Abstract
Age and growth analysis is essential to fisheries management. Indirect methods to calculate growth are widely used; however, length frequency data analysis in sea cucumbers is complicated by high data variability caused by body wall elasticity. Here we calculated Isostichopus badionotus parameters of the von Bertalanffy growth function. In order to address bias produced by body wall elasticity, we compared the performance of four measurements and one compound index that combines different biometric parameters: the square root of the length-width product (SLW). Results showed that variability in length data due to body wall elasticity was controlled by using body length (Le) from the SLW compound index. Growth in I. badionotus follows a negative allometric tendency. Slow or zero growth periods were observed during October and November, when weather conditions were adverse.
Similar content being viewed by others
Introduction
Most of the targeted holothurian species by fishers through the world are reported as over-exploited1,2. A recent worldwide analysis reveals that 20% of fisheries were found to be depleted, 38% over-exploited, 14% fully exploited (i.e. no potential margin for expansion) and only 27% moderately or underexploited3. In order to maintain stocks at levels capable of producing maximum sustainable yield, countries should adopt appropriate measures based on available scientific data4. Holothurians need particular attention due to their vulnerability to overfishing, biological characteristics and commercial value1.
In recent times some species are being targeted in Latin America, where scientific knowledge is limited. Specifically, ten years ago, a large Isostichopus badionotus5 stock was identified on the northern Yucatan Peninsula continental shelf6. This stock has attracted the interest of sea cucumber traders and fishers in developing this potential new fishery. However, lack of regulations and scientific knowledge on the species in the region has hindered development of any fishery sustainability guidelines, threatening the stock with overexploitation. In the western Atlantic, I. badionotus has been captured in Cuba7, Venezuela and Panama8. To date, studies on this species have focused on distribution, abundance and biomass assessment6,9, however, other biological characteristics, such as age and growth have not yet been studied.
Isostichopus badionotus is widely distributed in the Atlantic, from Central America to West Africa and from Bermuda to Ascension Island10. This species is found at depths of 0 to 65 m on shallow muddy-sand and sea grass beds11, bare hard surfaces, coral rubble, dead coral, pavements, erect macroalgae and turf algae12. Few data on biometric relationships are available for I. badionotus6,8,13,14.
Most methods address growth through length frequency data analysis, which depends on body measurements and biometric relationships. Precision measurements of weight and length in sea cucumbers are challenging. Body wall elasticity, digestive system content and coelomic fluid produce bias in size frequency data analysis15. This challenge can be addressed by using three alternative set of variables: 1) weight, immersed weight16,17, drained weight15,18,19 and/or gutted weight13,15,20; 2) length, either contracted21,22 or relaxed13,23; and 3) compound indices that combine different biometric parameters17,24,25,26,27,28,29,30; the latter one allows calculation of basal area and volume that can then be applied to generate more precise biometric relationships.
The objective of the present study was to document biometry and growth for a I. badionotus populations of the northern Yucatan Peninsula continental shelf (Mexico). Dorsal length, total weight and gutted weight were measured. A compound index and its transformation were analyzed and compared, as were the resulting biometric relationships. Isostichopus badionotus growth parameters were calculated by indirect methods.
Results
Morphological measurements and population structure
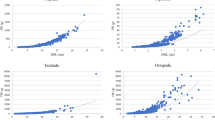
A total of 2347 sea cucumbers were measured during the study period and five frequency distributions analyzed from the body length (L), width (W), total weight (TW), muscle wet weight or gutted weight (GW) measurements and the compound index SLW (the square root of the length-width product) (Fig. 1). Mean body length ± SD (25.3 ± 5.2 cm; Fig. 1a) was almost twice mean body width (14.2 ± 3.1 cm; Fig. 1b), with sizes ranging from 3 to 45 cm and a population mode of 23 cm. The SLW frequency distribution showed the minimum coefficient of variance (18.6%; Fig. 1c), whereas the maximum coefficient of variance (56.9%) was observed in the GW frequency distribution (Fig. 1e). The SLW distribution was multimodal and ranged from 2.4 to 28.3 cm. Both the TW and GW frequency distributions were unimodal (mode = 600 g and 400 g, respectively). The TW frequency distribution had a mean of 552 ± 263 g with weights ranging from 1 to 1800 g (Fig. 1d). Weight loss from TW to GW was 37.5% (Fig. 1f) and, contrary to expectations, coefficient of variation (CV) was higher for GW than for TW.
Frequency distributions of measurements and a compound index for sea cucumber Isostichopus badionotus.
A total of n = 2347 sea cucumbers I. badionotus were measured during the study period in 2009 and 2010 off the Yucatan Peninsula, Mexico. (a) L distribution; (b) W distribution; (c) SLW distribution; (d)TW distribution; (e) GW distribution; and (f) weight loss from TW to GW of n = 1165 sea cucumbers, loss is indicated as a percentage and shown in bold. L: dorsal body length; W: dorsal body width; SLW: square root of length multiplied by width; TW: total weight; GW: gutted weight; SD: standard deviation; and CV: coefficient of variation in percentage, lowest value is shown in bold.
Biometric relationships
The regression equation between L and SLW used to generate estimated length was: Le = 1.11 + 1.28 SLW (r2 = 0.75, p ≪ 0.001). The overall Student t-test showed that I. badionotus grows allometrically (β ≠ 3, p < 0.05), with negative allometric β values ranging from 1.5 to 2.7 (Fig. 2). However, the length-weight relationships by location showed β values near 3 (isometric value) at Progreso. In all cases, the relationship between body length and weight was significant (p < 0.001). The Le measurement explained almost 80% of variation in body weight and was a more effective predictor of weight than L (Table 1). This is because Le was derived from two measurements (square root of length multiplied by width) rather than one, which improved the accuracy of body weight estimates (Figs 2c and 2d). The equation (1) produced poor body weight estimates at lengths < 18 cm and >30 cm when using length alone (Figs. 2a and 2b). The L-GW relationships showed a point cloud (points inside the dotted circle in the Figs. 2b and 2d) that does not fit the general tendency of most observations. This point cloud corresponded to measurements recorded in 2009, 52% of which were taken in September.
Relationships between estimated lengths and weights from sea cucumber Isostichopus badionotus.
Points inside the dotted circle are data recorded during 2009. TW: total weight; GW: gutted weight; and Le: estimated length from the equation Le = 1.11 + 1.28 SLW, where SLW is square root of length multiplied by width.
Analysis of mean condition factor (Kn ± SD) showed that Kn values were lower in September 2009 than in other months, overall and by location. Mean Kn values generally ranged between 0.37 ± 0.25 in September 2009 and 1.98 ± 0.25 in November 2010 (Table 2). However, when the data were broken down by location, mean Kn values were higher during September 2010.
Growth analysis
The ELEFAN I method (Electronic Length Frequency Analysis) yielded the best estimated growth parameter results both in the pooled sample and for Celestun: Le∞ = 31.6 cm, k = 0.6 yr−1 (overall); Le∞ = 31.9 cm, k = 0.5 yr−1 (Celestun) (Table 3). For Sisal and Progreso, the best results were produced by the PROJMAT method (Projection Matrix): Le∞ = 38 cm, k = 0.2 yr−1 (Sisal); Le∞ = 23.5 cm, k = 0.7 yr−1 (Progreso). Asymptotic gutted weight (GW∞) by location was 599 g for the pooled sample, 638 g for Celestun, 929 g for Sisal and 192 g for Progreso. Parameter C was 1 for Celestun, 0.7 for Sisal, 0.6 for Progreso and 0.8 for the pooled sample. These results indicate intense intra-annual oscillations in growth. Winter point (WP) parameter values were 0.8 (overall and Celestun) and 0.9 (Sisal and Progreso), implying minimum growth was present in October (overall and Celestun) and November (Sisal and Progreso).
The likelihood ratio tests indicated differences (χ2 test, df = 3, p < 0.001) in growth between locations. All comparisons showed differences, both in isolated VBGF parameters (χ2 test, df = 1, p < 0.015) and when combining Le∞ and k (χ2 test, df = 2, p < 0.015). The exception was the Sisal vs. Progreso comparison. In both the isolated Le∞ and k parameters, as well as their combination, the values were not significant (p > 0.015). These two locations may have similar environmental features, while those at Celestun are different. However, the to value showed a significant difference between Sisal and Progreso, whether in isolated Le∞ and k or when combined.
Graphic representations of growth (Figs. 3a and 3b) suggest that sea cucumbers younger than one year in age had Le < 18.9 cm (GW < 151 g) in Celestun, Le < 9.8 cm (GW < 30 g) in Sisal and Le < 15.3 cm (GW < 56 g) in Progreso. The largest recorded specimens were about five years of age in Celestun (Le > 30 cm and GW > 400 g), ten years of age in Sisal (Le > 35 cm and GW > 700 g) and four years of age in Progreso (Le > 22 cm and GW > 140 g). These results indicate that individuals measured during the study period were older than two years at both Celestun (mean ± SD: 25 ± 4.1 cm and 304.2 ± 158 g) and Progreso (mean ± SD: 20 ± 2.9 cm and 124.1 ± 81.1 g) and older than four years at Sisal (mean ± SD: 26.3 ± 4.8 cm and 350.8 ± 10.2 g).
Individual growth and relative ages by location of sea cucumber Isostichopus badionotus.
Left: seasonalized VBGF (von Bertalanffy growth function) by location in cm yr−1; Right: seasonalized VBGF by location in g yr−1. Relative ages were generated for each Le and GW datum by replacing the growth parameters calculated by ELEFAN I and PROJMAT with their respective values in the seasonalized VBGF, using sub-routines programmed in Wolfram Mathematica 9®. Le: Estimated length; GW: gutted weight.
Discussion
Biometric relationships are useful to carry out transformations of length to weight or vice versa and then calculate growth parameters. Length and weight relationships have not been established for all sea cucumber species and this is the first study that applies a compound index on I. badionotus. Length, as well as width, is another important biometric characteristic. Body length is a poor estimator of body weight in sea cucumbers and width provides little useful data per se. However, when length and width are combined to produce the SLW index, a modified version of the method proposed by Yamana and Hamano25, the analyses improved.
Using estimated length (Le) from SLW enhanced weight estimation accuracy by 80% in I. badionotus. This improvement in body weight estimation might be due to a significant variance reduction in length and width during organism handling. When measurements are carried out on live sea cucumbers, the act of handling can cause them to change shape elastically. As a result, the organism can deform along different symmetry axes (e.g. from left to right, top to bottom and forward to backward), changing individual measurements. Other advantages of SLW are that it facilitates in situ sea cucumber length and width measurements, is less time consuming to take measures and thus lowers organism stress level. The SLW compound index also showed multimodal frequency distribution, another advantage when identifying cohorts in growth studies through indirect methods.
The growth parameters reported here for I. badionotus are similar to those reported for other species in the family Stichopodidae (Table 4). A study done by Herrero-Perezrul et al.19 in the Pacific Ocean (Mexico), reports a growth rate for Isostichopus fuscus minor to the one found for I. badionotus in our study. This difference could be due to various reasons: 1) the deep water temperature in the East Pacific is colder than in the Gulf of Mexico, 2) I. fuscus specimens were measured underwater and, according to our field experience, the contraction of animals is inevitable, even underwater. 3) Herrero-Pérezrul et al.19 used a simple version of the VBGF, while we used a version that considers seasonality. Different authors have shown the existence of shrinking in sea cucumbers during certain periods as consequence of seasonal growth patterns due to changing environmental conditions17,31,32. As above, it is important to consider the version of the von Bertalanffy growth function with seasonality for sea cucumbers.
Isostichopus badionotus exhibits a negative allometric growth and our results provide strong evidence that growth in this species vary seasonally. The Eq. (2) results showed that growth was slower during October and November (WP = 0.8 and 0.9, respectively). Seasonal growth variations in the pooled sample showed growth to increase about 80% (C = 0.8) in the peak “growth season” (June to September) with an immediate 20% decline in October. In conclusion, the I. badionotus population on the northern continental shelf of the Yucatan Peninsula displays a variable growth pattern, with a rapid growth rate prior to October and a slower growth rate during October and afterwards.
Intra-annual oscillations in I. badionotus growth may be related to environment (e.g. water temperature, salinity, dissolved oxygen and transparency), as well as to biological processes such as seasonal cycles in feeding behavior for energy storage16. Growth variations coincided with seasonal changes (e.g. rain and cold front seasons) on the Yucatan coast, so these fluctuations and food availability may in turn impact condition factor. Growth rate was enhanced when environmental conditions were favorable and the organisms accumulated energy for an eventual reproduction season. In the present study, relative condition factor (Kn) did not decrease during the slow or zero growth seasons; on the contrary, it improved, perhaps due to nutrient storage in the body wall. There are evidences that physicochemical properties of the surface sediments linked to food availability for deposit-feeding detritivores determined the distribution pattern of Australostichopus mollis juveniles33. When organic matter availability was low, A. mollis juveniles ceased feeding and moved faster over the sea floor to new areas where the level of organic matter in the sediment was sufficiently high to stimulate feeding34.
Another characteristic related to seasonal growth in I. badionotus are inter-annual changes in biological condition. Relative condition factor was higher in 2010 than in 2009, principally during September. Gutted weight (GW) gave important information about biological condition of organisms. GW had a high coefficient of variation (CV = 56.9%), caused by individuals measured in 2009, as shown in the L-GW relationships (Fig. 2). The large variations in GW indicate that body walls were thinner in 2009 than in 2010; a trend confirmed by lower relative condition factor (Kn) values in 2009, particularly in July and September (Table 2). These variations occurred from June to November. Similar to condition factor, growth rate decreased to the point of body shrinkage during periods of environmental stress (e.g. algal blooms, hurricanes and winter storms), most likely in response to limited food availability.
Hurricanes have been shown to have short-term impacts on marine environments35. In September 2010, tropical storm Matthew36 impacted Belize and southern Mexico, producing torrential rainfall on the northern Yucatan coast in September and October. It was during September 2010 that the highest Kn values were recorded. Another phenomenon that may have affected sea cucumber stocks in this period was a large harmful algal bloom in summer 2008 off the Yucatan coast37. It negatively affected food availability in the regional marine ecosystem and caused widespread mortality in different marine organisms groups. Indeed, sea cucumbers disappeared from a number of locations and abundance was decimated in the northern and western coastal areas38. Small I. badionotus individuals were collected in summer 2009, an indication of recruitment and stock recovery. The thinner body walls recorded in 2009 may therefore be the aftermath of the widespread mortality and food scarcity caused by the harmful algal bloom.
The sea cucumber body wall is an important organ for nutrient storage during feeding periods39. A laboratory study showed that I. badionotus enters a 12-month dormant phase in response to adverse environmental conditions. Coelomic fluid protein concentration increased, suggesting optimization of the protein synthesis process40. A similar phenomenon has been reported for A. japonicus during dormancy periods, when body wall tissue crude protein, lipids and carbohydrates contents reach maximum values41. These authors found that body wall biochemical composition was significantly influenced by the environment, particularly by seasonal changes. This has been observed also in other species of sea cucumbers of the family Stichopodidae, like A. mollis, whose growth and survival rates fluctuate significantly and are suboptimal at poor-quality environments or low food availability42.
Our results confirm that I. badionotus has developed physiological mechanisms to face adverse conditions, for instance with a radically reduction of metabolism40. Nonetheless, our data also suggest that the species still remains sensitive to meteorological and oceanographic phenomena due probably to changes of water temperature and food availability. Zamora and Jeffs43 have showed that sea cucumbers can respond negatively to an increase in seawater temperature in terms of feeding, metabolism and growth.
Even though most sea cucumber fisheries are managed without age and growth studies, understanding these parameters can help in improving management measures. The SLW index reduces the variability of body length, which facilitates the calculation of growth parameters by indirect methods. The seasonal growth patterns in sea cucumbers can be well approached by one seasonally oscillating version of the VBGF, which may have implications in fisheries management. The data provided here will be vital to fishery managers, who need to consider environmental variability and biological stock condition when developing sustainable management strategies. Furthermore, these results are potentially relevant to develop aquaculture systems for this and other similar sea cucumber species in tropical ecosystems.
Methods
Organism collection and measurement were done at three locations off the northwest coast of Yucatan state, Mexico: (i) 20° 51′ 48″N, 90° 24′ 00″ W (off Celestun); (ii) 21° 09′ 15″ N, 90° 02 09″ W (off Sisal); and (iii) 21° 17 11 N, 89° 39′ 07″ W (off Progreso). Isostichopus badionotus individuals were collected during four months in 2009 (July, September, November and December) and 11 months in 2010 (all months but March). Unfavorable weather prevented collection in August and October 2009, March 2010 and May to December 2010 at Progreso. Individuals were collected at 10 to 18 m depth by SCUBA diving. The smallest individuals (from 3 to 12 cm length) were taken between 15 and 18 m depth on rocky reef areas with encrusting algae. The largest individuals were found on rocks, pavements and sandy beds with turf algae and hard corals (mainly blushing star and rose corals). Once collected, individuals were relaxed by placement in shaded containers with 10 L seawater and 24 g magnesium chloride (MgCl2) for 5 to 10 min. They were then measured.
Length (L), width (W) and total weight (TW) were measured for each individual. Length was measured dorsally from the anus to the center of the tentacle crown (curved length). Width was also measured dorsally, at the widest point17,29, excluding papillae on the ventrolateral margin and the ventral surface. Both length and width were measured to the nearest 0.1 cm with a tailor's tape. Total weight was measured with a digital balance (Torrey LEQ-5/10 Scale, ±1 g). In some individuals, the gut, gonad (if present), respiratory trees and coelomic fluid were removed and body wall weight (muscle wet weight or gutted weight) (GW) measured. Body length and width were used to calculate the compound index SLW (square root of length multiplied by width)25. Coefficients of variation were calculated for this index and each body measurement parameter. Collections were granted by fishing license no. DGOPA.08263.290709.2467 from CONAPESCA (Comision Nacional de Acuacultura y Pesca).
Biometric relationships
Lengths were recalculated (Le) using the regression between Lvs. SLW to increase growth parameter accuracy25. Description of relative changes in morphology was done using the allometric equation:

Where α and β are parameters, x is length (L or Le) and y is weight (TW or GW). Furthermore, four simple regression analyses were applied: L vs. TW, L vs. GW, Levs. TW and Le vs. GW. Parameters were calculated with non-linear regression analysis, an effective tool in allometric studies since it simultaneously considers variation in both x and y variables44. The loss functions were evaluated with Marquardt's compromise algorithm45, which minimizes the sum of squares (SSQ) of the difference between sample weights and pseudo-weights46. Convergence was reached at 50 iterations in each regression. A Student t-test was conducted to test the hypothesis of isometry (β = 3). Allometric growth occurred when either β > 3 (negative) or β < 3 (positive). Additionally, organism condition was analyzed using the relative condition factor47Kn as follows: Kn = GW/GW′, where GW is gutted weight of individual sea cucumbers and GW′ is predicted gutted weight. The Eq. (1) was applied to predict GW′ as follows: GW′ = α(Le)β.
Growth analysis
Different Le class intervals were evaluated and two indirect methods were applied: 1) Electronic Length Frequency Analysis (ELEFAN I)48; and 2) Projection Matrix (PROJMAT)49. One seasonally oscillating version of the von Bertalanffy growth function (VBGF) was applied as follows50:

Where Lt is predicted size at age t; L∞ is asymptotic size; k is curvature parameter per year−1, expressing the rate at which L∞ is approached; and to is theoretical “age” if organism were to have a size equal to zero. Considering seasonal growth oscillations, ts defines the start of the convex segment of a sinusoid oscillation with respect to t = 0 and C is the parameter reflecting the intensity of seasonal growth oscillation. To calculate Le∞, Le was used rather than L∞.
Growth parameters were generated with the Length Frequency Distribution Analysis (LFDA) Version 5 software package (http://www.fmsp.org.uk/Software.htm) and compared using the growth performance index51 ϕ′. The method was chosen with lowest variation ϕ′ between intervals and the interval with the best goodness-of-fit. The influence of each monthly sample in estimating growth parameters was determined with the estimator Δ% and a jackknifed coefficient of variation CVJ, as described by Levi et al.52. Based on the growth coefficient β, estimated from the allometric equation GW′ = α (Le)β, the Eq. (2) was also expressed in terms of GW46.
Relative ages at certain lengths and weights were generated by replacing the growth parameters with their respective values in the Eq. (2), using sub-routines programmed in Wolfram Mathematica 9® (license number 3422–6765).
Growth for individuals from the three sampled locations was compared by likelihood ratio tests53 and three simultaneous tests (Celestun vs. Sisal, Celestun vs. Progreso and Sisal vs. Progreso) were performed to evaluate pair-wise differences in growth. These tests were equivalent to testing h independent hypotheses at α significance level. By applying Bonferroni inequality to the three tests resulted in an experiment wise significance level ≤ h·α. An α = 0.015 was chosen, resulting in a 0.045 experiment wise significance level. First, a simultaneous comparison was done of all three parameters under the null hypothesis: Ho: L∞1 = L∞2; K1 = K2; to1 = to2. The model selection process was then done by sequentially altering the number of parameters in the comparison.
References
Purcell, S. Managing Sea Cucumber Fisheries with an Ecosystem Approach. (FAO Fisheries and Aquaculture Technical Paper No. 520., 2010).
Anderson, S. C., Flemming, J. M., Watson, R. & Lotze, H. L. Serial exploitation of global sea cucumber fisheries. Fish and Fish. 12, 317–339 (2011).
Purcell, S. W. et al. Sea cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish and Fish. 14, 34–59 (2013).
Food and Agriculture Organization of the United Nations. Code of Conduct for Responsible Fisheries Special Edition. (FAO, 2011).
Zetina, C. et al. Sea Cucumber (Astichopus multifidus, Isostichopus badionotus and Holothuria floridana) biomass estimation in two areas of Yucatán coast between October of 2000 to March of 2001 Year. Proc. of the GCFI 60, 298–306 (2003).
Alfonso, I., Frías, M. P., Aleaga, L. & Alonso, C. R. Current status of the sea cucumber fishery in the south eastern region of Cuba. In: Lovatelli A., et al., eds. Advances in Sea Cucumber Aquaculture and Management. (FAO, 2004).
Guzmán, H. M. & Guevara, C. A. Population Structure, Distribution and Abundance of Three Commercial Species of Sea Cucumber (Echinodermata) in Panama. Caribb. J. Sci. 38, 230–238 (2002).
Rodríguez, L. A., Reyes, C. F., Alpizar, R. & Tello, J. Sea Cucumber Population and Biomass Estimate for New Fishing Limit Assignation in Sisal Fishing Cooperative, through the Yucatan State Coast. Proc. of the GCFI 60, 547–553 (2007).
Selenka, E. Beiträgezur Anatomie und Systematik der Holothurien. Z. Wiss. Zool. 17, 291–374, pls. 17–20 (1867).
Miller, J. E. & Pawson, D. L. Holothurians (Echinodermata: Holothuroidea). Memoirs of the Hourglass Cruises. (Marine Research Laboratory. Florida, USA, Vol VII, Part 1, 1–79, 1984).
Pawson, D. L., Pawson, D. J. & King, R. A. A taxonomic guide to the Echinodermata of the South Atlantic Bight, USA: 1. Sea cucumbers (Echinodermata: Holothuroidea). Zootaxa 2449, 1–48 (2010).
Hammond, L. S. Patterns of feeding and activity in deposit-feeding holothurians and echinoids (Echinodermata) from a shallow back-reef lagoon, Discovery Bay, Jamaica. Bull. Mar. Sci. 32, 549–571 (1982).
Guzmán, H. M., Guevara, C. A. & Hernández, I. C. Reproductive cycle of two commercial species of sea cucumber (Echinodermata: Holothuroidea) from Caribbean Panama. Mar. Biol. 142, 271–279 (2003).
Frías del P, M., Alfonso, I., Castelo, R. & Blas, Y. Length-weigth variations of sea cucumber Isostichopus badionotus (Selenka, 1867) in the south eastern and south western regions of Cuba. Rev. Cub. Investig. Pesq. 25, 38–45 (2008).
Conand, C. 1990. The Fishery Resources of Pacific Island Countries. Part 2. Holothurians. (FAO Fisheries Technical Paper No. 272.2., 1990).
So, J. J., Hamel, J.-F. & Mercier, A. Habitat utilization, growth and predation of Cucumaria frondosa: implications for an emerging sea cucumber fishery. Fish. Manag. Ecol. 17, 473–484 (2010).
Hannah, L., Duprey, N., Blackburn, J., Hand, C. M. & Pearce, C. M. Growth rate of the California sea cucumber Parastichopus californicus: measurement accuracy and relationships between size and weight metrics. N. Am. J. Fish. Manag. 32, 167–176 (2012).
Conand, C. Reproductive biology of the holothurians from the major communities of the New Caledonia Lagoon. Mar. Biol. 116, 439–450 (1993).
Herrero-Pérezrul, M. D., Reyes, H., García-Domínguez, F. & Cintra-Buenrostro, C. E. Reproduction and growth of Isostichopus fuscus (Echinodermata: Holothuroidea) in the southern Gulf of California, México. Mar. Biol. 135, 521–532 (1999).
Conand, C. Reproductive cycle and biometric relations in a population of Actinopyga echinites (Echinodermata: Holothuroidea) from the lagoon of New Caledonia, western tropical Pacific. In: Lawrence J. M. ed. Echinoderms: Proceedings of the International Conference, Tampa Bay. 14–17 September 1981. (AA Balkema, Rotterdam 437–442 1982).
Mercier, A., Battaglene, S. C. & Hamel, J.-F. Periodic movement, recruitment and size-related distribution of the sea cucumber Holothuria scabra in Solomon Islands. Hydrobiologia 440, 81–100 (2000).
Laboy-Nieves, E. N. & Conde, J. E. A new approach for measuring Holothuria mexicana and Isostichopus badionotus for stock assessments. SPC Beche-de-mer Inf. Bull. 24, 39–44 (2006).
Conand, C. Sexual cycle of three commercially important Holothurian species (Echinodermata) from the lagoon of New Caledonia. Bull. Mar. Sci. 31, 523–543 (1981).
Battaglene, S. C., Seymour, J. E. & Ramofafia, C. Survival and growth of cultured juvenile sea cucumbers, Holothuria scabra. Aquaculture 178, 293–322 (1999).
Yamana, Y. & Hamano, T. New size measurement for the Japanese sea cucumber Apostichopus japonicus (Stichopodidae) estimated from the body length and body breadth. Fish. Sci. 72, 585–589 (2006).
Slater, M. J. & Carton, A. G. Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with green-lipped mussel farms. Aquaculture 272, 389–398 (2007).
Wheeling, R. J., Verde, E. A. & Nestler, J. R. Diel cycles of activity, metabolism and ammonium concentration in tropical holothurians. Mar. Biol. 152, 297–305 (2007).
Paltzat, D. L., Pearce, C. M., Barnes, P. A. & McKinley, R. S. Growth and production of California sea cucumbers (Parastichopus californicus Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas Thunberg). Aquaculture 275, 124–137 (2008).
Watanabe, S., Zarate, J. M., Sumbing, J. G., Hazel, M. J. & Nievales, M. F. Size measurement and nutritional condition evaluation methods in sandfish (Holothuria scabra Jaeger). Aquacult. Res. 43, 940–948 (2011).
Purcell, S. W., Gossuin, H. & Agudo, N. N. Status and management of the sea cucumber fishery of la Grande Terre, New Caledonia. (Programme ZoNéCo. World Fish Center Studies and Reviews No. 1901., The World Fish Center, Penang, Malaysia, 2009).
So, J. J., Hamel, J.-F. & Mercier, A. Habitat utilisation, growth and predation of Cucumaria frondosa: implications for an emerging sea cucumber fishery. Fish. Manag. Ecol. 17, 473–484 (2010).
Uthicke, S. & Benzie, J. A. H. A genetic fingerprint recapture technique for measuring growth in ‘unmarkable’ invertebrates: negative growth in commercially fished holothurians (Holothuria nobilis). Mar. Ecol. Prog. Ser. 241, 221–226 (2002).
Slater, M. J., Carton, A. G. & Jeffs, A. G. Highly localised distribution patterns of juvenile sea cucumber Australostichopus mollis. N. Z. J. Mar. Freshw. Res. 44, 201–216 (2010).
Slater, M. J., Jeffs, A. G. & Sewell, M. A. Organically selective movement and deposit-feeding in juvenile sea cucumber, Australostichopus mollis determined in situ and in the laboratory. J. Exp. Mar. Biol. Ecol. 409, 315–323 (2011).
Herrera-Silveira, J. A. & Morales-Ojeda, S. Evaluation of the health status of a coastal ecosystem in southeast Mexico: Assessment of water quality, phytoplankton and submerged aquatic vegetation. Mar. Pollut. Bull. 59, 72–86 (2009).
Brenannan, M. J. Tropical cyclone report: Tropical Storm Matthew (ALI52010) 23–26 September 2010. (http://www.nhc.noaa.gov/pdf/TCR-AL152010_Matthew.pdf). National Hurricane Center. Retrieved on 25th April 2014.
Enriquez, C., Mariño-Tapia, I. J. & Herrera-Silveira, J. A. Dispersion in the Yucatán coastal zone: Implications for red tide events. Cont. Shelf. Res. 30, 127–137 (2010).
Zetina-Ríos, K. E., Moreno-Mendoza, R., Domínguez-Cano, R. & Ríos-Lara, G. V. Co-management for study of rocky habitats affected for red tide in Yucatan coast, Mexico. Proc. of the GCFI 61, 283–286 (2008).
Mercier, A. & Hamel, J.-F. Endogenous and Exogenous Control of Gametogenesis and Spawning in Echinoderms (Advances in Marine Biology. Vol. 55, Elsevier, Amsterdam, 2009).
Gullian, M. Physiological and immunological conditions of the sea cucumber Isostichopus badionotus (Selenka, 1867) during dormancy. J. Exp. Mar. Biol. Ecol. 444, 31–37 (2013).
Gao, F., Xu, Q. & Yang, H. Seasonal biochemical changes in composition of body wall tissues of sea cucumber Apostichopus japonicus. Chin. J. Oceanol. Limnol. 29, 252–260 (2011).
Zamora, L. N. & Jeffs, A. G. A review of the research on the Australasian sea cucumber, Australostichopus mollis (Echinodermata: Holothuroidea) (Hutton 1872), with emphasis on aquaculture. J. Shellfish. Res. 32, 613–627 (2013).
Zamora, L. N. & Jeffs, A. G. Feeding, metabolism and growth in response to temperature in juveniles of the Australasian sea cucumber, Australostichopus mollis. Aquaculture 358–359, 92–97 (2012).
Ebert, T. A. & Russel, M. P. Allometry and model II non-linear regression. J. Teor. Biol. 168, 367–372 (1994).
Marquardt, D. W. An algorithm for least-squares estimation of non linear parameters. J. Soc. Indust. Appl. Math. 11, 431–41 (1963).
Gayanilo, F. C. & Pauly, D. FAO-ICLARM Stock Assessment Tools. Reference Manual. (FAO Comput. Inf. Ser. Fisheries 8 1997).
Le Cren, E. D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219 (1951).
Gayanilo, F. C., Sparre, P. & Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised version. User′s guide. (FAO Comput. Inf. Ser. Fisheries 8, 2005).
Shepherd, J. G. 1987. A weakly parametric method for the analysis of length composition data. In: Pauly D., Morgan G. P., eds. Length-based Methods in Fisheries Research. (ICLARM Conf. Proc. 13, Manila, 1987).
Hoenig, N. A. & Hanumara, R. C. An empirical comparison of seasonal growth models. Fishbyte 8, 32–34 (1990).
Pauly, D. & Munro, J. L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 2, 21 (1984).
Levi, D., Andreoli, M. G. & Cannizzaro, L. Use of ELEFAN I for Sampling Design. In: Pauly D., Morgan G. P., eds. Length-based Methods in Fisheries Research. (ICLARM Conf. Proc. 13, Manila, 1987).
Kimura, D. K. Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 77, 765–775 (1980).
Sulardiono, B., Prayitno, S. B. & Hendrarto, I. B. The growth analysis of Stichopus vastus (Echinodermata: Stichopodidae) in Karimunjawa waters. J. Coast. Dev. 15, 315–323 (2012).
Hamano, T., Amio, M. & Hayashi, K. Population dynamics of Stichopus japonicas Selenka (Holothuroidea, Echinodermata) in an intertidal zone and on the adjacent subtidal bottom with artificial reefs for Sargassum. Suisanzoshoku 37, 179–186 (1989).
Conand, C. Les holothuries Aspidochirotes du Lagon de Nouvelle Calédonie: biologie, écologie et exploitation. (Etudes et Thèses. Editions de l'ORSTOM Paris, France, 1989).
Acknowledgements
The research reported here constitutes partial fulfillment of the requirements for the doctoral degree of the first author at Cinvestav, Mexico. The authors thank Leonardo Arellano Mendez, Lucio Loman Ramos, Enrique Poot Salazar, Angeles Heredia and the local fishermen at Celestun, Sisal and Progreso for their assistance in the field. Special thanks are due Dr. Juan M. Camacho for his assistance with Wolfram Mathematica 9® and his comments on earlier versions of the manuscript. Thanks are also due to Dr. Gabriela Galindo and Dr. Omar Defeo Gorospe for their suggestions. Financial support was provided by the research fund of the Laboratorio de Bentos, Cinvestav and a scholarship from CONACYT (Mexico) to APS.
Author information
Authors and Affiliations
Contributions
A.P.-S. designed the study, analyzed the data, performed the statistical analyses and wrote the manuscript. P.-L.A. designed and supervised the study. A.H.-F. contributed to the analysis and interpretation of the results and wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Poot-Salazar, A., Hernández-Flores, Á. & Ardisson, PL. Use of the SLW index to calculate growth function in the sea cucumber Isostichopus badionotus. Sci Rep 4, 5151 (2014). https://doi.org/10.1038/srep05151
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05151
This article is cited by
-
Population Index and Growth Estimation Based On SLW Index of the Sea Cucumber (Holothuria arguinensis Koehler & Vaney, 1906) in the Moroccan Waters of the Atlantic Coast
Thalassas: An International Journal of Marine Sciences (2022)
-
Allometric relationships to assess ontogenetic adaptative changes in three NE Atlantic commercial sea cucumbers (Echinodermata, Holothuroidea)
Aquatic Ecology (2021)
-
To Estimate Growth Function by the Use of SLW Index in the Sea Cucumber Holothuria arenicola (Holothuroidea: Echinodermata) of Pakistan (Northern Arabian Sea)
Thalassas: An International Journal of Marine Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.