves. Additionally we show that egg parasitoids are repelled by mulberry leaf volatiles and specifically by two volatile compounds, valencene and α-humulene, present in the headspace of mulberry leaves. Finally, eggs on oviposition substrates treated with valencene or α-humulene suffered reduced parasitization.
A million years of evolutionary pressure is a better teacher than a thousand years of domestication. Through evolutionary time, simple innate behaviors like oviposition site-selection become sophisticated and appear to have a purpose. Such behaviors are products of millions of years of behavioral refinement through natural selection. In this study, we found that the oviposition site-selection behavior in B. mori is not impaired even after 5000 years of domestication. Specific volatile cues from its host plant, mulberry, M. alba may instigate oviposition and aid the moth in finding favorable oviposition sites.
Selecting a suitable oviposition site is a complex behavior where a female insect may use inputs from multiple sensory modalities to make the right decision to maximize the fitness of her progeny. Although decision-making requires complex sensory inputs, our findings suggest that insects make use of dedicated innate recognition templates tuned to specific olfactory cues during oviposition site selection. Innate recognition templates are like a direct relay cable embedded into the genome of an organism during evolution and are passed on from parents to their offspring. Silkmoths prefer to oviposit on mulberry, which has remained its host for millions of years. As there are no B. mori in the wild, it is quite difficult for us to study the evolutionary plant-insect interaction between the silkmoth, B. mori and mulberry, M. alba. Our study gives us an empirical glimpse of how silkmoth may have evolved to choose mulberry as its host and the discovered oviposition stimulants may aid future studies on dedicated neural circuits underlying this innate behavior. Apart, the oviposition stimulants may also be used to enhance egg laying in this economically important insect.
Methods
Insects and chemicals
B. mori (PM × CSR2 hybrid) moths were obtained from a sericulture farm in Bangalore, India. The moths were allowed to mate until they naturally dislodged from their mating position. They were immediately used for bioassays. Trichogramma chilonis wasps were purchased from NBAII, Bangalore, India, cultured on Corcera eggs. Wasps fed with 10% honey solution and water dispensed in cotton pads were maintained in culture chambers at 25°C, 75% RH and 14 D/10 N h photoperiod. Emerged adult wasps were allowed to mate for 24 h before using them for bioassays. Authentic compounds (>95% purity) listed in Supplementary Table S1 (online) were purchased from Sigma Aldrich, St. Louis, MO, USA.
Volatile collection and chemical analysis
Headspace volatiles emitted from mulberry leaves (cv. V1) were collected using a push-pull air-entrainment system. Charcoal-filtered air was pumped into a PET bag containing the leaves at a flow rate of 0.8 L/min and at the same time a portion of the air was pumped out of the bag at a flow rate of 0.5 L/min. The air pumped out was passed through a mini glass column packed with Porapak Q (50 mg) to capture the volatile compounds. Prior to use, Porapak Q columns were washed twice with redistilled diethyl ether and were activated by heating at 100°C for 2 h in a customized dry block heater. Nitrogen gas was passed through the Porapak Q columns during activation. Volatiles of mulberry leaves absorbed on to Porapak Q at 27°C for 12 h were eluted twice with 500 μl of redistilled diethyl ether. The collected volatiles were subjected to GC-MS analysis using a HP 6890 system (Agilent Technologies, USA) coupled with a mass selective detector (HP 5973; Agilent Technologies, USA) operated in electron impact mode (source temperature - 230°C; transfer line temperature - 250°C). HP 5 MS phenyl methyl siloxane non-polar capillary column (Length: 30 m; ID: 0.25 μm) was used. The mobile phase was Helium (99.9% pure; Praxair, India) with a flow rate of 1 ml/min. Split inlet with a split ratio of 50:1 and temperature of 280°C was set before injecting the samples. Oven temperature program was set to 70°C min−1 with 2 min hold and a ramp of 10°C/min till 260°C. The MS detector was maintained at 280°C. Mass spectra of detected compounds were compared using spectral libraries (Wiley 2012 and NIST 2012 versions).
Electrophysiology studies
Studies on electrophysiological activities of Porapak Q elute and individual compounds of mulberry leaves at different concentrations were carried out as described earlier9,10.
Insect behavioral bioassays
Behavioral assays were divided into three parts. First, using binary-choice assay we studied the oviposition preference of B. mori. Second, using EAD we identified electrophysiologically active volatile compounds and their oviposition stimulating activity. Third, we studied if the selected volatile compounds repelled general egg parasitoid, T. chilonis.
Preliminary binary choice oviposition assay
Mulberry leaves or filter paper were used as oviposition substrate to determine the site-selection choice of silkmoths. An arena measuring 30 cm × 20 cm × 15 cm was divided into two equal parts with one part containing filter paper (control) and the other part containing either mulberry leaves or EAD active volatiles. A gravid female silkmoth was placed in the center of the arena and was allowed for 24 h to make a choice. All assays were conducted in a controlled chamber maintained at 25°C, 75% RH and 14 D/10 N h photoperiod. A single insect was used per trial, 10 trials were conducted with each trial lasting for 24 h.
Screening of oviposition stimulants
Using GC-EAD and GC-MS analysis, we identified 7 electrophysiologically active headspace volatiles from mulberry leaves. To test that these compounds function as oviposition stimulants, we conducted binary-choice oviposition assay (see Preliminary binary choice oviposition assay). EAD-active volatiles on filter paper discs (90 mm disc diameter; see Table S1 (online) for test concentration of electrophysiologically active compounds) were presented to gravid silkmoths singly along with a control. Chemical standards of EAD-active volatiles namely cis-hexanol, terpine-4-ol, cis-jasmone, cis-geraniol, α-gurjunene, α-humulene and valencene were screened for their oviposition stimulation activity. Oviposition index (OI) was calculated as described previously9,10. Number of eggs laid and number of eggs retained in the abdomen of silkmoths were recorded. For retained eggs, each silkmoth was dissected after the assay (24 h) and eggs retained in the abdomen were enumerated under a stereomicroscope. To determine that volatile compounds were responsible for oviposition stimulation and not physical cues like leaf texture, an oviposition assay with filter paper, filter paper with headspace volatiles (Porapak Q elute) and leaf were presented to gravid females for 24 h. The number of eggs laid in each treatment was enumerated.
Olfactometer bioassays for parasitic wasps
To test the preference of T. chilonis towards mulberry volatiles, a 4-arm Perspex olfactometer was used (60 mm diameter) to measure behavioral responses of parasitoid wasps to headspace volatiles and EAD active synthetic standards. Glasswares were washed thoroughly with distilled water, rinsed with acetone and oven dried overnight at 150°C. Perspex components were washed thoroughly with distilled water, rinsed with ethanol (70% v/v) and air dried in a clean room. Experiments were conducted in a controlled environment (25°C, 75% RH). The central area of the olfactometer was fitted with a filter paper base (Whatman No. 1; 50 mm diameter) to provide traction for the walking insects. The olfactometer was placed in a black box (60 cm × 60 cm × 60 cm) to remove any external visual stimuli and illuminated from above by a uniform lighting from a diffusing light to provide homogenous lighting. Olfactometer bioassay was conducted as described by Kamala Jayanthi et al. (2012). Briefly, individual gravid female wasps (24–48 h old mated females) were introduced through a hole on top of the olfactometer. The wasps were allowed to acclimatize for 2 min in the olfactometer, after which the experiment was run for 10 min for each replicate. Air was drawn through the central hole at 0.3 l min−1 and subsequently exhausted from the room. The central arena of the olfactometer was divided into four discrete odor fields corresponding to each of four glass inlet arms. Of four glass arms, one contained the treatment and the other three served as controls. Test samples (10 μl; see Materials and Methods) were pipetted onto filter paper strips and the solvent was allowed to evaporate prior to placement into the treatment arm. Filter paper strips with solvent (diethyl ether) served as controls in the remaining three arms. Time spent in each olfactometer arm was recorded with Olfa software (F. Nazzi, Udine, Italy). Six replicates were carried out for each odor source tested.
Egg parasitization bioassay
For egg parasitization bioassay, we used a similar setup as described in preliminary binary-choice oviposition assay. Silkmoth eggs on filter paper (450 eggs each) were placed in control and treatment parts of the arena. The filter paper with eggs on the treated part received volatiles (100 μl of headspace volatiles of mulberry leaves or 100 μl of predetermined concentrations of synthetic volatile compounds; see Method and supplementary Table S1 online). Wasps (1000 mated females) were released into the arena and allowed to parasitize silkmoth eggs for 24 h. Percentage of parasitization was recorded after 7 days of parasitization.
References
Ponnusamy, L. et al. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl. Acad. Sci. U S A. 105, 9262–9267 (2008).
Joseph, R. M., Devineni, A. V., King, I. F. G. & Heberlein, U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. U S A. 106, 11352–11357 (2009).
Stensmyr, M. C. et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357 (2012).
Dweck, H. K. et al. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 23, 1–9 (2013).
Del Solar, E., Guijon, A. M. & Walker, L. Choice of colored substrates for oviposition in Drosophila melanogaster. Bull. Zool. 41, 253–260 (1974).
Schwartz, N. U., Zhong, L., Bellemer, A. & Tracey, W. D. Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS ONE 7, e37910 (2012).
Dillon, M. E., Wang, G., Garrity, P. A. & Huey, R. B. Review: Thermal preference in Drosophila. J. Therm. Biol. 34, 109–119 (2009).
Bisch-Knaden, S., Daimon, T., Shimada, T., Hansson, B. S. & Sachse, S. Anatomical and functional analysis of domestication effects on the olfactory system of the silkmoth Bombyx mori. Proc. R. Soc. B. 281, 20132582 (2014).
Kamala Jayanthi, P. D. et al. Oviposition site-selection by Bactrocera dorsalis is mediated through an innate recognition template tuned to γ-Octalactone. PLoS ONE 9, e85764 (2014).
Kamala Jayanthi, P. D. et al. Specific volatile compounds from mango elicit oviposition in gravid Bactrocera dorsalis females. J. Chem. Ecol. 40, 259–266 (2014).
Kamala Jayanthi, P. D., Woodcock, C. M., Caulfield, J., Birkett, M. A. & Bruce, T. J. Isolation and identification of host cues from mango, Mangifera indica that attract gravid female oriental fruit fly, Bactrocera dorsalis. J. Chem. Ecol. 38, 361–369 (2012).
Kacsoh, B. Z., Lynch, Z. R., Mortimer, N. T. & Schlenke, T. A. Fruit flies medicate offspring after seeing parasites. Science 339, 947–949 (2013).
Calatayud, P. A. et al. Importance of plant physical cues in host acceptance for oviposition by Busseola fusca. Entomol. Exp. Appl. 126, 233–243 (2008).
Konno, K. et al. Mulberry latex rich in antidiabetic sugar-mimic alkaloids forces dieting on caterpillars. Proc. Natl. Acad. Sci. U S A. 103, 1337–1341 (2006).
Conner, W. E. Tiger moths and wooly bears – behavior, ecology and evolution of Arctiidae [Conner, W. E. (ed.)] (Oxford University Press, New York, 2009).
Fatouros, N. E., Dicke, M., Mumm, R., Meiners, T. & Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 19, 677–689 (2008).
Janz, N. [Evolutionary ecology of oviposition strategies]. Chemoecology of insect eggs and egg deposition [Hilker, M. & Meiners, T. (ed.)] [349–376] (Blackwell publication, Berlin, 2002).
Fox, C. W. & Lalonde, R. G. Host confusion and the evolution of insect diet breadths. Oikos 67, 577–581 (1993).
Larsson, S. & Ekbom, B. Oviposition mistakes in herbivorous insects: confusion or a step towards a new host plant. Oikos 72, 155–160 (1995).
Nylin, S. & Janz, N. Oviposition preference and larval performance in Polygonia calbum (Lepidoptera: Nymphalidae): the choice between bad and worse. Ecol. Entomol. 18, 394–398 (1993).
Nylin, S., Bergstrom, A. & Janz, N. Butterfly host choice in the face of possible confusion. J. Insect Behav. 13, 469–482 (2000).
Kotler, B. P. & Mitchell, W. A. The effect of costly information on the diet choice. Evol. Ecol. 9, 18–29 (1995).
Acknowledgements
We thank Mr. Vinay for the B. mori strain (PM × CSR2 hybrid), Mr. Rajanna TS, Miss Nagarathna M for their help with maintaining insects and plants. We thank Mr. Sagar J and Miss Lakshmi HS for their help in conducting olfactometer bioassays. We thank Dr. Toby J.A. Bruce of Rothamsted for proofreading the manuscript. This work was supported by grants from the Indian Council for Agricultural Research's [ICAR] National Fellow Project awarded to K.J.P.D. and research fellowships granted to V.K. and R.M.A.
Author information
Authors and Affiliations
Contributions
K.J.P.D. and V.K. designed the study, V.K., S.B.R. and R.M.A. conducted bioassays and other experiments, V.K., R.K.V. and B.N. designed and conducted electrophysiology study, V.K. wrote the first draft of the manuscript and all authors contributed to the final version of the manuscript. A.V. provided certain instruments. K.J.P.D. and V.K. contributed equally to the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Details of electrophysiologically active volatile fractions from mulberry leaves
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Damodaram, K., Kempraj, V., Aurade, R. et al. Centuries of domestication has not impaired oviposition site-selection function in the silkmoth, Bombyx mori. Sci Rep 4, 7472 (2014). https://doi.org/10.1038/srep07472
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07472
This article is cited by
-
Two odorant receptors regulate 1-octen-3-ol induced oviposition behavior in the oriental fruit fly
Communications Biology (2023)
-
Stage-specific expression of an odorant receptor underlies olfactory behavioral plasticity in Spodoptera littoralis larvae
BMC Biology (2021)
-
Forewarned is forearmed: Queensland fruit flies detect olfactory cues from predators and respond with predator-specific behaviour
Scientific Reports (2020)
-
Induced Plant Defenses Against Herbivory in Cultivated and Wild Tomato
Journal of Chemical Ecology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.