Abstract
The European eel is a highly migratory fish. After the reproduction in the Sargasso Sea early larval-stages start a passive ocean migration towards European and Mediterranean continental waters. After several years as yellow eels, mature adults change to silver stage and then start their return trip. The trajectory of their backward migration is unknown, because of low probability of capturing migrating individuals, having this capture never been reported in the Mediterranean. Recently, 8 silver eels were collected in the Strait of Sicily. Using literature information about possible individual route and speed, their geographical position was projected up to the spawning site during reproductive season. Despite using optimal and continuous migration swimming speed, none of the specimens may have been able to reach the Sargasso Sea in time for mating. Subsequently, to identify putative Mediterranean areas from which eels could have been reaching the spawning grounds on time, a backward scenario was postulated using the previous scientific assumptions. Our results suggests that just a small quota of Mediterranean silver males successfully reaches the Sargasso area and only females from the westernmost and central parts of the basin could be able to fruitfully pond their eggs during the supposed spawning period.
Similar content being viewed by others
Introduction
The European eel (Anguilla anguilla L., 1758) is a catadromous species whose life cycle is considered unique due to the amplitude of the spawning migration. Males mature at a smaller size than females (i.e., at 30–45 cm and 80–180 g versus 60–80 cm and 400–1000 g1. Silver eels (adult life-stage) migrate in autumn from European and North African coastal and inland waters to the Sargasso Sea, where they spawn and die. The resulting larvae (the leptocephali) drift backward to the Atlantic and Mediterranean continental shelf, then metamorphose into glass eels and colonize coastal and inland waters. Older juveniles (yellow eels) undergo a second transformation to adulthood and complete the life cycle by migrating back to the Sargasso Sea, where in late spring they complete the maturation processes, mate, spawn and then die2,3.
The European eel represents an important fishery commodity within its distribution area. Presently, the species is seriously threatened, as inferred from both the sharp recruitment decline, which is now less than 10% of what was observed in the past decades4 and the resulting decrease of fishery yields5. In 2007, the European Union released the Council Regulation 1100/2007/EC, for the recovery of the European eel stock, imposing on each member country to adopt an Eel Management Plan (EMP). A crucial aspect of such plans is to ensure an escapement to sea of at least 40% of the pristine silver eel biomass, i.e., when no human activities affected the fishing area or the eel stock.
Such coordinated management measures have been enforced assuming that, in general, the species is a single and large panmictic population: in fact, the most recent and comprehensive study reported a very low and non significant genetic differentiation between geographic areas across Europe and a total lack of genetic structuring among larvae of European eel collected in the Sargasso Sea6.
That notwithstanding, several authors do not agree, suggesting that Mediterranean individuals may have a significantly reduced role on the actual spawning stock than the European Atlantic ones7,8,9. Since the probability of offshore capture of migrating adults is an extremely rare event (few dozens have been captured in the last 100 years and none in the Mediterranean)9, these hypotheses should be confirmed with the support of both field data and theoretical simulations.
GIS technology offers the possibility of combining biological and spatial information in order to investigate fish movement and habitat use. In analyzing fish migration, the most usual applications of this technique concern the possibility to treat and plot the large amount of data acquired from animal tags and utilize them to generate models for individual species or eventually the whole ecosystems10,11. In addiction some software (e.g., the Animal Movement Analyst Extension, AMAE12 has been especially developed in order to integrate new tools in GIS programs allowing to analyzing tracking data with analysis tools for a large collection of animal movement.
Here, we report the outcomes of geographic simulations performed with GIS software, carried out on 8 adult eels captured offshore in the Strait of Sicily (central Mediterranean), in order to identify putative trajectories and final points of the reproductive migration and verifying if these specimens could have been reaching the spawning grounds on time. Using the most favorable parameters from literature about swimming speed, silver migration time and possible individual routes, our aim is to show if, virtually, all Mediterranean silver male and female eels could successfully reach the Sargasso area in time, or if only a quota of them is able to fruitfully pond their eggs.
Results
Migrating silver eel catches and “forwarded scenario”
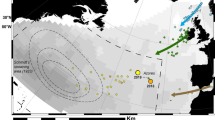
For the first time, 8 silver eels (Tab. 1) were incidentally collected in 5 different occasions on the slopes of the Strait of Sicily (Fig. 1a), by deep-water commercial bottom trawlers that operate in one of the heaviest exploited areas of the world13. The specimens were caught in daylight with only 1-2 specimens per haul (lasting a few hours each).
The eels were determined morphologically and genetically as Anguilla anguilla; the Sr/Ca of the otoliths confirmed that they had lived in freshwater; their muscles still had 30% (dry matter) of lipid reserves13. The eels showed signs of advanced maturation, such as extremely high ocular indexes14 (Fig. 1b).
To assess whether these eels would have been able to join the Sargasso Sea to mate and spawn, their geographical position was projected “forwarded” up to April 1st (Fig. 2), the month when several oceanic campaigns collected the most part of youngest leptocephali2,15, since the exact spawning site has not yet been discovered.
The putative location of the European eels caught in the Strait of Sicily at the forwarded date of April 1st, the time limit for successful spawning.
Individual routes, that are calculated as the shortest possible, are not straight since reflecting the globe curvature. The maps were drawn using the software ArcGIS Desktop: Release 10.
This scenario was built using literature data on speed (0.65 and 0.40 m s−1 for females and males respectively, corresponding to 0.75 and 1.05 body length s−1)16,17, stamina, routes and currents and the resulting most favorable parameters (i.e., continuous swimming, shortest route, etc.). However, none of the Sicilian specimens could have reached the Sargasso Sea on time, missing the spawning site by 5 weeks or more (eel #1 F, 52 d; eel #2 F, 31 d; eel #3 F, 31 d; eel #8 M, 108 d) or - as in the case of eel #4, 5, 6 and 7, fished June 1st - they would have been able to reach the Sargasso Sea only in the next mating season, about 10 months later (Tab. 2).
Backward retrospective scenarios
The question remained whether any other Mediterranean eel could participate in the reproductive event. Therefore, two “backward” scenarios, respectively for silver males and silver females, were built using the previous assumptions and some other ancillary information (e.g., size, latitude, hydrology, meteorology, temperature, all linked to the migration starting time/place and marine currents), to show the areas of the Mediterranean coasts from which eels could have been reaching the spawning grounds on time. Also, we considered a different optimum swimming speed for both sexes16,17. Our retrospective scenario was built with the most favorable assumptions: geographical (direct, shortest routes), temporal (early start of the migration, presence of opposite sex, short hatching lapse) and physiological (continuous swimming, optimum swimming speed, etc.). The results were overlaid on a geographical map using ArcGIS 10 (ESRI. ArcGIS desktop: release 10. Redlands, CA: Environmental Systems Research Institute, 2011).
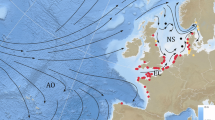
The retrospective scenarios, based on possible/virtual time of departure during the migration seasons (i.e., from inland water) and swimming speed (Fig. 3), reveal that just a small quota of silver males escaping from the Mediterranean (Alboran Sea) could reach the Sargasso area in time to join the breeding event. On the contrary, silver females, with a higher average cruising speed, cover the same distance to the Sargasso Sea in less time and therefore may reach the spawning area, theoretically from all the Mediterranean coastal areas.
Areas from where, depending on the starting period of the catadromic journey, European eels could reach the spawning grounds on time for successful reproduction; the two scenarios (upper panel, ♂; lower panel, ♀) reflect the differences in speed between sexes.
The polygon encompasses the area where small leptocephali [<10 mm] have been collected; eels do not live in Black Sea nor in Red Sea areas. The maps were drawn using the software ArcGIS Desktop: Release 10.
Discussion
The capture of 8 silver eels in deep waters of the Central Mediterranean Sea is a unique event and a fundamental occasion to unveil some aspects of the still unknown life-cycle of one of the most intricate migratory fish of the world.
Apart the unquestionable rarity, the specimens were captured in deep water between 275 and 660 m in daylight. Silver eels are supposed to undertake distinct diel vertical migrations in the water column, in order to travel in warm, shallower waters at night and in the deeper, cooler, disphotic zone during daylight18. Several explanations have been proposed for these daily dives: silver eels may swim into cooler waters in order to delay gonad development until later in the migration18 and also as a consequence of a visual predator-avoidance strategy19. Since continued residence in low temperatures reduces their swimming efficiency, silver eels may require a daily ascent to warmer water to increase metabolic rate and muscle efficiency18,20,21. Besides, Righton et al.22 recently affirmed that Anguilla spp. muscle works well also on depths, since low temperature and high pressure favor aerobic energy production, thus reducing cost of swimming and improving the migratory efficiency.
All sampled individuals yielded a large amount of lipids in the muscular tissues (ca. 30%), a characteristic that in silver eels is crucial for completing the reproductive migration, not only for sustaining long-distance swimming, but also for gonad development and gametes production. In fact, silver eels should have at least 20% (out of total weight) of fat to migrate and reproduce successfully23.
Simulations indicate that none of the captured silver eels (“forwarded” scenario) and only a very low percentage of silver males from the Mediterranean coasts (“backward retrospective” scenario) could reach the spawning area in due time. On the other hand, all female silver eels - even from the easternmost part of the basin - may be able to spawn, on condition that they do not delay up to November the beginning of migration.
In order to synchronize the arrival at a hypothetical breeding ground and maximize reproductive success, males, which have a slower swimming speed, should leave earlier22, or travel a shorter route to fertilize the eggs. However, the migrating time for silver eels to leave the continental domain and start the marine catadromic journey depends on many triggering factors24: the season, the lunar phase, the decreasing temperature, the rain amount and the river water flow. Apart from possible exceptions, the leaving time goes from October to February, with in general a strong peak in November25. The ample temporal “tail” may be due to the actual starting geographical location, which could be far upstream of long, undammed rivers (e.g., the Rhône, the Po, the lower Nile). Furthermore, migration of silver males and females from continental waters starts in general simultaneously1 and the postulated differential migratory start, does not result - at least at Mediterranean latitudes - from data on traditional fisheries landings, where males and females are contemporarily fished26. Differently from the Atlantic and north European countries27, weather conditions postpone the start of the silver eels migration until winter time in the whole Mediterranean basin, especially in eastern areas (A. Goda, Fish Nutrition Laboratory, National Institute of Oceanography and Fisheries, Egypt, personal communication 2011; E. Genc, Department of Fisheries and Aquaculture, Ankara University, Turkey, personal communication 2011). This phenomenon would make ineffective the journey of the majority of Mediterranean silver males (along with eastern females), which may need to leave the shores not later than November. In fact, the specimens caught in June in the Strait of Sicily are probably not stray eels, but may belong to such late, “useless” group.
The most recent sampling campaign in the Atlantic looking for the smallest European eel larvae proved that silver eel spawning period occurs from March through May, with a peak of reproductive activity in April2. Although Schmidt collected intensively in the spawning area in June and July 1920 and June 1922 and captured28 - among a few thousand larger specimens – also about 85 leptocephali <10 mm long in June and another 4 in July, there is little evidence from field studies of spawning in the latter half of the year.
Maes et al.29 found significant genetic differentiation between early and late glass eels recruiting in a Mediterranean coastal lagoon in France. They suggested several alternative hypotheses for such temporal genetic differentiation, among which the existence of two discrete spawning events in the Sargasso Sea. This scenario was depicted also by Wang and Tzeng30 who, studying otoliths of European glass eels, reported a protracted spawning period, but peaking earlier, in January. However, temporal genetic differences could be the consequence of the offspring from two different reproductive years too. Another possible scenario, inducing such patterns, is genetic patchiness31,32,33, at present the most likely hypothesis: the combined effect of an effectively restricted number of parents and differential seasonal survival rates of offspring during the long transoceanic migration that is affected also by fluctuating oceanic condition.
Concerning the duration of their travel towards the Sargasso Sea, endurance and stamina of the migrating eels are critical factors for successfully reaching the breeding place. Their journey was previously believed to last around 6 months, while more recently the estimation fell to just 4 months16. In fact, there are no field data at present concerning the duration of the oceanic catadromic migration. However, since silver eels cease feeding when starting their migration1, they must rely on their energy storage for both swimming and reproduction. In this scenario, it seems probable that they should reach the spawning ground during the first possible mating window, to optimize the use of reserves accumulated during the continental life. An alternative scenario, which seems not valid for the Mediterranean, is that the eels would reach the Sargasso Sea in the following mating season, with a traveling period greater than one year. This hypothesis, of a migrating journey towards the Sargasso Sea longer than one winter, may be used to offer a chance of reproduction also to silver eels from the Mediterranean Sea. Without this assumption, individuals would not be able to reach the reproductive site in the spring. Although it cannot be neither confirmed nor rejected by (inexistent) experimental/field data, a scenario where a migration period longer than the 6–9 months is commonplace seems difficult to accept. Since silver eels cease feeding during the catadromic migration34, in this last situation the limited energy storage of eels must provide not only fuel for swimming and for gonad maturation, but also for routine metabolism35. Given that fat levels in silver eels from many in Mediterranean environments36,37,38 are similar to what estimated to be just enough to successfully complete the reproduction23, this fact seems inconsistent with the possibility of spending more than one year at sea without any energy intake.
Moreover, gonad development must be delayed or even stopped while waiting for the next spawning period. In fact, silver eels descending to deeper waters during the oceanic migration might delay gonad development thanks to temperatures lower than 11°C18,39. On the contrary, the Mediterranean temperatures, unlike those of the Atlantic Ocean, remain largely uniform at around 12.5–14.5°C at all depths below the thermocline (200 m depth40), being thus ineffective for delaying maturation.
It is still uncertain whether eels swim continuously during their migration, whether they rest regularly or whether they profit from the oceanic currents41. There are contrasting data on the daily activity, with some tracking experiments showing uninterrupted swimming42, while other authors observed in shallow waters, just after leaving the coastal environments, only nocturnal swimming, with the animals resting on the bottom at daylight43,44.
About the use of currents to promote migration by increasing swimming speeds, it should be consider that no substantial westward drift exist in the Mediterranean. In the Atlantic two migration routes to the Sargasso Sea have been proposed: a northern migration route via the central Atlantic Ocean45, which is the same hypothesis used in the present research and a southern route via the Azores Current42. In fairness, it should be stated that present knowledge about the Atlantic Ocean phase of the migration is very limited46. Although A. anguilla could potentially make use of the North Equatorial Current (NEC) to facilitate long distance swimming18,47 we decide to consider a northern migration route via the central Atlantic Ocean45 because, according to our aim of understanding the timing of arrival of silver eels, the use of a southern route via the NEC would increase the migration distance41, from Gibraltar to the spawning point, of about 30%, nullifying any possible and difficult to calculate, advantage arising from entering in the NEC.
In conclusion, the annual arrival of larval and glass eels on every coast in the distribution range is proof enough that European eels do overcome all the challenges of their life history, such as synchronicity of arrival and spawning success. That notwithstanding, according to our results, part of the Mediterranean stock may be unable to contribute to the panmixia. This would indicate that A. anguilla distribution may follow a modified single-species Tucker's mechanism, based on swimming energetic arguments48. Accordingly, only a portion of the entire eel population is responsible for replenishing the whole distribution area, in agreement with results showing no evidence for a genetic bottleneck in the European eel. A stable, effective population size of around 3000–12000 individuals, as calculated through forward-time simulations, might be sufficient to sustain the observed levels of genetic diversity49.
As described above in details, our results come from GIS-based theoretic scenarios, rather than mathematical models, which allow, for the first time, to figure out the fate of male and female silver eels leaving the Mediterranean coastal environments towards their reproductive ground in the Sargasso sea. The data and assumption considered for building these scenarios are quite permissive (early start of silver migration, continuous optimal swimming speed, direct routes) in order to adopt a precautionary approach; nonetheless, results point out clues that should be considered not only for further studies of basic biology, but also in the perspective of the complex international framework of policies striving to protect and manage this declining resource. In fact such indications should be confirmed in the near future by additional biological data from both larvae and silver eels catches during the oceanic migration in order to better understand the complex life cycle of this species.
Methods
Biological material
Since the present work did not rely on actual animals, there was no need of approved guidelines for animal welfare by any institutional and/or licensing committee.
All the eels were caught in the Strait of Sicily by commercial bottom trawlers targeting red shrimps, in daylight on grounds 300–650 m deep; fishermen froze the animals without any precise protocol and brought them to the CNR lab in Mazara del Vallo (southern Sicily).
Eels were measured for biometric analyses, sexed histologically, X rayed for vertebral count and their otoliths extracted. Otoliths were ground to thin sections and age was assessed with a light microscope. In order to verify the freshwater history, the sections were also microanalysed for Sr and Ca with quantitative X ray mapping, using a Cambridge S360 SEM connected to an Inca Energy 200 Energy Dispersive Spectrometer. Samples of flesh were analysed for their proximate composition; lipids were extracted and the fatty acids profile determined by gas chromatography. Total genomic DNA was extracted from other flesh samples and mtDNA (cytochrome b) sequences were amplified via PCR; amplification products were finally sequenced and compared with GenBank records.
Mapping
Simulating individual movements on the seascape requires a model which allows both to consider the presence of terrain obstacles and to minimize the distortion introduced by the use of projected spatial data in calculating distances on a geoid surface.
Based on these assumptions we choose a network-based approach in combination with the use of geodetic primitives. All the procedures were implemented using ESRI ArcGIS (rel. 10.1) tools.
Network analysis is a widely applied framework in geography, information technology and computer science. It is primarily concerned with the minimization of transportation costs in the movement of people and goods50 but more recently it was also used to analyze ecological processes concerned with the analysis of potential pathways and connectedness51,52.
According to graph theory53, a network is a lattice consisting of a set of objects, called nodes, with certain pairs of these objects connected by links, called edges54. The links might be of various kinds, representing a logical or a geographical connection between two nodes; commonly in the geographical case they represents the potential movement of an organism among the network and they are associated with an attribute (impedance) representing the cost of passing from a node to another.
A common problem in network analysis is to find the shortest route between two points. The classical solution is performed by using the Dijkstra's55 algorithm. To determine the best path, the algorithm scans the road network for all nodes adjacent to the origin node. All links to these nodes are assessed and the lowest cumulative impedance from the origin is selected at each node until the destination node is reached. When working with networks concentrated in a relatively small area and with an appropriate projected coordinate system that minimizes distance distortion, the Euclidean distance can be used as impedance in order to express the shortest route from a point to another, but in the case of long distances the overall route deviates significantly from the shortest path. In order to minimize this deviation the edges used to build the network need to be geodetic vectors representing the shortest line linking two points in the space considering earth curvature56.
In our case we built a network dataset representing the area of Mediterranean and Sargasso Seas. Initially a grid of points, placed at different distances ranges from 5 to 15 km, was positioned in the geographical space representing the study area, created by masking all land features obtained from a world vector map57; then each point was connected with its 8 neighbours using geodetic vectors and for each of them the corresponding geodetic distance was calculated using Vincenty's equations58.
Forwarded eels scenario
The first GIS scenario (Fig. 2) was built in order to identify the potential geographic positions in the study area where each eel, starting from its catch point, could have been found during the migration to the spawning site.
The calculation has been performed by first identifying for each individual the shortest route from the original positions to the spawning point and then calculating the accumulated geodetic distance that it could cover during the considered time period according to its sex, size and swimming velocity.
All the paths were determined by using the network analyst tool (NAT) available in ArcGIS and then smoothed in order to assign a more realistic shape. The starting points for each path are the catch locations and the arrival point the site where the aggregations of larvae appear as the densest (26°N, 67.5°W)59. That location was indentified during several scientific cruises over the wide area of the Sargasso Sea (23–30°N, 48–74°W) since 191160.
Our computations took into account the date of the April 1th as theoretical arrival date of silver eels to the spawning site. Evidence from sampling in the Sargasso Sea for leptocephali of European eels, that has occurred over different seasons, decisively suggests a restricted spawning season, to a few months mainly in late winter and spring2,3,28,58,59,60,61.
Eels geographic positions were “forwarded” up to April 1st, using the shortest routes from each catch point to the spawning site and considering different continuous cruise swimming speed for each sexes. Experimental studies on swimming performance and energy consumption simulating the migration in swim tunnel (Blazka type 127-l) established that A. anguilla is very efficient endurance swimmer by estimating the optimum swimming speed, i.e., the speed at which the cost of transport (mg O2 kg−1 km−1) is minimal, finding a mean value for wild female silver eels around 0.65 m s−1 (or, equivalently, 0.75 body length s−1)16 and for farmed silver males of 0.40 m s−1 (or, equivalently, 1.10 body length s−1)17. These values, at least for females, agrees with the sustained swimming speed cruise currently observed in field tracking studies on migrating females1,19,20,41,62, in particular when considering the fastest measured records (even considering that swimming speeds might have been impaired by the tag burden)63,64.
Backward retrospective scenarios
The second GIS scenario (Fig. 3) was a “backward” displacement computation, which identified the corresponding potential period of starting migration time from each zone in the study area of the southern distribution area of A. anguilla. The computation performed assumes that mature individuals should arrive in the spawning site (26°N, 67.5°W) at the Sargasso Sea on the following April 1st, with respect of the time of departure from Mediterranean coastal environments. Also in this case, two different swimming speeds were considered, using published speed data (0.65 and 0.40 m s−1 for females and males, respectively)16,17.
The calculation was performed in two steps. Initially a distance map of the study area was produced by generating a raster layer where each cell represents the geodetic distance from the spawning site. Geodetic distances were computed using iteratively NAT tools, considering as starting point the spawning site and as arrivals a grid of points spaced from 30 to 45 km. In this way we obtained about 12,000 routes representing the optimal paths from the spawning site to a series of location equally distributed on the study area. For each route the accumulated geodetic distance was calculated and the obtained value was assigned to the corresponding point. Finally, the point layer was converted in raster format by interpolating point values with a spline algorithm.
The distance map was then used to produce the two backward scenarios, respectively for males and females, by calculating for each cell the corresponding period of migration considering the two different optimum swimming speeds. Finally, the two raster maps were classified in monthly ranges, showing in the study area the time period when an eel should have been starting its migration in order to reach the spawning point on April 1st.
References
Tesch, F. W. The eel. [Thorpe J.E. (ed.)] (Blackwell Science, Oxford, 2003).
McCleave, J. D. Contrasts between spawning times of Anguilla species estimated from larval sampling at sea and from otolith analysis of recruiting glass eels. Mar. Biol. 155, 249–262; 10.1007/s00227-008-1026-8 (2008).
van Ginneken, V. J. T. & Maes, G. E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev. Fish Biol. Fish. 15, 367–398; 10.1007/s11160-006-0005-8 (2005).
ICES/EIFAC. Report of the 2007 Session of the Joint EIFAC/ICES, Working Group on Eels, FAO European Inland Exploration of the Sea, Bordeaux, 3-7 September 2007. EIFAC Occasional Pap. 39, ICES CM 2007/ACFM:23, 524 pp (2007).
ICES/EIFAC. Report of the 2006 session of the Joint EIFAC/ICES Working Group on eels (Rome 23-27 January 2006). EIFAC Occasional Pap. 38 (2006), ICES CM2006/ACFM:16, FAO (Rome) & ICES (Copenhagen).
Als, T. D. et al. All roads lead to home: panmixia of European eel in the Sargasso Sea. Mol. Ecol. 20, 1333–1346; 10.1111/j.1365-294X.2011.05011.x (2011).
Belpaire, C. et al. Decreasing eel stocks: survival of the fattest? Ecol. Freshw. Fish 18, 197–214; 10.1111/j.1600-0633.2008.00337.x (2009).
Kettle, A. J., Bakker, D. C. E. & Haines, K. Impact of the North Atlantic oscillation on the trans-Atlantic migrations of the European eel (Anguilla anguilla). J. Geophys. Res. 113, 1–26; 10.1029/2007JG000589 (2008).
Kettle, A. J., Asbjørn Vøllestad, L. & Wibig, J. Where once the eel and the elephant were together: decline of the European eel because of changing hydrology in southwest Europe and northwest Africa? Fish Fish. 12, 380–411; 10.1111/j.1467-2979.2010.00400.x (2011).
Hooge, P. N., Eichenlaub, W. M. & Solomon, E. K. [Using GIS to Analyze animal movements in the marine environment]. Spatial processes and management of marine populations [Kruse, G.H. et al. (eds.)] [37–51] (Alaska Sea Grant College Program, Anchorage, 2001).
Block, B. A. et al. Migratory movements, depth preferences and thermal biology of Atlantic bluefin tuna. Science 293, 1310–1314 (2001).
Erisman, B. et al. Spatio-temporal dynamics of a fish spawning aggregation and its fishery in the Gulf of California. Scientific Reports 2, 284 (2012).
Bianchini, M. L. et al. European eels from deep Mediterranean waters. Am. Fish. Soc. Symp. 69, 871–874 (2009).
Pankhurst, N. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 21, 127–140 (1982).
Sparholt, H., Sounds of the Sargasso Sea (2014). (Date of access: 05/05/2014). http://www.ices.dk/Forum/Sargasso%20Sea/_layouts/15/start.aspx#/default.aspx.
Palstra, A. P., van Ginneken, V. & van den Thillart, G. Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp. Biochem. Physiol. Part A 151, 37–44; 10.1016/j.cbpa.2008.05.011 (2008).
Burgerhout, E. et al. Schooling reduces energy consumption in swimming male European eels, Anguilla anguilla L. J. Exp. Mar. Biol. Ecol. 448, 66–71; 10.1016/j.jembe.2013.05.015 (2013).
Aarestrup, K. et al. Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325, 1660; 10.1126/science.1178120 (2009).
Jellyman, D. & Tsukamoto, K. First use of archival transmitters to track migrating freshwater eels Anguilla dieffenbachii at sea. Mar. Ecol. Prog. Ser. 233, 207–215 (2002).
Jellyman, D. & Tsukamoto, K. Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii. Mar. Ecol. Prog. Ser. 286, 261–267 (2005).
Scaion, D. & Sébert, P. Glycolytic fluxes in European silver eel, Anguilla anguilla: sex differences and temperature sensitivity. Comp. Biochem. Physiol. Part A 151, 687–90 (2008).
Righton, D. et al. The Anguilla spp. migration problem: 40 million years of evolution and two millennia of speculation. J. Fish Biol. 81, 365–386; 10.1111/j.1095-8649.2012.03373.x (2012).
van den Thillart, G. E. E. J. M., Palstra, A. P. & van Ginneken, V. Simulated migration of European silver eel: swim capacity and cost of transport. J. Mar. Sci. Technol. 15, 1–16 (2007).
Haro, A. [Downstream migration of silver-phase anguillid eels]. Eel Biology [Aida, K., Tsukamoto, K. & Yamauchi, K. (eds.)] [215–221] (Springer, Tokyo, 2003).
van Ginneken, V. J. T. et al. Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Anim. Biol. 57, 63–77 (2007).
Ciccotti, E. [Interactions between capture fisheries and aquaculture: the case of the eel (Anguilla anguilla L., 1758)]. Interactions between aquaculture and capture fisheries: a methodological perspective [Cataudella, S., Massa, F. & Crosetti (eds.)] [190–203] (FAO, Rome, 2005).
Tsukamoto, K. Oceanic migration and spawning of anguillid eels. J. Fish Biol. 74, 1833–1852; 10.1111/j.1095-8649.2009.02242.x (2009).
Boëtius, J. & Harding, E. F. A re-examination of Johannes Schmidt's Atlantic eel investigations. Dana 4, 129–162 (1985).
Maes, G. E., van Vo, B., Crivelli, A. J. & Volckaert, F. A. M. Morphological and genetic seasonal dynamics of European eel Anguilla anguilla recruitment in southern France. J. Fish Biol. 74, 2047–2068; 10.1111/j.1095-8649.2009.02294.x (2009).
Wang, C. H. & Tzeng, W. N. The timing of metamorphosis and growth rates of American and European eel leptocephali: a mechanism of larval segregative migration. Fish. Res. 46, 191–205 (2000).
Hedgecock, D. Temporal and spatial genetic-structure of marine animal populations in the California current. Cal. Coop. Ocean. Fish. 35, 73–81 (1994).
Pujolar, J. M., Maes, G. & Volckaert, F. Genetic patchiness among recruits in the European eel Anguilla anguilla. Mar. Ecol. Prog. Ser. 307, 209–217 (2006).
Pujolar, J. M., Maes, G. E. & Volckaert, F. A. M. Genetic and morphometric heterogeneity among recruits of the European eel, Anguilla anguilla. B. Mar. Sci. 81, 297–308 (2007).
Sébert, P., Scaion, D. & Belhomme, M. High hydrostatic pressure improves the swimming efficiency of European migrating silver eel. Respir. Physiol. Neurobiol. 165, 112–114 (2009).
Boetius, I. & Boetius, J. Experimental maturation of female silver eels, Anguilla anguilla: estimates of fecundity and energy reserves for migration and spawning. Dana 1, 1–28 (1980).
Bettinetti, R. et al. Use of Anguilla anguilla for biomonitoring persistent organic pollutants (POPs) in brackish and riverine waters in central and southern Italy. Water, Air, Soil Poll. 217, 321–331; 10.1007/s11270-010-0590-y (2010).
Ferrante, M. et al. Polychlorinated biphenyls and organochlorine pesticides in European eel (Anguilla anguilla) from the Garigliano river (Campania region, Italy). Chemosphere 78, 709–716 (2010).
Quadroni, S. et al. Contamination, parasitism and condition of Anguilla anguilla in three Italian stocks. Ecotoxicology 22, 94–108; 10.1007/s10646-012-1006-0 (2012).
Boetius, I. & Boetius, J. Studies in the European eel, Anguilla anguilla (L.): experimental induction of male sexual cycle, its relation to temperature and other factors. Medd. Danm. Fisk Havunders NS 4, 339–415 (1967).
Hopkins, T. S. Physics of the sea. Key environments: Western Mediterranean [Margalef, R. (ed.)] [100–125] (Pergamon Press, New York, 1985).
Fricke, H. & Kaese, R. Tracking of artificially matured eels (Anguilla anguilla) in the Sargasso Sea and the problem of the eel's spawning site. Naturwissenschaften 82, 32–36 (1995).
Tesch, F. W. Changes in swimming depth and direction of silver eels (Anguilla anguilla L.) from the continental shelf to the deep sea. Aquat. Living Resour. 2, 9–20 (1989).
Westerberg, H., Lagenfelt, I. & Svedang, H. Silver eel migration behaviour in the Baltic. ICES J. Mar. Sci. 64, 1457–1462 (2007).
Davidsen, J. G. et al. Early marine migration of European silver eel Anguilla anguilla in northern Norway. J. Fish Biol. 78, 1390–1404 (2011).
Tesch, F. W. Der Aal als Konkurrent von anderen Fischarten und von Krebsen. Oesterr. Fisch. 39, 5–20 (1986).
Clevestam, P. D., Ogonowski, M., Sjöberg, N. B. & Wickström, H. Too short to spawn? Implications of small body size and swimming distance on successful migration and maturation of the European eel Anguilla anguilla. J. Fish Biol. 78, 1073–89 (2011).
Knights, B. A review of the possible impacts of long-term oceanic and climate changes and fishing mortality on recruitment of anguillid eels of the northern hemisphere. Sci. Total Environ. 310, 237–244 (2003).
Tucker, D. W. A new solution to the Atlantic eel problem. Nature 183, 495–501 (1959).
Pujolar, J. M. et al. No apparent genetic bottleneck in the demographically declining European eel using molecular genetics and forward-time simulations. Conserv. Genet. 12, 813–825; 10.1007/s10592-011-0188-y (2011).
Menesatti, P. et al. Cost and waste comparison of reusable and disposable shipping containers for cut flowers. Packag. Technol. Sci. 25, 203–215 (2012).
Halpin, P. N. & Bunn, A. G. Using GIS to compute a least-cost distance matrix: a comparison of terrestrial and marine ecological applications. Paper presented at the 20th Annual Esri User Conference, San Diego. Esri press. (2000, June 26–30).
Urban, D., & Keitt, T. Landscape connectivity: a graph-theoretic perspective. Ecology 82, 1205–1218 (2001).
Harary, F. Graph theory (Addison-Wesley, Reading, 1969).
Easley, D. & Kleinberg, J. Networks, crowds and markets: reasoning about a highly connected world (Cambridge University Press, 2010).
Dijkstra, E. W. A note on two problems in connexion with graphs. Numer. Math. 1, 269–271 (1959).
Raynaud, X. [The shortest path between two points, geodesics and mechanics] Trasference. Interdisciplinary Communications 2008/2009 (Centre for Advanced Study, Oslo, 2010).
Global Administrative Areas. GADM database of Global Administrative Areas, version 2.0. [online]. (2012). (Date of access: 28/04/2014). www.gadm.org
Vincenty, T. Direct and inverse solutions of geodesics on the ellipsoid with application of nested equations. Surv. Rev. 23, 88–93 (1975).
McCleave, J. D., Kleckner, R. & Castonguay, M. Reproductive sympatry of American and European eels and implications for migration and taxonomy. Am. Fish. Soc. Symp. 1, 286–297 (1987).
Schmidt, J. The breeding places of the eel. Philos. T. Roy. Soc. B. 211, 179–208 (1923).
Tesch, F. W. & Wegner, G. The distribution of small larvae of Anguilla sp. related to hydrographic conditions between Bermuda and Puerto Rico. Int. Rev. Gesamten Hydrobiol. 75, 845–858 (1990).
McCleave, J. D. & Arnold, G. P. Movements of yellow and silver phase European eels (Anguilla anguilla L.) tracked in the western North Sea. ICES J. Mar. Sci. 56, 510–536 (1999).
Burgerhout, E. et al. Dramatic effect of pop-up satellite tags on eel swimming. Naturwissenschaften 98, 631–634; 10.1007/s00114-011-0805-0 (2011).
Methling, C., Tudorache, C., Skov, P. V. & Steffensen, J. F. Pop up satellite tags impair swimming performance and energetics of the European eel (Anguilla anguilla). PLoS ONE 6, e20797; 10.1371/journal.pone.0020797 (2011).
Acknowledgements
Thanks to the skippers of the Mazara fleet who provided the eels; to E. Ciccotti of University of Roma “Tor Vergata” for framing indications and concepts; to L. Sola of University of Roma I “Sapienza” for the DNA analysis; to G. Vaggelli of IGG-CNR for the otolith microchemistry; to G.B. Palmegiano of ISPA-CNR for the lipids determinations.
Author information
Authors and Affiliations
Contributions
All the authors wrote the main manuscript text. F.C., C.C., E.C. and M.L.B. analyzed data. M.L.B., S.R. and E.C. prepared figure 1, E.C. prepared figure 2 and 3. F.C., J.A., M.L.B., F.A., S.R. and C.C. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Capoccioni, F., Costa, C., Canali, E. et al. The potential reproductive contribution of Mediterranean migrating eels to the Anguilla anguilla stock. Sci Rep 4, 7188 (2014). https://doi.org/10.1038/srep07188
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07188
This article is cited by
-
Estimation of the spawning time of Japanese eels in the open ocean
Scientific Reports (2020)
-
First evidence of European eels exiting the Mediterranean Sea during their spawning migration
Scientific Reports (2016)
-
Selection of best-performing reference gene products for investigating transcriptional regulation across silvering in the European eel (Anguilla anguilla)
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.