Abstract
The carbon nanotubes (CNTs) filled polydimethylsiloxane (PDMS) hybrid membrane was fabricated to evaluate its potential for butanol recovery from acetone-butanol-ethanol (ABE) fermentation broth. Compared with the homogeneous PDMS membrane, the CNTs filled into the PDMS membrane were beneficial for the improvement of butanol recovery in butanol flux and separation factor. The CNTs acting as sorption-active sites with super hydrophobicity could give an alternative route for mass transport through the inner tubes or along the smooth surface. The maximum total flux and butanol separation factor reached up to 244.3 g/m2·h and 32.9, respectively, when the PDMS membrane filled with 10 wt% CNTs was used to separate butanol from the butanol/water solution at 80°C. In addition, the butanol flux and separation factor increased dramatically as temperature increased from 30°C to 80°C in feed solution since the higher temperature produced more free volumes in polymer chains to facilitate butanol permeation. A similar increase was also observed when butanol titer in solution increased from 10 g/L to 25 g/L. Overall, the CNTs/PDMS hybrid membrane with higher butanol flux and selectivity should have good potential for pervaporation separation of butanol from ABE fermentation broth.
Similar content being viewed by others
Introduction
Butanol is considered an advanced biofuel for its advantages of high energy content, hydrophobic properties and compatibility with current gasoline transport infrastructure1,2. However, butanol production costs in conventional acetone-butanol-ethanol (ABE) fermentation were higher than that of the petrochemical process, due to its low butanol titer of less than 2% (w/v) which led to an extremely high recovery cost in downstream process by conventional distillation3,4.
Alternative separation technologies, such as liquid-liquid extraction, adsorption, gas stripping and pervaporation, are considered to be energy-efficient improvements of butanol titer, yield and productivity since they could be integrated with ABE fermentation to continuously remove toxic products5,6,7,8. Among them, pervaporation is a membrane-based technology with great potential to recover solvents from the dilute fermentation broth with low energy consumption9,10. One of the main challenges faced by pervaporation is its deficiencies in desirable permeation flux, chemical resistance and butanol selectivity. Many studies have demonstrated that hydrophobic fillers or a hydrophobic layer composited with the membrane could improve the permeation flux and separation factor of butanol, outperforming the homogenous membrane11,12.
Carbon nanotubes (CNTs) are novel carbonaceous nanomaterials with exceptional properties, such as high mechanical stiffness and a large specific surface area etc.13. Therefore, membranes composed of CNTs may overcome the shortcomings of homogenous membranes and have thus been intensively studied for a wide range of applications, including gas separation and water purification14,15. Sae-khow et al. immobilized CNTs into polyvinylidene fluoride (PVDF) membrane pores for the alternation of analyte-polymer interactions16. The presence of CNTs led to higher permeation, as well as a faster rate of mass transfer for the removal of volatile organics, such as dichloromethane, chloroform, benzene, trichloroethylene and toluene, from water. The PDMS composite membranes with high hydrophobicity and good thermal, chemical and mechanical stability also showed excellent performance over the PVDF membranes9,10. In addition, the CNTs/PDMS composite materials have been used and well studied for gas separation, nanosensors and electrodes13,17,18,19. However, there has been no attempt to apply the CNTs/PDMS membrane for butanol recovery from dilute solution or ABE fermentation broth.
In this work, PDMS membranes filled with various amounts of CNTs were fabricated and used to study pervaporation for butanol recovery from aqueous solution and ABE fermentation broth. The effects of CNTs on the membrane properties and pervaporation performance were investigated. The addition of CNTs in the PDMS membrane was effective to enhance mass transfer and butanol separation, demonstrating beneficial use of CNTs for butanol recovery from ABE fermentation broth for the first time.
Results
Characterization of the CNTs/PDMS membrane
Field emission scanning electron microscopy (FESEM) was used to investigate the morphologies of PDMS and CNTs filled PDMS membranes. From the FESEM images, it can be seen that the surface of the membranes was smooth and homogeneous with no defect or void. Since there was no obvious difference of the surface views among the membranes with/without CNTs filling, the surface views are not given and only the cross-section images are shown in Figure 1. In general, the color of the membranes darkened with increasing the CNT filling weights. As seen in Figure 1, dispersed CNTs in the membrane were intimately enclosed in the surrounding PDMS phase. It appeared that the CNTs had a good interface compatibility with the hydrophobic PDMS. The CNTs were uniformly dispersed in the PDMS polymer with the integral structure, which gave stable pervaporation performance for butanol recovery without any leakage problem. This can be attributed to the hydrophobic nature of the CNTs, their favorable association with the prior dispersed silicone elastomeric base and also the high volatility of the doping solvent used in membrane preparation.
The performance of the CNTs/PDMS membrane in butanol/water solution
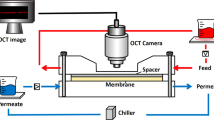
The pervaporation system with an effective membrane area of 58 cm2 is illustrated in Figure 2. To investigate the effect of CNTs addition on pervaporation performance, the hybrid membranes with different loadings of 2 wt%, 5 wt% and 10 wt% CNTs were investigated in butanol/water solution at 37°C and 80°C, respectively and the results are shown in Figure 3.
The total flux, butanol flux and separation factor of the PDMS membrane were 38.8 g/m2·h, 4.1 g/m2·h and 8.0 at 37°C, respectively, as shown in Figure 3a. When 2 wt% and 5 wt% CNTs were added into the PDMS membrane, the total flux, butanol flux and separation factor were slightly enhanced. Furthermore, when 10 wt% CNTs was presented in the membrane, the total flux, butanol flux and separation factor were significantly improved to 61.1 g/m2·h, 12.5 g/m2·h and 18.0, respectively, with increases by 57.5%, 204.9% and 125.0% compared to those in the PDMS membrane. However, when the addition of CNTs was 12 wt% in the PDMS solution, the membrane cure failed due to the decreased viscosity of the PDMS solution.
As seen in Figure 3b, with a higher temperature at 80°C in feed solution, the total flux, butanol flux and separation factor dramatically increased for both PDMS and CNTs/PDMS hybrid membranes, which can be attributed to the fact that the higher temperature would produce more free volumes in polymer chains to facilitate the permeation of the compounds. When 10 wt% CNTs were filled into the PDMS membrane, the total flux, butanol flux and separation factor reached the maximum values of 244.3 g/m2·h, 75.7 g/m2·h and 32.9, respectively, increases of 20.4%, 38.6% and 25.1% compared to the PDMS membrane. However, the improvement of hybrid membrane performance at the higher temperature of 80°C was less than that at 37°C, indicating that the presence of CNTs was more effective in enhancing PDMS membrane performance at 37°C than at 80°C. Therefore, the CNTs/PDMS hybrid membrane would be effective for separating butanol produced in ABE fermentation by Clostridium, which is usually conducted at 37°C.
The performance in ABE solution and fermentation broth
To further explore the potential application of the CNTs/PDMS membranes in conventional ABE fermentation, the ABE aqueous solution and fermentation broth as feed were employed to evaluate their pervaporation performance. As illustrated in Figures 4a and 4b, the CNTs/PDMS membranes have high selectivity for butanol and acetone, but low selectivity for ethanol. Increasing the CNTs amount in the membrane also increased the separation factors for butanol and acetone, but not ethanol. The maximum separation factors of butanol, acetone and ethanol were 16.6, 13.2 and 2.4, respectively, when 10% CNTs was added into the membrane. Therefore, increasing the CNTs addition in the PDMS membrane was beneficial for increasing ABE solvent selectivity in pervaporation. In addition, when the addition of CNTs was less than 5% in the membrane, there was no significant change in acetone, ethanol and water fluxes, whereas butanol flux increased gradually with increasing the CNTs addition. Moreover, maximum total and butanol fluxes of 93.7 g/m2·h and 17.7 g/m2·h, respectively, were achieved when there was 10% CNTs in the PDMS membrane. So, 10% CNTs in the PDMS membrane was optimal for achieving the desirable pervaporation performance for ABE recovery from ABE aqueous solution.
As shown in Figures 4c and 4d, compared to ABE aqueous solution as feed, similar trends were observed with solvent recovery from ABE fermentation broth. The separation factor of butanol and acetone increased with increasing the content of CNTs in the membrane. The maximum butanol separation factor and flux of 15.3 and 15.8 g/m2·h, respectively, occurred in the presence of 10% CNTs in the membrane. It can be concluded that the CNTs/PDMS membrane is suitable for butanol recovery from the ABE fermentation broth.
Effects of temperature and butanol titer in feed
The effects of temperature and butanol titer in feed on the performance of PDMS membrane filled with 10% CNTs were investigated with butanol/water solution and the results are shown in Figure 5. As shown in Figure 5a, the total flux and butanol flux increased dramatically from 29.0 g/m2·h and 2.8 g/m2·h to 241.0 g/m2·h and 77.5 g/m2·h, respectively, with increasing temperature from 30°C to 80°C. Butanol titer in the permeate and separation factor also increased from 101.1 g/L and 7.6 to 310.0 g/L and 32.9, respectively. It was clear that the pervaporation performance can be enhanced at the higher temperature, which could be attributed to the enhanced thermal motion of polymer chains induced by the increased temperature, allowing more butanol and water easily penetrating through the membrane. In other words, at the higher temperature, the plasticizing effect on the membranes facilitated mass transport because of the weaker interactions between the ABE solvent, water and membranes.
The effect of butanol titer in feed on the pervaporation performance was evaluated at 37°C and the results are shown in Figure 5b. When the butanol titer in solution increased from 10 g/L to 25 g/L, the total flux and butanol flux increased dramatically from 27.1 g/m2·h and 3.2 g/m2·h to 71.0 g/m2·h and 29.0 g/m2·h, respectively. Butanol titer in permeate and separation factor also increased from 166.7 g/L and 13.5 to 408.8 g/L and 32.0, respectively. It was clear that higher butanol titer in feed was beneficial for the improvement of butanol flux and butanol titer in permeate. The higher gradient of butanol caused by a higher butanol titer in feed could offer higher driving forces to facilitate butanol molecules diffusing and permeating through the membrane. Therefore, a high feed butanol titer would be highly desirable for butanol recovery by pervaporation to obtain a high butanol titer in the permeate, which would require less energy in the downstream dehydration process.
Mechanical property and membrane stability
The tensile stress-strain curves for the CNTs/PDMS membranes filled with various weights of CNTs are shown in Figure 6. More stress was required for CNTs added PDMS to produce the same strain, indicating that the addition of nanotubes stiffened the PDMS matrix. In general, the Young's modulus (E) of the CNTs/PDMS membranes increased with increasing the CNTs weights in the membrane up to 5%, as indicated by the increased slopes in the plots. However, E decreased when the CNTs weight fraction further increased to 10%, although it was still higher than that of the PDMS membrane. A similar phenomenon of tensile stress-strain property was also observed in other studies about the CNTs/PDMS composite materials used for electrodes and nanosensors18,19.
The stability of 10% CNTs/PDMS membrane in pervaporation for ABE recovery from fermentation broth was investigated at 37°C for ~200 h and the results showed relatively stable performance with less than 10% fluctuation in ABE separation factor, total flux and butanol flux for all 10 samples taken during the tested period (data not shown). There was no obvious decrease in total and butanol fluxes during the entire tested period, indicating no fouling or clogging of the membrane. Since the membrane surface was smooth and nonporous (see Figure 1), it would be difficult for biomass to stick to or penetrate through the membrane under the cross-flow conditions. Therefore, the PDMS membranes were stable and could keep a long life-time for butanol recovery from ABE fermentation broth.
Discussion
Pervaporation is considered an energy efficient alternative to conventional distillation for removing solvents from the dilute fermentation broth. The application of pervaporation for butanol recovery is based on the high selective permeation of butanol in preference to water through the hydrophobic membrane, due to its hydrophobic property. For comparison, some selected studies with various kinds of membranes are summarized in Table 1. In general, the main problem of the homogeneous membranes such as polytetrafluoroethylene (PTFE) and poly(ether block amide) (PEBA 2533) membranes is their deficiencies in high butanol separation factor or flux20,21. Although the PDMS membrane has good thermal and mechanical stability with superior performance compared to other hydrophobic membranes, its butanol selectivity over water is still not satisfactory4.
Applying hydrophobic fillers or a layer composited with the PDMS membrane is an effective way to improve the mass flux and separation factor of butanol in pervaporation. As can be seen in Table 1, the PDMS/PE/brass support membrane with a tri-layer structure had higher total flux and separation factor than those of the PDMS/brass support membrane, suggesting that the large surface porosity of the PE layers could effectively decrease mass transfer resistance and promote mass transfer for the improvement of membrane performance22. Niemistö et al. fabricated a PDMS membrane with a support layer of polyacrylonitrile (PAN) to separate ABE from ABE aqueous solution with butanol and acetone separation factor of 22, demonstrating its potential for solvent recovery from ABE fermentation broth10. However, although the porous support layers were effective for improving membrane performance, their porosity structure may lead to a fouling problem induced by biomass or macromolecules detained in the pores of the membranes.
Mix matrix membrane (MMM) is a class of membranes with highly selective nanoparticles dispersed in polymeric matrix. In recent years, nanoparticles, such as zeolite, silicalite and carbon molecular sieve etc., have been extensively studied for the fabrication of hybrid membranes. MMM such as poly(ether-block-amide) (PEBA) filled with ZIF-71, a subclass of metal-organic framework (MOFs) with superhydrophobic properties, gave a high total flux of 520 g/m2·h and butanol separation factor of 18.8 using ABE solution as feed at 37°C23. With the addition of 5% CNTs in PEBA, the hybrid membrane increased the total flux and butanol separation factor from 85 g/m2·h and 17.4 to 153 g/m2·h and 19.4, respectively, compared to the control without CNTs addition24.
For the first time, the CNTs/PDMS hybrid membrane was fabricated and tested for n-butanol recovery and its advantages and potential for use in the advanced biofuels production were demonstrated in this study. The CNTs can be uniformly dispersed in the PDMS polymer membrane with excellent compatibility. The total flux and separation factor reached 244.3 g/m2·h and 32.9, respectively, with 10 wt% CNTs in the CNTs/PDMS membrane. It has also been reported that the PDMS mixed with carbon nanotubes played a role in the enhancement of the hydrophobic surface25. Rodzi et al. (2013) demonstrated that the addition of CNTs on PDMS thin films increased their contact angle to >110.0° with excellent hydrophobic property26. The hydrophobic CNTs, as well as their interaction with PDMS polymer, may contribute to the high butanol permeance. CNTs fillers have good compatibility with the PDMS polymer matrix, so the CNTs/PDMS hybrid membrane exhibited high butanol selectivity and flux, not only in the butanol or ABE aqueous solution, but also in the ABE fermentation broth.
It is important to get the fillers well dispersed in the mixed matrix structure27,28. In case fillers agglomerated, the “channel flow” would dominate the mass transport across the agglomerates region, where the inter-filler free channel space was generally too large to be molecularly selective. As a result, the highly discriminative flow, which takes place in the inner, or along the smooth, surface of the CNTs, would lose their chance in improving the membrane selectivity, depending on the fraction of the fillers formed in the agglomerates. Furthermore, it should be noted that the dispersed fillers also needed to have a good interfacial compatibility with the polymer matrix to exclude the non-selective “leaky flow” for the necessarily guarantee of the highly selective performance.
Interestingly, compared to the PDMS membrane without CNTs addition, butanol flux and separation factor of the PDMS membrane with 10 wt% CNTs filling were enhanced by 204.9% and 125.0% at 37°C, respectively, while the increase was only 38.6% and 25.1% at 80°C. Our results indicated that the presence of CNTs in the PDMS membrane could play a more important role for the enhanced pervaporation performance at low rather than high temperatures. In general, the presence of CNTs in the membrane could alter the analyte-PDMS polymer interactions, the major physicochemical factors affecting the permeability and separation factor of the polymer composite membrane29. In addition, as shown in Figure 2, the CNTs acting as active sorption sites that interacted with the analytes and provided an alternative route for mass transport via diffusion through the inner channel tubes or along its smooth and hydrophobic surface, allowing the analytes to penetrate the membrane more easily30,31. Therefore, at low temperature, the presence of CNTs led to significant enhancement of permeability through the hybrid membrane. This is because in the compact PDMS membrane mass transfer is strongly controlled with serious resistance from the PDMS polymer chain structure. However, the higher temperature produced more free volumes in polymer chains and accelerated thermal motion of the polymer chains, thus significantly facilitating the analytes to permeate or diffuse through the membrane. So, even if the CNTs could provide more flexible routes in the PDMS membrane, their effects are less pronounced at high temperature.
According to molecular dynamics (MD) simulations on slip flow of water inside CNTs, water slip could contribute to the increase in water transport through the CNTs32. However, the underlying mechanism of water transport through the CNTs is more complicated, since it should be characterized by some theoretical models such as a standard 6–12 Lennard-Jones (LJ) potential and the TIP3P model etc. for elucidation of the interaction between water and carbon atoms. More importantly, there is a large difference between the water flow speeds in the experiments and in the MD simulations31,33. In the present study, the water flux slightly increased with increasing the amount of CNTs in the PDMS membrane and reached its maximum value at 10% CNTs filling, indicating CNTs in the membrane were beneficial for the improved water permeation through the membrane, which was consistent with the MD simulation results that water transport through the CNTs could be increased due to water slip. In addition, the butanol flux also increased with increasing the amount of CNTs in the PDMS membrane. Furthermore, the improvement of butanol flux was much greater than that of water flux at the same amount of CNTs filling. Therefore, butanol separation factor was enhanced when the PDMS membranes were filled with CNTs, especially at 10% weight fraction.
The presence of CNTs was especially effective in improving butanol separation by the PDMS membrane at 37°C. Since the conventional ABE fermentation by solvent-producing Clostridium spp. is usually conducted at 37°C, our CNTs/PDMS hybrid membrane is practical to mitigate inhibitory products and recover butanol from fermentation broth in ABE fermentation integrated with pervaporation. Therefore, the CNTs/PDMS hybrid pervaporation membrane should have great potential to enhance butanol productivity and yield when applied to the ABE fermentation process.
In conclusion, novel CNTs/PDMS hybrid membranes with enhanced and stable pervaporation performance were fabricated for butanol recovery. The presence of the CNTs in the PDMS membrane led to enhanced butanol flux and separation factor, which may be attributed to the smooth and hydrophobic surface of the CNTs as well as their interaction with PDMS polymer to facilitate butanol permeation through the membrane. The CNTs/PDMS membrane thus has great potential if applied in ABE fermentation integrated with pervaporation.
Methods
Preparation of PDMS and CNTs/PDMS membrane
For the PDMS membrane fabrication, the base solution from the Sylgard®184 silicone elastomer kit (Dow Corning, USA) was mixed with the curing agent in a 10:1 ratio, using pentane as the solvent to dilute the mixture. The mixture was stirred completely for 5 min and then sonicated in an ultrasonic bath for 0.5 h. The mixture was placed on a clean glass plate and cast evenly using a micron film applicator (Paul N. Gardner Company, USA) and then placed in vacuum to degas. The mixture on the glass plate was then heated in an oven for 3 h at 100°C. After curing, the membrane was carefully peeled off for use in pervaporation.
The CNTs/PDMS hybrid membrane was fabricated with the CNTs (Flotude 9000, CNano Technology Ltd, USA) added into the PDMS membrane. The base solution and curing agent in the ratio of 10:1 was mixed using pentane as the solvent and CNTs with a mass ratio of 2%, 5% and 10% were dispersed into the solution, respectively. Firstly, the CNTs were dispersed in pentane to facilitate effective and uniform dispersion in the PDMS viscous matrix. The pentane/CNTs suspension was then added to PDMS base solution followed by vigorous manual mixing. The mixture was then added to the curing agent before being mechanically stirred to evaporate the pentane solvent and sonication in an ultrasonic bath for 0.5 h. The mixture was uniformly coated on a clean glass plate with a micron film applicator and then placed in vacuum to degas. After heating at 100°C for 3 h to cure the membrane, the membrane was peeled off the glass plate. The thickness of the PDMS and CNTs/PDMS hybrid membranes was 200 μm. The field emission scanning electron microscopy (FESEM) (Nova Nano SEM450, FEI, USA) was used to analyze the CNTs composite membranes morphologies.
Pervaporation experiment
Either ABE solution or fermentation broth containing ~15 g/L butanol was used as the feed solution at 37°C to evaluate the pervaporation performance of PDMS and CNTs/PDMS membranes. Fermentation broth was collected from ABE fermentation with P2 medium by Clostridium acetobutylicum ATCC 55025, described by Xue et al34. The ABE ratio in ABE solution was 3:6:1, same as the usual ABE fermentation broth. To evaluate the effects of temperature and butanol titer on pervaporation performance, temperature and butanol titer ranges of 30–80°C and 10–25 g/L were investigated, respectively. All the pervaporation experiments were conducted at the feed flow rate of 1.2 L/min and a vacuum of <20 kPa on the permeate side of the membrane. The permeate was collected in a cold trap immersed in liquid nitrogen.
Analytical methods
The titers of acetone, butanol and ethanol were assayed with a gas chromatograph (Alilgent 6890A GC) following the method described previously35.
The flux and separation factor (SF) were calculated as follows:


Where W is the weight (g) of the permeate; A is the membrane area (m2), t is the time (h) and x and y are the weight fractions of components in the feed and permeate, respectively.
References
Gheshlaghi, R., Scharer, J. M., Moo-Young, M. & Chou, C. P. Metabolic pathways of clostridia for producing butanol. Biotechnol. Adv. 27, 764–781 (2009).
Xue, C. et al. Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresour. Technol. 135, 396–402 (2013).
Ezeji, T. C., Milne, C., Price, N. D. & Blaschek, H. P. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl. Microbiol. Biotechnol. 85, 1697–1712 (2010).
Xue, C. et al. Integrated butanol recovery for an advanced biofuel: current state and prospects. Appl. Microbiol. Biotechnol. 98, 3463–3474 (2014).
Evans, P. J. & Wang, H. Y. Enhancement of butanol formation by Clostridium acetobutylicum in the presence of decanol-oleyl alcohol mixed extractants. Appl. Environ. Microbiol. 54, 1662–1667 (1988).
Nielsen, D. R. & Prather, K. J. In situ product recovery of n-butanol using polymeric resins. Biotechnol. Bioeng. 102, 811–821 (2009).
Xue, C. et al. High-titer n-butanol production by Clostridium acetobutylicum JB200 in Fed-batch fermentation with intermittent gas stripping. Biotechnol. Bioeng. 109, 2746–2756 (2012).
Vane, L. M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 80, 603–629 (2005).
Wu, H. et al. Acetone-butanol-ethanol (ABE) fermentation using Clostridium acetobutylicum XY16 and in situ recovery by PDMS/ceramic composite membrane. Bioproc. Biosyst. Eng. 35, 1057–1065 (2012).
Niemistö, J., Kujawski, W. & Keiski, R. L. Pervaporation performance of composite poly (dimethylsiloxane) membrane for butanol recovery from model solutions. J. Membr. Sci. 434, 55–64 (2013).
Peng, F. B. et al. Hybrid organic–inorganic membrane: solving the trade off between permeability and selectivity. Chem. Mater. 17, 6790–6796 (2005).
Li, Y. L. et al. Recent advances in the fabrication of advanced composite membranes. J. Mater. Chem. A. 1, 10058–10077 (2013).
Sears, K. et al. Recent developments in carbon nanotube membranes for water purification and gas separation. Materials. 3, 127–149 (2010).
Choi, J. H., Jegal, J., Kim, W. N. & Choi, H. S. Incorporation of multiwalled carbon nanotubes into poly (vinyl alcohol) membranes for use in the pervaporation of water/ethanol mixtures. J. Appl. Polym. Sci. 111, 2186–2193 (2009).
Kim, S., Pechar, T. W. & Marand, E. Poly (imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination. 192, 330–339 (2006).
Sae-Khow, O. & Mitra, S. Carbon nanotube immobilized composite hollow fiber membranes for pervaporative removal of volatile organics from water. J. Phys. Chem. C. 114, 16351–16356 (2010).
Nour, M. et al. CNT/PDMS composite membranes for H2 and CH4 gas separation. Int. J. Hydrogen Energ. 38, 10494–10501 (2013).
Lu, J. et al. Study of piezoresistance effect of carbon nanotube-PDMS composite materials for nanosensors. Proceedings of the 7th IEEE International Conference on Nanotechnology, Hong Kong., 1240–1243; 10.1109/NANO.2007.4601407 (2007).
Jung, H. C. et al. CNT/PDMS composite flexible dry electrodes for long-term ECG monitoring. IEEE T. Bio-med. Eng. 59, 1472–1479 (2012).
Vrana, D. L., Meagher, M. M., Hutkins, R. W. & Duffield, B. Pervaporation of model acetone-butanol-ethanol fermentation product solutions using polytetrafluoroethylene membranes. Sep. Sci. Technol. 28, 1–14 (1993).
Liu, F. F., Liu, L. & Feng, X. S. Separation of acetone-butanol-ethanol (ABE) from dilute aqueous solution by pervaporation. Sep. Purif. Technol. 42, 273–282 (2005).
Li, S. Y., Srivastava, R. & Parnas, R. S. Separation of 1-butanol by pervaporation using a novel tri-layer PDMS composite membrane. J. Membr. Sci. 363, 287–294 (2010).
Liu, S. N., Liu, G. P., Zhao, X. H. & Jin, W. Q. Hydrophobic-ZIF-71 filled PEBA mixed matrix membranes for recovery of biobutanol via pervaporation. J. Membr. Sci. 446, 181–188 (2013).
Yen, H. W., Chen, Z. H. & Yang, I. K. Use of the composite membrane of poly (ether-block-amide) and carbon nanotubes (CNTs) in a pervaporation system incorporated with fermentation for butanol production by Clostridium acetobutylicum. Bioresour. Technol. 109, 105–109 (2012).
Ma, M. & Hill, R. M. “Superhydrophobic surfaces”. Curr. Opin. Colloid. In. 11, 193–202 (2006).
Rodzi, N. H. M., Shahimin, M. M., Poopalan, P., Man, B. & Nor, M. N. M. Hydrophobicity studies of polymer thin films with varied CNT concentration. Proc. SPIE, 8923, 89235G; DOI:10.1117/12.2034283 (2013).
Sircar, S., Rao, M. B. & Thaeron, C. M. A. Selective surface flow membrane for gas separation. Sep. Sci. Technol. 34, 2081–2093 (1999).
Mahajan, R. & Koros, W. J. Mixed matrix membrane materials with glassy polymers. Part 1. Polym. Eng. Sci. 42, 1420–1431 (2002).
Polotskaya, G. A., Penkova, A. V. & Toikka, A. M. Fullerene-containing polyphenylene oxide membranes for pervaporation. Desalination. 200, 400–402 (2006).
Striolo, A. The Mechanism of Water Diffusion in Narrow Carbon Nanotubes. Nano. Lett. 6, 633–639 (2006).
Thomas, J. A. & McGaughey, A. J. Reassessing fast water transport through carbon nanotubes. Nano. Lett. 8, 2788–2793 (2008).
Ma, M. D. et al. Friction of water slipping in carbon nanotubes. Phys. Rev. E. 83, 036316 (2011).
Chen, X. et al. Nanoscale fluid transport: Size and rate effects. Nano. Lett. 8, 2988–2992 (2008).
Xue, C. et al. Characterization of gas stripping and its integration with acetone-butanol-ethanol fermentation for high-efficient butanol production and recovery. Biochem. Eng. J. 83, 55–61 (2014).
Wu, Y. D., Xue, C., Chen, L. J. & Bai, F. W. Effect of zinc supplementation on acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. J. Biotechnol. 165, 18–21 (2013).
Huang, J. C. & Meagher, M. M. Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes. J. Membr. Sci. 192, 231–242 (2001).
Acknowledgements
Thank Dr Xiang-Cun Li and Gao-Hong He for the support of tensile stress test. This work was financially supported by the National Natural Science Foundation of China (NSFC) with grant numbers of 21306020 and 21376044, the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130041120027), the Scientific Research Fund of Liaoning Provincial Education Department (L2013022), Doctor startup fund of Liaoning Province (20141197), China Postdoctoral Science Foundation (2013M530904 and 2014T70257) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry.
Author information
Authors and Affiliations
Contributions
C.X. led the project and conducted the data analysis. G.D. performed the experiments and collected the data. C.X. and G.D. wrote the paper. S.Y., L.C., J.R., J.S. and F.B. discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Xue, C., Du, GQ., Chen, LJ. et al. A carbon nanotube filled polydimethylsiloxane hybrid membrane for enhanced butanol recovery. Sci Rep 4, 5925 (2014). https://doi.org/10.1038/srep05925
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05925
This article is cited by
-
Replicating Arabidopsis Model Leaf Surfaces for Phyllosphere Microbiology
Scientific Reports (2019)
-
Stretchable strain sensor facilely fabricated based on multi-wall carbon nanotube composites with excellent performance
Journal of Materials Science (2019)
-
A novel close-circulating vapor stripping-vapor permeation technique for boosting biobutanol production and recovery
Biotechnology for Biofuels (2018)
-
Gas permeation through rubbery polymer nano-corrugated membranes
Scientific Reports (2018)
-
Organosolv pretreatment of sorghum bagasse using a low concentration of hydrophobic solvents such as 1-butanol or 1-pentanol
Biotechnology for Biofuels (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.