Abstract
The innovative design and synthesis of nanofiber based hydro-philic/phobic membranes with a thin hydro-phobic nanofiber layer on the top and a thin hydrophilic nanofiber layer on the bottom of the conventional casted micro-porous layer which opens up a solution for membrane pore wetting and improves the pure water flux in membrane distillation.
Similar content being viewed by others
Introduction
Membrane distillation (MD) has not been widely used for a large scale industrial operation despite the fact that it was introduced in 19631, due to the limitation of the membranes. Generally, MD membranes should be hydrophobic and microporous with high liquid entry pressure. The development of MD membranes with higher flux has been a primary goal of MD research while another important goal is the reduction of pore-wetting. As well. energy consumption can be lowered by designing membranes of reduced temperature polarization and heat conduction. Pore-wetting is caused by penetration of feed liquid into the membrane pores or by vapor condensation in the pores, which leads to flux decay and salt deposition2,3,4.
In order to enhance the membrane performance, several MD membrane designs have been proposed based on the concept of single layer membrane and dual layer membrane. Composite dual layer MD membranes consisting of a hydrophobic top layer and a hydrophilic sub layer were patented in the 1980s by Cheng and Wiersma5,6,7. While hydrophilic layer is wetted with liquid (normally water), transport of vapor takes place the hydrophobic layer. The higher mass transport in the MD by using the dual layer membrane is due to the shorter path required for the vapor to move between the liquid/vapor interfaces8,9,10.
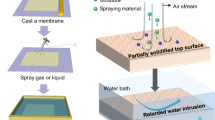
Much research has focused on improving MD membranes by increasing its hydrophobicity. Recently, the technique of electro-spinning has been harnessed to produce nanofiber mats and their use as MD membranes have been proposed11,12,13. These mats typically have a high porosity and a highly hydrophobic surface, which make them ideal for MD membranes. However, the liquid entry pressure of water (LEPw) of these nanofiber mats is usually low, which might suggest a higher propensity of pore wetting over time. A recent work showed that a PVDF nanofiber membrane of thickness 0.3 mm had a LEPw of only 90 kPa and salt passage through the membrane was observed when operated in direct contact MD (DCMD) mode after 1 h12. However, it was possible to improve this layer further and by controlling the operating conditions a prolonged use of at least 15 h with little change in the permeate quality was possible despite a LEPw of only 35 kPa13. However, these nanofiber membranes could potentially encounter pore wetting issues during air gap MD (AGMD) and vacuum MD (VMD), due to the large pressure difference across the membrane and high feed water velocities14.To further enhance the performance of the nanofiber membranes and the conventional dual layer membranes for MD use, we propose a triple layer nanofiber based membrane fabricated by a combination of wet casting and electro-spinning. Fig. 1 shows schematically the structure of the triple layer membrane.
Results
The average water contact angles of the membranes are presented in Table 1 together with the error range. Comparing the contact angles of the top surface (CAwT) of the intermediate layers whose thickness is 100 µm (from S1 to S4), the contact angle of the top surface increased with an increase in SMM(hydrophobic) content from S1 (control, SMM(hydrophobic) = 0 wt.%) of 84.6 ± 3.4° to S4 (SMM(hydrophobic) = 3 wt.%) of 120 ± 3.5°. Thus, the addition of SMM(hydrophobic) effectively modified the top surface of the intermediate layer and made it 42% more hydrophobic. After coating the top surface of the intermediate membrane with PVDF nanofiber, one of the membranes (S6) showed a further increase in CAwT up to 143 ± 2.7°, which is 69% higher than the control membrane S1. It has already been proven that the PVDF nanofiber membrane has a very high contact angle and it repels liquid water from its surface due to low surface energy and its structure11,12,13,15,16,17. In a recent study it was revealed that nano or hierarchical structured membrane surfaces are unstable and easy to roll off the water droplets16. Regarding the bottom surface of the intermediate layer, the increase in the contact angle (CAwB) was only 10% from S1 to S4. When the bottom surface was coated with PVDF-SMM(hydrophilic) nanofiber mat the contact angle decreased remarkably to 21.5 ± 4.8°, which is 74.5% lower than the control S1 membrane. Thus, the bottom layer which is far more hydrophilic than the intermediate layer will help to absorb the condensed water molecule from the middle layer, thus preventing the pore wetting2,3,4,17,18,19. Fig. 2 and Fig. 3 shows, summarily, how the contact angles of the top and the bottom layer changed from the control S1 membrane to those of the very top and very bottom of the triple layer S7 membrane.
Table 1 also shows average LEPw of the membranes together with its error range. Comparing the LEPw, the single layer membrane for which the thickness was maintained at 100 µm, LEPw increased from 220 kPa of S1 to 400 kPa of S4 with an increase in the amount of SMM(hydrophobic) added, which is 81% increase. The increase in LEPw thus parallels to the increase in contact angle (hydrophobicity) of the top membrane surface15,17. According to YL equation, the LEPw of the novel triple layer membrane is supposed to be around ~140% higher than the casted membrane due to the addition of the highly hydrophobic nanofiber layer. When the LEPw of the triple layer membrane is compared with the control membrane, the increase is not so spectacular. However, as shown in Fig. 2, LEPw increased from 220 kPa to 300 kPa, which is only 36% increase. Nevertheless, the latter LEPw allowed the use of S7 membrane even for the long term testing14,15,17.
The mean surface pore size of the middle casted layer is slightly decreased with the increase of SMM concentration; This may be due to the increased viscosity of the casting solution23,24. The pore size for sample S1 is 0.17 ± 0.06 and for sample S4 the pore size is 0.08 ± 0.03. In addition to the contact angle, this little reduction in pore size will also help the membrane to have a higher LEPw.
SEM images of S2 to S6 are shown Fig (ESI†). The cross-sectional SEM image of the triple layer membrane (S7) is shown in Fig 3 together with the top surface view of the PVDF nanofiber coated top layer, top hydrophobic side of the intermediate layer and the PVDF-SMM (hydrophilic) coated bottom layer. It should be noted that the membrane was flipped upside down, so the top side of the triple layer membrane appears at the bottom and the bottom side appears at the top in Fig. 3. Thus, the intermediate cast membrane is sandwiched between the two nanofiber layers. The top layer, appearing at the bottom, has a thickness of 30 ± 5 μm and the bottom layer appearing at the top has a thickness of 20 ± 5 μm. Including the sandwiched layer thickness of 50 ± 10 μm, the total thickness of the triple layer membrane becomes 100 μm. Some of the previous works showed that the optimum thickness of the hydrophobic selective layer in the dual layer membrane should be in the range of 25–55 μm9,18. The thickness of 30 μm for the electro-spun nanofiber top layer is at the lower range of these values. Each layer within the triple layer configuration serves a specific function. The top selective layer consisting of electro-spun PVDF nanofibers, which are highly hydrophobic are expected to prevent liquid water from entering from the feed side into the membrane pores. The high porosity (70–90%) of the top nanofiber layer is postulated to reduce the heat loss across the membrane and be saturated with water vapor. The intermediate layer will help increase the LEPw of the membrane by narrowing the pore size and the bottom SMM(hydrophilic) layer will help to draw water vapor from the middle layer by absorption19,20,21. This will also help to remove the condensed vapor (liquid water molecules) from the intermediate layer, reducing the chance of pore wetting2,3,4,19,20,21.
All the prepared membranes were tested for DCMD and the results of S1, S3, S5, S6 and S7 were chosen and their performance data are shown in Fig. 4, in which the feed solution temperature was changed from 50 to 80°C. S1 is the control membrane. S3 and S5 are the single layer membranes from which S6 and S7 triple layer membranes were made, respectively, by coating nanofiber layers. Note that the thickness of S5 is one half of S3. (The data of S1, S2, S3 and S4 that were prepared by the phase inversion method are shown in Fig. 6 of ESI.)
The following trends in the DCMD flux Jw are clearly observed.
-
1
The control memrane shows the lowest flux.
-
2
S5's flux is higher than S3. This is because the thickness of S5 is a half of S3.
-
3
S6's flux is higher than S3 and S7's flux is higher than S5, despite the increase in total thickness due to the addition of the coated nanofiber layers. As a result, one of the triple layer membrane (S7) showed about 6 fold increase in the DCMD flux compared to the control S1 membrane. Thus, sandwiching the cast intermediate layer by two layers of hydrophobic and hydrophilic nanofibers increased the DCMD flux enormously.
-
4
There is an exponential increase of the flux with an increase in feed temperature. This is due to the vapor pressure increase of water. On the other hand, the average salt rejections of all five membranes were more than 99% as shown in Fig. 5b.
A long term DCMD experiment was carried out (ESI†) for the control sample (S1) and the best performing triple layer membrane (S7). The warm feed side temperature was kept between 70°C and 80°C while the cold side temperature was kept at 17 ± 2°C. The DCMD flux and the salt rejection of both membranes are shown in Figs. 6a and 6b, respectively. The initial flux of S7 membrane is about 6 fold as high as S1, as mentioned earlier. S7 maintained the stable flux of 50 to 70 L/m2h during the entire operation period of 97 h, while the flux of S1 membrane decreased from about 10 to 7 L/m2h after 35 h where the experiment was stopped. Most strikingly, the salt rejection of S7 membrane remained above 99% over the entire period of operation, while that of S1 membrane went down steeply after 15 h.
It is important to note that the triple layer membrane can prevent pore wetting in MD by adjusting the membrane surface chemistry and engineering. It is proven that the highly hydrophobic PVDF nanofiber layer is repelling liquid and the highly hydrophilic PVDF-SMM nanofiber is absorbing the water molecule (condensed vapor) from the intermediate layer, which enhances the vapor flux across the membrane. Based on these results, it is concluded that the triple layer composite membrane is a solution for pore wetting in membrane distillation.
Discussion
In this paper, a novel nanofiber based triple layer hydrophilic/-phobic membrane is synthesized and experimentally tested for desalination of a synthetic salt solution using direct contact membrane distillation (DCMD). The triple layer membrane consists of a poly(vinylidene fluoride) (PVDF) nanofiber top layer, a microporous PVDF intermediate layer produced by conventional phase inversion technique and a nanofiber bottom layer electro-spun from PVDF/hydrophilic surface modifying macromolecule (SMM(hydrophilic)) blend. These three layers are bound to each other by heat pressing. The unique triple layer membrane was characterized thoroughly. The top side contact angle of the triple layer membrane is around 69% higher than the control PVDF membrane prepared by the phase inversion method. Similarly, the contact angle on the bottom side is 74.5% lower than the control membrane. These differences in contact angle enhance the driving force for the vapor transport. The liquid entry pressure of the triple layer membrane was found to be 27% higher than the control membrane. The DCMD flux of the triple layer membrane was found to be around 6 times (600%) higher than that of the control membrane while the salt rejection was > 99.9%. Most importantly, the triple layer membrane could be operated continuously for more than 95 h without any significant change to permeate quality, whereas the control membrane could be operated only for 15 h before pore wetting occurred. Based on the results it is proposed that the triple layer configuration with nanofiber based selective layers could be a promising solution for pore wetting issue in the MD processes.
Methods
Materials and chemicals: Poly(vinylidene fluoride) (PVDF) Kynar® 761 grade with a melting point of 165–172°C was purchased from Arkema Pte. Ltd., Singapore. Poly(vinyl pyrrolidone) (PVP) K17 technical grade was purchased from Shanghai Welltone Material Technology Co. Ltd., Shanghai, PR China. Methylene bis(p-phenyl isocyanate) (diphenylmethanediisocyanate, MDI, 98%), polyethylene glycol (PEG, typical Mn200 Daltons), poly(propylene glycol) (PPG, typical Mn 425 Daltons) were supplied from by Sigma-Aldrich, Inc., St. Louis, MO, USA. Zonylfluorotelomer intermediate, 2-(Perfluoroalkyl)ethanol (oligomeric fluoro-alcohol, OFA, BA-L of average Mn 443 Daltons and 70 wt% fluorine) is a DuPont product supplied by Aldrich Chemical Company, Inc., Milwaukee, WI, USA. The details of SMM synthesis, characterization and function are referred our earlier research25,26,27,28,29. Ethanol, acetone, N,N′-dimethyl acetamide (DMAc) and sodium chloride were analytical grade from Sigma, Singapore. The water used was distilled and de-ionized (DI) with a Milli-Q plus system from Millipore, Bedford, MA, USA.
The method of the triple layer membrane fabrication is as follows.
-
1
The intermediate layer was fabricated by the conventional phase inversion technique, to prepare the casting dope, PVDF was dissolved in DMAc/ethanol solvent mixture and PVP was added as pore former. Furthermore, a small amount (0–3 wt%) of SMM(hydrophobic) was added to modify the membrane surface. The dope compositions are given in Table 2 for 5 such membranes. It should be noted all of these membranes were used as single layer membranes. But two of them S3 and S5 were further subjected to nanofiber coating to fabricate triple layer membranes. The polymer and the additives were added into the mixed solvent in a RB flask and the mixture was stirred at 250 ~ 350 rpm for at least 24 h at 80°C to make the polymer completely dissolved. The polymer solution was then cast to two different thicknesses of 100 µm (S1, S2, S3, S4) and 50 µm (S5), before being subjected to immersion precipitation process22(ESI†). It should be noted that the top layer turned out to be much more hydrophobic than the bottom layer by the contact angle measurement.
Table 2 Composition of the casting dope -
2
The bottom side of the intermediate layer was then coated by PVDF-SMM(hydrophilic) composite nanofibers by electro-spinning. The composition of the spinning dope was; PVDF 7 wt.%, SMM(hydrophilic) 13 wt%, DMAc 32 wt.% and acetone 48 wt.%, where DMAc:acetone (2:3) mixture is used as the solvent. The spinning dope was prepared by stirring the mixture at 80°C for about 20–24 h. Ten mL of the spinning dope was electro-spun at a rate of 1 mL/h by applying a voltage of 22 kV between the tip of the spinneret and the rotating metal drum (collector) with a distance of 150 mm with a uniform thickness of 20 ± 5 µm. After electro-spinning, the membrane was dried for a day at room temperature for the solvent to evaporate. The membrane was then heat-pressed at 140°C and 200 kPa to bind the two layers.
-
3
Finally, the top side of the intermediate layer was coated with a PVDF nanofiber mat by electro-spinning. The spinning dope composition is; PVDF, 14 wt.%, DMAc 34.4 wt.% and acetone 51.6 wt.%. The conditions of electro-spinning and heat-pressing are exactly the same as in step 2. The heat-pressed membranes were stored in a desiccator before use. It should be noted that both step 2 and step 3 elecro-spinning was applied to S3 and S5 single layer (intermediate layer) membranes and they are called S6 and S7 triple layer membranes, respectively. The fabrication procedure and the thickness of the membranes are summarized in Table 3.
Table 3 Summary of the membrane preparation procedure
The results of SMM characterization are summarized in Table (ESI†). The prepared membranes were characterized by scanning electron microscopy (SEM), by the measurement of liquid entry pressure of water (LEPw), pore size, membrane thickness and surface water contact angle (CAw) (ESI†). Furthermore, all the prepared membranes were tested for Direct Contact Membrane Distillation (DCMD) experiments using a feed solution containing 3.5wt% NaCl (ESI†). Fig. 6 shows the P&ID diagram of the DCMD system.
References
Bodell, B. R. Silicone rubber vapor diffusion in saline water distillation, United States Patent Serial No. 285032 (1963).
Lawson, K. W. & Lloyd, D. R. Membrane distillation. J. Membr. Sci. 124, 1–25 (1997).
Burgoyne, A. & Vahdati, M. M. Direct contact membrane distillation. Sep. Sci Technol. 35, 1257–1284 (2000).
El-Bourawi, M. S., Ding, Z., Ma, R. & Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 285, 4–29 (2006).
Cheng, D. Y. & Wiersma, S. J. Composite membrane for a membrane distillation system, United States Patent Serial No. 4, 316–772 (1982).
Cheng, D. Y. & Wiersma, S. J. Composite membrane for a membrane distillation system, United States Patent Serial No. 4419242 (1983).
Cheng, D. Y. & Wiersma, S. J. Apparatus and method for thermal membrane distillation, United States Patent Serial No. 4419187 (1983).
Wu, Y., Kong, Y., Lin, X., Liu, W. & Xu, J. Surface-modified hydrophilic membranes in distillation. J. Membr. Sci. 72, 189–196 (1992).
Bonyadi, S. & Chung, T. S. Flux enhancement in membrane distillation by fabrication ofdual layer hydrophilic–hydrophobic hollow fiber membranes. J. Membr. Sci. 306, 134–146 (2007).
Chanachai, A., Meksup, K. & Jiraratananon, R. Coating of hydrophobic hollow fiberPVDF membrane with chitosan for protection against wetting and flavor loss in osmotic distillation process. Sep. Purif. Technol. 72, 217–224 (2010).
Feng, C. et al. Production of drinking water from saline water by air-gap membrane distillation using polyvinylidene fluoride nanofiber membrane. J. Membr. Sci. 311, 1–6 (2008).
Prince, J. A. et al. Preparation and characterization of highly hydrophobic poly(vinylidene fluoride) – Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 397–398, 80–86 (2012).
Liao, Y., Wang, R., Tian, M., Qiu, C. & Fane, A. G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 425–426, 30–39 (2013).
Prince, J. A., Anbharasi, V., Shanmugasundaram, T. S. & Singh, G. Preparation and characterization of novel triple layer hydrophilic–hydrophobic composite membrane for desalination using air gap membrane distillation. Sep. Purif. Technol. 118, 598–603 (2013).
Singh, G. & Prince, J. A. Ngee Ann Polytechnic, A triple layer composite nanofiber membrane for Membrane Distillation. (MD) applications, International publication number WO/2013/074040, 2013 may 23.
Liao, Y., Wang, R. & Fane, A. G. Engineering superhydrophobic surface on poly(vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 440, 77–87 (2013).
Prince, J. A. et al. Effect of hydrophobic surface modifying macromolecules on differently produced PVDF membranes for direct contact membrane distillation. Chem. Eng. J. 242, 387–396 (2014).
Zhao, Y.-H. et al. Modification of porous poly(vinylidene fluoride) membrane using amphiphilic polymers with different structures in phase inversion process. J. Membr. Sci. 310, 567–576 (2008).
Qtaishat, M., Rana, D., Khayet, M. & Matsuura, T. Effect of surface modifying macromolecules stoichiometric ratio on composite hydrophobic/hydrophilic membranes characteristics and performance in direct contact membrane distillation. AIChE J. 55, 3145–3151 (2009).
Khayet, M., Mengual, J. I. & Matsuura, T. Porous hydrophobic/hydrophilic composite membranes: application in desalination using direct contact membrane distillation. J. Membr. Sci. 252, 101–113 (2005).
Khayet, M. & Matsuura, T. Application of surface modifying macromolecules for the preparation of membranes for membrane distillation. Desalination 158, 51–56 (2003).
Matsuura, T. Synthetic Membranes and Membrane Separation Processes. CRC Press., Florida 33431, 1994
Young, T. H., Cheng, L. P., Lin, D. J., Fane, L. & Chuang, W. Y. Mechanisms of PVDF membrane formation by immersion-precipitation in soft (1-octanol) and harsh (water) non solvents. Polymer 40, 5315–5323 (1999).
Prince, J. A., Bhuvana, S., Boodhoo, K. V. K., Anbharasi, V. & Singh, G. Synthesis and characterization of PEG-Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J. Membr. Sci. 454, 538–548 (2014).
Kim, Y., Rana, D., Matsuura, T. & Chung, W.-J. Towards antibiofouling ultrafiltration membranes by blending silver containing surface modifying macromolecules. Chem. Commun. 48, 693–695 (2012).
Rana, D. et al. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J. Membr. Sci. 409–410, 346–354 (2012).
Qtaishat, M., Rana, D., Khayet, M. & Matsuura, T. Preparation and characterization of novel hydrophobic/hydrophilic polyetherimide composite membranes for desalination by direct contact membrane distillation. J. Membr. Sci. 327, 264–273 (2009).
Pezeshk, N., Rana, D., Narbaitz, R. M. & Matsuura, T. Novel modified PVDF ultrafiltration flat-sheet membranes. J. Membr. Sci. 389, 280–286 (2012).
Rana, D. et al. Development of novel charged surface modifying macromolecules blended PES membranes to remove EDCs and PPCPs from drinking water sources. J. Mater. Chem. A 2, 10059–10072 (2014).
Acknowledgements
We gratefully acknowledge funding support from Singapore's Ministry of Education under the Innovation Fund. The authors also gratefully acknowledge the financial support from Natural Sciences and Engineering Research Council of Canada for the partial support of this work.
Author information
Authors and Affiliations
Contributions
J.A.P. and G.S. conceived the project and planned the experiments. D.R. synthesized and characterized the surface modifying macromolecules (SMMs). J.A.P. and N.A. prepared the triple layer membrane samples. J.A.P. and T.S.S. carried out most of the experiments (contact angle measurement, membrane Distillation (MD) flux and long time performance studies). D.R. and J.A.P. carried out the NMR, FTIR and SEM analysis. J.A.P., D.R., T.M. and G.S. analyzed the data and wrote the paper. All authors discussed the results and commented on the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary informations
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Prince, J., Rana, D., Matsuura, T. et al. Nanofiber based triple layer hydro-philic/-phobic membrane - a solution for pore wetting in membrane distillation. Sci Rep 4, 6949 (2014). https://doi.org/10.1038/srep06949
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06949
This article is cited by
-
Structural design of the electrospun nanofibrous membrane for membrane distillation application: a review
Environmental Science and Pollution Research (2022)
-
Pervaporation membrane for desalination derived from tetraethylorthosilicate-methyltriethoxysilane
Journal of Sol-Gel Science and Technology (2022)
-
Preparation and characterization of triple layer membrane for water filtration
Environmental Science and Pollution Research (2020)
-
Advances in Membrane Materials and Processes for Desalination of Brackish Water
Current Pollution Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.