Abstract
Tungsten sulfide (WS2)–carbon composite powders with superior electrochemical properties are prepared by a two-step process. WO3-carbon composite powders were first prepared by conventional spray pyrolysis and they were then sulfidated to form WS2-carbon powders. Bare WS2 powders are also prepared by sulfidation of bare WO3 powders obtained by spray pyrolysis. Stacked graphitic layers could not be found in the bare WS2 and WS2–carbon composite powders. The amorphous bare WS2 and WS2–carbon composite powders have Brunauer–Emmett–Teller (BET) surface areas of 2.8 and 4 m2 g−1, respectively. The initial discharge and charge capacities of the WS2–carbon composite powders at a current density of 100 mA g−1 are 1055 and 714 mA h g−1, respectively and the corresponding initial Coulombic efficiency is 68%. On the other hand, the initial discharge and charge capacities of the bare WS2 powders are 514 and 346 mA h g−1, respectively. The discharge capacities of the WS2–carbon composite powders for the 2nd and 50th cycles are 716 and 555 mA h g−1, respectively and the corresponding capacity retention measured after first cycle is 78%.
Similar content being viewed by others
Introduction
Transition metal sulfides (MxSy, M = W, Mo, Zn, Mn, Ni, Fe) with various morphologies prepared by conventional liquid solution processes have been investigated as promising anode materials for lithium-ion batteries (LIBs)1,2. In particular, layered dichalcogenide materials (MoS2 and WS2), which have van der Waals forces across the gaps between the S–M–S sheets, thus allowing the Li ions to diffuse without a significant increase in volume expansion, have higher structural stabilities during repeated lithiation and delithiation processes as compared with transition metal oxides and tin oxide studied widely as anode materials for LIBs3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19. In addition, transition metal dichalcogenides have better electronic and ionic conductivities than transition metal oxides and tin oxide. However, the electronic conductivity of transition metal dichalcogenides is still lower as compared to carbon-based materials15. Carbonaceous materials, including graphene, carbon nanotubes and amorphous carbon, have been composited with transition metal dichalcogenides in order to improve their electrochemical properties5,6,7,8,9,10,11,12,13,14,15,16,17. Various structured MoS2–carbon composite materials prepared by liquid solution methods have been studied as anode materials for LIBs5,6,7,8,9,10,11,12,13. However, the preparation of WS2–carbon composite materials and their electrochemical properties have been scarcely studied4,5,6,7,8,9,10,11,12,13,14,15,16,17. WS2–graphene composite materials had better cycling and rate performances than bare WS2 powders15.
Commercial LIB anode materials typically comprise spherical powders with sizes of several microns. However, micron-sized metal sulfide–carbon composite powders with spherical particles and superior electrochemical properties have been scarcely studied in the conventional liquid solution processes. In this study, tungsten sulfide (WS2)–carbon composite powders with superior electrochemical properties were prepared by a two-step process. WO3–carbon composite powders prepared by spray pyrolysis (Figure S1) were sulfidated to form the WS2–carbon composite powders20,21. The spherical shape and micron size of the WO3–carbon composite powders were maintained even after the sulfidation process. The electrochemical properties of the WS2–carbon composite powders were compared to those of the bare WS2 powders. The WS2–carbon composite powders had higher capacities than the bare WS2 powders.
Results
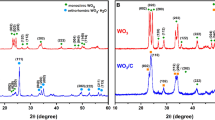
The WO3 and WO3–carbon composite powders prepared by spray pyrolysis were transformed into WS2 and WS2–carbon composite powders, respectively, by a sulfidation process. The morphologies and crystal structures of the bare WO3 and WO3–carbon composite powders prepared by spray pyrolysis under nitrogen atmosphere are shown in Figure 1. The carbon component was formed by polymerization and the carbonization of sucrose dissolved into the spray solution. The powders had a spherical shape and nonaggregation characteristics, regardless of the carbon component. One particle with spherical shape was formed from one droplet by gas phase reaction. The WO3–carbon composite powders with high carbon content had a larger mean size than the bare WO3 powders, as shown in the SEM images in Figures 1a and 1b. The bare WO3 powders had a sharp monoclinic crystal structure without impurity peaks as shown in Figure 1c. On the other hand, the WO3–carbon composite powders had an amorphous structure with low-intensity crystalline peaks.
Figure 2a shows the XRD patterns of the sulfidated powders. The WS2 and WS2–carbon composite powders had pure crystal structures of a hexagonal WS2 phase without impurity peaks of WO3, even though the two samples had different crystal orientations. The (002) reflection indicating stacking of the WS2 layers was only observed in the WS2–carbon composite powders. The XRD patterns had broad peaks with low intensities. The amorphous WS2 and WS2–carbon composite powders were prepared because of a low sulfidation temperature of 400°C. Figure 2b shows the thermogravimetry (TG) curve of the WS2–carbon composite powders. The TG curve had two distinct weight losses below 700°C. The weight loss by complete oxidation of WS2 into WO3 under air atmosphere was as low as 6.5 wt%. Therefore, the two-step weight losses observed near 200 and 400°C were mainly due to the decomposition of carbon components. The composition of the WS2–carbon composite powders with a dense structure occurred in two steps. The calculated carbon content of the WS2–carbon composite powders calculated from the TG results was about 30 wt%.
The morphologies of the WS2–carbon composite powders are shown in Figure 3. The composite powders had morphology similar to that of the WO3–carbon composite powders, indicating that the sulfidation process did not significantly change the morphology of the powders. The high-resolution TEM images shown in Figures 3c and 3d reveal slightly crystalline WS2 that consists of a few graphitic layers near the surface of the powder. The interlayer distance of the (002) plane measured from the TEM image is 0.63 nm14. However, the stacked graphitic layers were not found in the inner part of the composite powders, as shown by the dotted rectangular in Figure 3d. The dot-mapping images shown in Figure 3e show the uniform distributions of the W, S and C components all over the WS2–carbon composite powder. Complete sulfidation of the powders occurred. The uniform distribution of carbon all over the powders disturbed the growth of WS2 crystals. Figure 4 shows the morphologies of the bare WS2 powders. The bare WS2 powders also had a spherical shape like the WS2–carbon composite powders. The high-resolution TEM images shown in Figures 4c and 4d show the non-stacked WS2 layers and amorphous structure, respectively. The bare WS2 and WS2–carbon composite powders had Brunauer–Emmett–Teller (BET) surface areas of 2.8 and 4 m2 g−1, respectively. The dense structures of the WS2 and WS2–carbon composite powders without pores, as shown by the TEM images in Figures 3 and 4, resulted in low BET surface areas.
The electrochemical properties of the bare WS2 and WS2–carbon composite powders are shown in Figure 5. Figure 5a shows the cyclic voltammograms (CVs) of the WS2–carbon composite powders measured at a scan rate of 0.4 mV s−1 in the voltage range of 0.001–3 V. The first discharge curve had two broad reduction peaks near 0.5 and 0.1 V. The mesoporous WS2 powders with high crystallinity prepared by a vacuum assisted impregnation route had sharp reduction and oxidation peaks in their CV curves14. However, the amorphous WS2–carbon composite powders had broad reduction and oxidation peaks in all cycles. The clear reduction peaks for the lithium insertion to WS2 to form LixWS2 (WS2 + xLi+ + xe− = > LixWS2) and the conversion reaction (WS2 + 4Li+ + 4e− = > W + 2Li2S) were not observed15. The steady CV profiles after the initial cycle indicated high stability and reversibility of the WS2–carbon composite powders for Li+ insertion and extraction. Figure 5b shows the charge and discharge curves of the WS2–carbon composite powders at a constant current density of 100 mA g−1. The charge and discharge curves for all cycles, including the first cycle, had no distinct plateaus. These results well coincide with those of the CVs shown in Figure 5a. The cycle curves of the bare WS2 powders with amorphous structure shown in Figure S2 also had no distinct plateaus. The initial discharge and charge capacities of the WS2–carbon composite powders were 1055 and 714 mA h g−1, respectively and the corresponding initial Coulombic efficiency was 68%. On the other hand, the initial discharge and charge capacities of the bare WS2 powders were 514 and 346 mA h g−1, respectively and the corresponding initial Coulombic efficiency was 67%. The rate performance of the WS2-carbon composite powders is shown in Figure S3, wherein the current density increased stepwise from 50 to 500 mA g−1 and then returned to 50 mA g−1. The WS2-carbon composite powders exhibited the final discharge capacities of 761, 630, 561, 524 and 502 mA h g−1 at current densities of 50, 150, 300, 400 and 500 mA g−1, respectively. When the current density returned to 50 mA g−1, the discharge capacity recovered to 678 mA h g−1.
Discussion
Electrochemical impedance spectroscopy was conducted to determine the Li+ ion transfer behavior in the bare WS2 and WS2–carbon composite powders. The Nyquist impedance plots obtained before cycling are shown in Figure 5c. The semicircular diameter, which shows the charge-transfer resistance (Rct) obtained in the medium-frequency region for the WS2–carbon composite powders, was similar to that in the case of the bare WS2 powders. Generally, the high BET surface area increased the Rct of the powders. However, the high conductivity decreased the Rct of the WS2–carbon composite powders. The relationship between the real part of the impedance (Zre) and ω−1/2 (where ω is the angular frequency in the low-frequency region, ω = 2πf) in the low-frequency region is shown in the Figure S422,23. The low slope (σ, Warburg impedance coefficient) of Zre versus ω−1/2 revealed the high lithium ion diffusion rate of the WS2–carbon composite powders. Figure 5d shows the cycling performances of the bare WS2 and WS2–carbon composite powders at a current density of 100 mA g−1. The discharge capacities of the WS2–carbon composite powders for the 2nd and 50th cycles were 716 and 555 mA h g−1, respectively and the corresponding capacity retention measured after the first cycle was 78%. On the other hand, the discharge capacities of the bare WS2 powders for the 2nd and 50th cycles were 356 and 304 mA h g−1, respectively and the corresponding capacity retention measured after the first cycle was 85%. The bare WS2 powders had good cycling performance even though they had low initial discharge and charge capacities. Figure S5 shows the morphologies and EDS spectra of the bare WS2 and WS2–carbon composite powders after 50 cycles. The two samples maintained their spherical morphologies even after cycling as shown by SEM images. The high structural stabilities of the powders improved their cycling performances. However, the TEM images showed the different structures of the bare WS2 and WS2–carbon composite powders after cycling. The thick layer owing to the formation of a polymeric gel-like film during cycling covered the bare WS2 material. The main component of the layer covering the bare WS2 powder observed by EDS spectrum was carbon.
Phase-pure WS2–carbon composite powders with uniform distribution of W, S and C components were prepared by applying ultrasonic spray pyrolysis. The WS2–carbon composite powders had a spherical shape, dense structure and amorphous crystal structure. Amorphous WO3–carbon composite powders with high carbon content were transformed into amorphous WS2–carbon composite powders. However, highly crystalline bare WO3 powders prepared by spray pyrolysis were transformed into amorphous bare WS2 powders by a sulfidation process. The cyclic voltammograms and initial discharge and charge curves revealed the amorphous structure of the WS2–carbon composite powders. The WS2–carbon composite powders had better electrochemical properties than the bare WS2 powders.
Methods
Material fabrication
Spherical WO3–carbon and WO3 powders were prepared directly by spray pyrolysis from spray solutions of ammonium metatungstate with and without a sucrose additive, respectively. The spray pyrolysis system consisted of a droplet generator, a quartz reactor and a powder collector. The length and diameter of the quartz reactor were 1000 and 55 mm, respectively. A 1.7 MHz ultrasonic spray generator with six vibrators was used to generate a large quantity of droplets, which were carried into the high-temperature tubular reactor by the carrier gas (nitrogen) at a flow rate of 10 L min−1. The reactor temperature was fixed at 900°C. The concentrations of the ammonium metatungstate and sucrose were 0.2 and 0.1 M, respectively. The as-prepared WO3–carbon and WO3 powders were converted to WS2–carbon and WS2, respectively, by adopting a sulfidation process under a reducing atmosphere with thiourea as the sulfur source. The as-prepared powders were placed in a small alumina boat, which was located inside a larger alumina boat. Thiourea was loaded outside of the small alumina boat in excess to form the WS2. The sulfidation process was performed at 400°C for 6 h in the presence of a 10% H2/Ar mixture gas.
Characterization
The crystal structures of the WS2–carbon and WS2 powders were investigated by performing X-ray diffractometry (XRD, Rigaku DMAX-33) using Cu Kα radiation at the Korea Basic Science Institute (Daegu). The morphologies of the powders were characterized using scanning electron microscopy (SEM, JEOL JSM-6060) and high-resolution transmission electron microscopy (TEM, JEOL JEM-2010). The specific surface areas of the bare WS2 and WS2-carbon composite powders were calculated by Brunauer–Emmett–Teller (BET) analysis of nitrogen-adsorption measurements (Micromeritics TriStar 3000).
Electrochemical measurements
The electrochemical properties of the WS2–carbon and WS2 powders were measured using 2032-type coin cells. The electrodes were prepared by mixing 35 mg of the active powders, 10 mg of carbon black (Super P) as a conductive material, 5 mg of sodium carboxymethyl cellulose (CMC) using distilled water. Lithium metal and a microporous polypropylene film were used as the counter electrode and separator, respectively. LiPF6 (1 M) in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) in a 1:1 volume ratio with 5 wt% fluoroethylene carbonate (FEC) was used as the electrolyte (Techno Semichem Co.). The entire cell was assembled under an argon atmosphere in a glove box. The charge/discharge characteristics and electrochemical impedance spectroscopy (EIS) measurements of the samples were measured at 100 mA g−1 in the voltage range of 0.001–3 V. Cyclic voltammetry (CV) measurements were carried out at a scan rate of 0.4 mV s−1.
References
Lai, C. H., Lu, M. Y. & Chen, L. J. Metal sulfide nanostructures: synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 22, 19–30 (2012).
Gao, M. R., Xu, Y. F., Jiang, J. & Yu, S. H. Nanostructured metal chalcogenides: synthesis,modification and applications in energy conversion and storage devices. Chem. Soc. Rev. 42, 2986–3017 (2013).
Chhowalla, M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013).
Wang, Q. H. et al. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Xiao, J. et al. Exfoliated MoS2 nanocomposite as an anode material for lithium ion batteries. Chem. Mater. 22, 4522–4524 (2010).
Sun, P., Zhang, W., Hu, X., Yuan, L. & Huang, Y. Synthesis of hierarchical MoS2 and its electrochemical performance as an anode material for lithium-ion batteries. J. Mater. Chem. A 2, 3498–3504 (2014).
Zhu, C. et al. Single-Layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium Storage. Angew. Chem. Int. Ed. 53, 2152–2156 (2014).
Chang, K. et al. Graphene-like MoS2/amorphous carbon composites with high capacity and excellent stability as anode materials for lithium ion batteries. J. Mater. Chem. 21, 6251–6257 (2011).
Shi, Y. et al. Self-assembly of hierarchical MoSx/CNT nanocomposites (2<x<3): towards high performance anode materials for lithium ion batteries. Sci. Rep. 3, 2169 (2013).
Pham, V. H. et al. Liquid phase co-exfoliated MoS2-graphene composites as anode materials for lithium ion batteries. J. Power Sources 244, 280–286 (2013).
Chang, K. et al. Ultrathin MoS2/nitrogen-doped graphene nanosheets with highly reversible lithium storage. Adv. Energy Mater. 3, 839–844 (2013).
Wang, Z. et al. CTAB-assisted synthesis of single-layer MoS2–graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 1, 2202–2210 (2013).
Zhou, X., Wan, L. J. & Guo, Y. G. Synthesis of MoS2 nanosheet–graphene nanosheet hybrid materials for stable lithium storage. Chem. Commun. 49, 1838–1840 (2013).
Liu, H., Su, D., Wang, G. & Qiao, S. Z. An ordered mesoporous WS2 anode material with superior electrochemical performance for lithium ion batteries. J. Mater. Chem. 22, 17437–17440 (2012).
Shiva, K. et al. Employing synergistic interactions between few-layer WS2 and reduced graphene oxide to improve lithium storage, cyclability and rate capability of Li-ion batteries. Nano Energy 2, 787–793 (2013).
Bhandavat, R., David, L. & Singh, G. Synthesis of surface-functionalized WS2 nanosheets and performance as Li-ion battery anodes. J. Phys. Chem. Lett. 3, 1523–1530 (2012).
Chen, D. et al. In situ nitrogenated graphene–few-layer WS2 composites for fast and reversible Li+ storage. Nanoscale 5, 7890–7896 (2013).
Wang, S. et al. Synthesis and characterization of cobalt-doped WS2 nanorods for lithium battery applications. Nanoscale Res. Lett. 5, 1301–1306 (2010).
Wang, G. X. et al. Tungsten disulfide nanotubes for lithium storage. Electrochem. Solid-State Lett. 7, A321–A323 (2004).
Choi, S. H. & Kang, Y. C. Synthesis for yolk-shell-structured metal sulfide powders with excellent electrochemical performances for lithium-ion batteries. Small 10, 474–478 (2014).
Ko, Y. N., Choi, S. H., Park, S. B. & Kang, Y. C. Preparation of yolk-shell and filled Co9S8 microspheres and comparison of their electrochemical properties. Chem. Asian J. 9, 572–576 (2014).
Shi, Y. et al. Hollow structured Li3VO4 wrapped with graphene nanosheets in situ prepared by a one-pot template-free method as an anode for lithium-ion batteries. Nano Lett. 13, 4715–4720 (2013).
Ko, Y. N., Park, S. B., Jung, K. Y. & Kang, Y. C. One-pot facile synthesis of ant-cave-structured metal oxide−carbon microballs by continuous process for use as anode materials in li-ion batteries. Nano Lett. 13, 5462–5466 (2013).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2012R1A2A2A02046367). This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (201320200000420).
Author information
Authors and Affiliations
Contributions
S.H.C., J.H.L. and Y.C.K. devised the concept, designed the experiment and wrote the manuscript. S.H.C. and S.J.B. performed the experiments and analyzed the data. Y.C.K. supervised the project. All authors discussed the results and contributed in this manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Choi, S., Boo, S., Lee, JH. et al. Electrochemical properties of tungsten sulfide–carbon composite microspheres prepared by spray pyrolysis. Sci Rep 4, 5755 (2014). https://doi.org/10.1038/srep05755
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05755
This article is cited by
-
Auxiliary ball milling to prepare WS2/graphene nanosheets composite for lithium-ion battery anode materials
Tungsten (2024)
-
Application of WS2-G composite as cathode for rechargeable magnesium batteries
Ionics (2020)
-
Synthesis and Performance of Tungsten Disulfide/Carbon (WS2/C) Composite as Anode Material
Journal of Electronic Materials (2018)
-
Scalable production of self-supported WS2/CNFs by electrospinning as the anode for high-performance lithium-ion batteries
Science Bulletin (2016)
-
Aerosol-assisted rapid synthesis of SnS-C composite microspheres as anode material for Na-ion batteries
Nano Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.