Abstract
The human murine double minute 2 (MDM2) is known as an oncoprotein through inhibiting P53 transcriptional activity and mediating P53 ubiquitination. Therefore, the amplification of MDM2 may attenuate the P53 pathway and promote tumorigenesis. The SNP309 T>G polymorphism (rs2279744), which is located in the intronic promoter of MDM2 gene, was reported to contribute to the increased level of MDM2 protein. In this hospital-based case-control study, which consisted of 573 cases and 588 controls, we evaluated the association between MDM2 SNP309 and the risk of colorectal cancer (CRC) in a Chinese population by using the TaqMan method to genotype the polymorphism. We found that the MDM2 SNP309 polymorphism was significantly associated with CRC risk. In addition, in our meta-analysis, we found a significant association between MDM2 SNP309 and CRC risk among Asians, which was consistent with our results. In conclusion, we demonstrated that the MDM2 SNP309 polymorphism increased the susceptibility of CRC in Asian populations.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of death from cancer worldwide, which accounts for an estimated 1,330,000 new cases and 608,000 cancer deaths in 20081. The incidence rates are high in Australia/New Zealand and Western Europe, low in Africa and South-Central Asia and intermediate in Latin America1. In the USA, CRC was the third leading cancer type for estimated new cancer cases and deaths in 20132. In China, epidemiological data showed that there was an annual increase of 3.33% in CRC incidence and 3.05% in CRC mortality during 2003~20073. The mechanisms underlying the development of CRC are complex. Both environmental and genetic factors play an important role in the occurrence and progression of CRC4. Genetic epidemiology and twin studies demonstrate that upwards of 35% of the CRC cases may be due to inherited factors, which indicates the importance of inherited genetic susceptibility in carcinogenesis5.
P53, the tumor suppressor protein, plays a crucial role in multi-cellular functions, including gene transcription, DNA synthesis and repair, growth arrest, cell senescence and apoptosis6. p53 mutations that disrupt the balance between cell apoptosis and repair are found in at least half of all human cancers, which highlight a critical role of P53 in tumor suppression7. The human homolog of the mouse double minute 2 (MDM2) functions as an important negative regulator of P53 through an autoregulatory feedback loop. The elevated nuclear P53 level will activate MDM2 gene transcription and increase the protein expression of MDM2. MDM2 will inhibit the transcriptional activity of P53 through its direct binding to P53 and also serve as an E3 ubiquitin ligase, promoting the degradation of P538,9,10,11. Thus, MDM2 overexpression may disturb this feedback loop and cause the deficiency of P53, which will result in inefficient growth arrest and/or apoptosis. Amplification of MDM2 is observed in many human tumor tissues, including CRC12,13,14. Consequently, up-regulated expression of MDM2 and attenuation of P53 pathway has been observed15.
MDM2 SNP309 (rs 2279744), which is located in the promoter of MDM2 gene, was identified as a functional single nucleotide polymorphism (SNP). This SNP is a novel T to G substitution located at the 309th nucleotide in the first intron, showing a greater binding affinity for the transcription factor Sp115. Therefore, it was hypothesized that the genetic variant might have an impact on the expression of MDM2 and affect the individual's susceptibility to developing tumors. Many studies have evaluated this association in different tumors, but their results are conflicting16,17,18. Some studies have reported a direct connection between MDM2 SNP309 and CRC risk19,20,21; however others have shown the opposite22,23.
Recently, Zhang et al. has shown no direct association between MDM2 SNP309 and CRC, but a combined effect of TP53 Arg72Pro and MDM2 SNP309 showed an increased CRC risk in a Chinese population24. Considering this conclusion is only based on the central Chinese demographics, more studies are needed to confirm this finding. Therefore, in this study, we genotyped the MDM2 SNP309 and evaluated its association with CRC risk in a population from the southeast of China.
Results
Study characteristics
The characteristics of our study are shown in Table 1. No significant differences were found between cases and controls for age [cases vs. controls (mean ± SD), 60.3 ± 12.5 vs. 59.3 ± 9.8 years; P = 0.136], sex (P = 0.824), smoking status (P = 0.191) and alcohol use (P = 0.082). And these variables were adjusted for in the multivariate logistic regression analysis. As expected, however, CRC patients had a higher rate of family history of cancer than that of the controls (P < 0.001). Of the 573 CRC cases, the frequencies of the Dukes A, B, C and D stage were 9.1%, 40.6%, 35.1% and 15.2%, respectively. For tumor grade, 6.5% of patients were with poor-differentiated tumors; 74.9% and 18.6% were found with moderate and well-differentiated tumors, respectively.
Association between MDM2 SNP309 and CRC risk
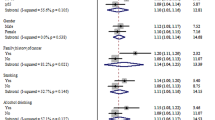
The genotype distributions of MDM2 SNP309 in the control group were in accordance with the HWE (P = 0.805). The genotype frequencies of MDM2 SNP309 were 19.4% (TT), 51.5% (TG) and 29.1% (GG) in cases, which were statistically different from that in the control group (25.5% TT, 49.5% TG and 29.1% GG) (P = 0.031). After adjusting for age, sex, smoking status and drinking status, multivariate logistic regression analysis revealed that the individuals carrying the TG or GG genotype had an increased CRC risk (OR = 1.36, 95% CI = 1.01–1.82 for TG vs. TT; OR = 1.53, 95% CI = 1.10–2.13 for GG vs. TT), compared with the TT genotype. We also found that the MDM2 SNP309 TG/GG genotypes were associated with higher CRC susceptibility (OR = 1.41, 95% CI = 1.07–1.87) (Table 2). In the stratified analyses based on the dominant model, we found individuals carrying MDM2 SNP309 (TG/GG) were associated with increased risk among older subjects (OR = 1.76, 95% CI = 1.17–2.64), males (OR = 1.52, 95% CI = 1.06–2.19), smokers (OR = 1.90, 95% CI = 1.11–3.27) and non-drinkers (OR = 1.42, 95% CI = 1.03–1.96) (Table 3). Furthermore, we also assessed the association between the MDM2 SNP309 polymorphism and clinicopathological characteristics of CRC. As shown in Table 4, the individuals carrying the TG/GG genotypes were found to have an increased risk in rectal cancer (OR = 1.50, 95% CI = 1.06–2.14), well-differentiated CRC (OR = 2.07, 95% CI = 1.16–3.69) and early stage cancer (Dukes A and B) (OR = 1.55, 95% CI = 1.08–2.21). In addition, the median age of tumor onset according to the genotype of MDM2 SNP309 was evaluated. No significant differences were found in the median ages among men [62.0 for TT, 63.0 for TG and 62.0 for GG (P = 0.895)]. Moreover, neither younger women (≤57 years) [46.0 for TT, 47.0 for TG and 48.0 for GG (P = 0.246)], nor older women (> 57 years) [66.5 for TT, 68.0 for TG and 68.5 for GG (P = 0.371)] showed statistical differences in the median ages of tumor onset.
Meta-analysis of MDM2 SNP309 and CRC risk
We performed a meta-analysis to evaluate the association between MDM2 SNP309 and CRC risk. A total of 11 studies were selected, which included 4 studies of Asian population and 7 studies in Europeans (Table 5). Then we pooled the previous published studies and our present study together and this meta-analysis consisted of 3744 cases and 3185 controls.
The MDM2 SNP309 (TG/GG) carriers among Asians were associated with higher CRC risks (OR = 1.20, 95% CI = 1.03–1.38) (Fig. 1C). And significantly increased risks of CRC were also observed in Asians with TG (OR = 1.20, 95% CI = 1.03–1.40) (Fig. 1A) or GG (OR = 1.21, 95% CI = 1.01–1.45) (Fig. 1B), when compared with SNP309 TT. However, these results were not found in Europeans (Table 6). In the total population, no statistical association between the MDM2 SNP309 polymorphism and CRC risk were found in all genetic models under random-effects model (P value for heterogeneity < 0.1). Thus we used a Galbraith plot to investigate the source of heterogeneity and found one article with an European population21, which could potentially be the cause of high heterogeneity (Fig. 2). After excluding that specific study, we analyzed the data again. With low heterogeneity, statistical associations with risk of CRC were found in the dominant model (Fig. 1), but the associations were still not observed in Europeans. In addition, publication bias was assessed by the Begg's and Egger's tests and no evidence of publication bias in all genetic models was found (t = 0.15, P = 0.880 for TG vs. TT; t = −0.19, P = 0.851 for GG vs. TT; t = 0.08, P = 0.937 for dominant model; t = −0.44, P = 0.672 for recessive model).
Discussion
As reported, MDM2 can directly bind to P53 and down-regulate its function as a tumor suppressor. The oncogenic properties of MDM2 are thought to be P53-dependent. However, some studies have shown that MDM2 may form complexes with other tumor suppressor proteins independent of P53 in vitro and in P53-deficient cells34,35. These findings demonstrate the oncogenic potential of MDM2 in P53-independent pathways. In addition, although MDM2 SNP309 is located on a P53-response intronic promoter, the P53-independent overexpression of MDM2 was still observed36. Moreover, MDM2 amplification might also be regulated in post-transcriptional ways37,38. All aforementioned findings indicate that complex mechanisms underlie the regulation of MDM2 gene during tumorigenesis. Considering that MDM2 SNP309 may regulate the MDM2 expression, it is meaningful for us to evaluate its association with cancer risk.
In CRC, whether MDM2 SNP309 has a direct effect on carcinogenesis is still controversial. Some studies show that the TG genotype is associated with higher CRC risk than the TT genotype19,20,21, whereas, others show no association22,23. Recently, Zhang et al. reported a combined effect of TP53 Arg72Pro and MDM2 SNP309 in a dose-response fashion, increasing CRC risk in the population from the central region of China, but no association between MDM2 SNP309 alone and CRC risk was found24. In our study population, the MDM2 SNP309 carriers, either with TG or GG, were found to have an increased CRC risk compared with those carrying TT genotype. To resolve this conflict and further validate our results, we pooled the published data and our current data together and then did a meta-analysis. In this meta-analysis, we found that MDM2 SNP309 contributed to an increased CRC susceptibility in Asians, which was consistent with our present study. And the similar association in the dominant model was also observed in the combined populations across studies if we excluded one article which was the main cause of the high heterogeneity. Interestingly, a meta-analysis published by Cao et al. also found a significantly increased CRC risk among the individuals with TG genotype, especially among Asians, when compared with TT genotype39. However, we found no association between MDM2 SNP309 and CRC risk among Europeans. Considering the frequencies of the MDM2 SNP309 G allele among the cases and controls were different by ethnicity (MAF: 0.47 in Asians and 0.39 in Europeans), it has indicated a possible ethnic difference in genetic backgrounds. Moreover, a second MDM2 promoter polymorphism named SNP285 (a G to C transversion located only 24 bp upstream of SNP309), which is present only among Caucasions, was reported to reduce Sp1 binding and antagonize the affinity of Sp1 with an enhanced effect by SNP30940. Thus, MDM2 SNP285 may be another explanation for the differential effect of MDM2 SNP309 between ethnicities.
The effect of MDM2 SNP309 on CRC risk was found more pronounced among the older people, which may reflect the accumulative effects of risk factors, such as prolonged red meat consumption41. Increased CRC risk associated with MDM2 SNP309 was found only in men but not in women, which was consistent with a previous study20 and we also did not have a biological explanation. Some authors reported that the interaction between TP53 Arg72Pro and MDM2 SNP309 was associated with elevated CRC risk in smokers but not in non-smokers24. In the stratified analysis, we found MDM2 SNP309 had a direct connection with CRC risk in smokers and also not in non-smokers. Long-term smoking has been reported as a risk factor for CRC42. MDM2 SNP309 might influence the activity of P53 and then increase the possibility that some colon cells damaged by tobacco carcinogens might escape the apoptosis triggered by P53. Therefore, smokers carrying MDM2 SNP309 are expected to have a higher risk of CRC but further validation is still needed. Alcohol consumption is also associated with CRC risk43 and has already been reported to be related with p53 mutations in breast cancer44. Therefore, drinkers with MDM2 SNP309 should be associated with higher CRC risk. However, in our study, this association was not found. The relative small sample size after stratifying for drinking status may be the reason. After stratifying the tumor stage and grade, we observed that the MDM2 SNP309 was associated with an increased risk in CRC patients with Duke's A/B stage or well-differentiated tumor grade, which indicated the involvement of SNP309 in the early stages of CRC. The family history of cancer in our study is not matched and it might be important for the better understanding of the genetic variants. However, in our analysis, the effect of family history on the association between MDM2 SNP309 and CRC risk was not observed.
A significant earlier age of onset was observed to be associated with MDM2 SNP309 in several tumors15. In CRC, several studies showed this association especially in women, but not in men22,45. The MDM2 promoter, where SNP309 is located, is regulated by hormonal signaling pathways. Therefore, it is hypothesized that the increased affinity of female-specific hormones such as estrogen, caused by the gene variant, might accelerate tumor formation46. And higher frequencies of the SNP309 G allele in CRC were found in women at a younger or premenopausal age than in women at a older or menopausal age and in men46, which supported the hypothesis in some extent. Because we did not have the data of menopausal age, we only compared the onset age of CRC in younger and older women based on the median age (60 years) separately. However, no statistical difference was observed between CRC onset age of the SNP309 carriers and individuals with TT genotypes in younger or older women. Several studies have shown conclusions consistent with ours20. But there is still one more thing we should consider. Menin et al. reported that MDM2 SNP309 may affect the age of cancer onset only in the tumors with wild-type P5327. The lack of the information of the p53 mutation status in the tumors might influence our results. Thus, further studies about p53 mutations are required to resolve this conflict.
In conclusion, we demonstrated that MDM2 SNP309 was associated with increased CRC risk in a Chinese population, which was concordant with our meta-analysis. Additionally, in the stratified analyses, we found that increased risk was more pronounced in males, older people, smokers, non-drinkers, people diagnosed with rectal cancer and patients with Duke's A/B stage or well-differentiated tumor grade. Moreover, the earlier age of cancer onset in patients carrying MDM2 SNP309 was not found in our study. Considering the correlation between MDM2 and P53, the status of P53 is necessary for further studies. Further validation of large population-based studies in different ethnicities is still needed.
Methods
Ethics statement
The study was approved by the institutional review board of Nanjing Medical University. Informed written consent was obtained from all subjects. The experimental protocol was carried out in accordance with the approved guidelines.
Study subjects
The characteristics of the CRC patients and cancer-free controls in this study have been previously described in detail25. Briefly, this study consisted of 573 patients with CRC and 588 cancer-free controls. All the patients with histologically-confirmed CRC were consecutively recruited from September 2010 at the First Affiliated Hospital of Nanjing Medical University, Nanjing, China, without age or sex restrictions. The cancer-free control patients, who were genetically unrelated to the CRC patients, were matched by age (±5 years) and sex to the CRC patients. A trained personnel interviewed each participant after obtaining the signed informed consent and a structured questionnaire on demographic information and environmental exposures. Individuals who smoked daily for at least one year were defined as smokers. People who consumed one or more alcoholic drinks per week for more than one year were defined as drinkers. After the interview, a 5 ml venous blood sample was obtained from each patient for genomic DNA extraction.
DNA extraction and genotyping
Genomic DNA was obtained from white-blood-cell fractions by using the Qiagen Blood Kit (Qiagen) following the manufacturer's protocol. We used the 384-well ABI 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) for the TaqMan SNP Genotyping assay. Two people achieved this genotype analysis independently in a blind fashion. We also randomly selected 10% of our samples for repeated genotyping to assess the reproducibility and the concordant rate was 100%.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) of alleles was evaluated by using a goodness-of-fit chi-square test. The differences in demographic characteristics, selected variables and frequencies of the genotypes were tested using a Student's t-test (for continuous variables) or Pearson's chi-square test (for categorical variables). The Kruskal-Wallis Test was used to compare the age of tumor onset according to the genotype of MDM2 SNP309. The association between MDM2 SNP309 and CRC risk was assessed by odds ratios (ORs) and 95% confidence intervals (CI) using unconditional logistic regression analysis with the adjustment for possible confounders. All data analyses were two-sided and performed with Statistical Analysis System software (version 9.1.3; SAS Institute Inc, Cary, NC, USA).
Meta-analysis
To further evaluate the association between the MDM2 SNP309 and CRC risk, we performed a meta-analysis based on the previous published studies and our current study. The databases of PubMed, Embase and Web of Science updated on April 1, 2013, were searched for articles based on the human associated case-control studies in English, using the terms: “MDM2”, “polymorphism(s) or genetic variation(s)”, “colorectal” and “cancer or carcinoma or tumor” as well as their combinations. Finally, we collected 11 studies consisting of a total of 3171 cases and 2597 controls19,20,21,22,23,24,26,27,28,29. Because the studie published by Chaar21 was found to be the potential cause of high heterogeneity, we excluded this study and analyzed the rest again. ORs and 95% CIs were used to determine the strength of association between MDM2 SNP309 and CRC risk and we used Z-tests to estimate the statistical significance of the pooled OR. A fixed-effects model was used, unless the heterogeneity of the study results tested by the Cochran's Q-test was considered significant (P < 0.1). Then, the random-effects model was used30,31. Weighting was applied to results calculated by the fix-effects or random-effects model, which represented the contribution of each study to the pooled analysis. We used a Galbraith plot to find the source of heterogeneity. Begg's test and Egger's test were used to assess the publication bias32,33. All analyses were calculated with Stata software (version 10.1; StataCorp LP, College Station, TX), using two-sided P values.
References
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J Clin 63, 11–30 (2013).
Qiong, C., Zhicai, L. & Lanping, C. An Analysis of Incidence and Mortality of Colorectal Cancer in China, 2003~2007. China Cancer 21, 179–182 (2012).
Lichtenstein, P. et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark and Finland. N Engl J Med 343, 78–85 (2000).
Markowitz, S. D. & Bertagnolli, M. M. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 361, 2449–2460 (2009).
Bargonetti, J. & Manfredi, J. J. Multiple roles of the tumor suppressor p53. Curr Opin Oncol 14, 86–91 (2002).
Olivier, M., Hussain, S. P., Caron de Fromentel, C., Hainaut, P. & Harris, C. C. TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. IARC Sci Publ, 247–270 (2004).
Momand, J., Zambetti, G. P., Olson, D. C., George, D. & Levine, A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237–1245 (1992).
Oliner, J. D. et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 362, 857–860 (1993).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Oliner, J. D., Kinzler, K. W., Meltzer, P. S., George, D. L. & Vogelstein, B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358, 80–83 (1992).
Onel, K. & Cordon-Cardo, C. MDM2 and prognosis. Mol Cancer Res 2, 1–8 (2004).
Tachibana, M. et al. Dysfunction of p53 pathway in human colorectal cancer: analysis of p53 gene mutation and the expression of the p53-associated factors p14ARF, p33ING1, p21WAF1 and MDM2. Int J Oncol 25, 913–920 (2004).
Bond, G. L. et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119, 591–602 (2004).
Lind, H., Zienolddiny, S., Ekstrom, P. O., Skaug, V. & Haugen, A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer 119, 718–721 (2006).
Hong, Y. et al. The role of P53 and MDM2 polymorphisms in the risk of esophageal squamous cell carcinoma. Cancer Res 65, 9582–9587 (2005).
Ma, H. et al. Polymorphisms in the MDM2 promoter and risk of breast cancer: a case-control analysis in a Chinese population. Cancer Lett 240, 261–267 (2006).
Alazzouzi, H. et al. Tumour selection advantage of non-dominant negative P53 mutations in homozygotic MDM2-SNP309 colorectal cancer cells. J Med Genet 44, 75–80 (2007).
Joshi, A. M. et al. TP53 R72P and MDM2 SNP309 polymorphisms and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Jpn J Clin Oncol 41, 232–238 (2011).
Chaar, I. et al. Impact of MDM2 polymorphism: increased risk of developing colorectal cancer and a poor prognosis in the Tunisian population. Eur J Gastroenterol Hepatol 24, 320–327 (2012).
Alhopuro, P. et al. The MDM2 promoter polymorphism SNP309T-->G and the risk of uterine leiomyosarcoma, colorectal cancer and squamous cell carcinoma of the head and neck. J Med Genet 42, 694–698 (2005).
Talseth, B. A. et al. MDM2 SNP309 T>G alone or in combination with the TP53 R72P polymorphism does not appear to influence disease expression and age of diagnosis of colorectal cancer in HNPCC patients. Int J Cancer 120, 563–565 (2007).
Zhang, Y. et al. Polymorphisms in TP53 and MDM2 contribute to higher risk of colorectal cancer in Chinese population: a hospital-based, case-control study. Mol Biol Rep 39, 9661–9668 (2012).
Zhu, L. et al. A Functional Polymorphism in miRNA-196a2 Is Associated with Colorectal Cancer Risk in a Chinese Population. DNA Cell Biol 31, 350–4 (2012).
Sotamaa, K. et al. p53 codon 72 and MDM2 SNP309 polymorphisms and age of colorectal cancer onset in Lynch syndrome. Clin Cancer Res 11, 6840–6844 (2005).
Menin, C. et al. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst 98, 285–288 (2006).
Chen, Y. L., Chang, Y. S., Chang, J. G. & Wu, S. M. Genotyping of single nucleotide polymorphism in MDM2 genes by universal fluorescence primer PCR and capillary electrophoresis. Anal Bioanal Chem 394, 1291–1297 (2009).
Sugano, N. et al. MDM2 gene amplification in colorectal cancer is associated with disease progression at the primary site, but inversely correlated with distant metastasis. Gene Chromosome Canc 49, 620–629 (2010).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
Mantel, N. & Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Martin, K. et al. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature 375, 691–694 (1995).
Xiao, Z. X. et al. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature 375, 694–698 (1995).
Ries, S. et al. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103, 321–330 (2000).
Gudas, J. M. et al. Differential expression of multiple MDM2 messenger RNAs and proteins in normal and tumorigenic breast epithelial cells. Clin Cancer Res 1, 71–80 (1995).
Maya, R. et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 15, 1067–1077 (2001).
Cao, X., Zhang, T., Zhao, Z. & Zhao, T. MDM2 SNP309 polymorphism and colorectal cancer risk: a meta-analysis. DNA Cell Biol 31, 355–359 (2012).
Knappskog, S. et al. The MDM2 promoter SNP285C/309G haplotype diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer Cell 19, 273–282 (2011).
zur Hausen, H. Red meat consumption and cancer: reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int J Cancer 130, 2475–2483 (2012).
Terry, P., Ekbom, A., Lichtenstein, P., Feychting, M. & Wolk, A. Long-term tobacco smoking and colorectal cancer in a prospective cohort study. Int J Cancer 91, 585–587 (2001).
Fedirko, V. et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 22, 1958–1972 (2011).
Freudenheim, J. L. et al. Diet and alcohol consumption in relation to p53 mutations in breast tumors. Carcinogenesis 25, 931–939 (2004).
Bond, G. L. et al. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet 43, 950–952 (2006).
Bond, G. L. et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res 66, 5104–5110 (2006).
Acknowledgements
This study was partly supported by National Natural Science Foundation of China (81072031, 81272712, 81230068, 81201570, 81373091 and 81102089), Natural Science Foundation of Jiangsu Province (BK2011773), the Key Program for Basic Research of Jiangsu Provincial Department of Education (12KJA330002 and 11KJB330002), Jiangsu Provincial Graduates Innovative Project (CXZZ13_0586) and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine). We thank Li Wang of Wake Forest University School of Medicine (Winston-Salem, NC) for review and scientific editing.
Author information
Authors and Affiliations
Contributions
Z.Z., Z.X. and M.W. conceived and designed the experiments. W.W., M.D. and D.G. performed the experiments. W.W., M.D. and L.Z. analyzed the data. M.W., H.C. and N.T. contributed reagents/materials/analysis tools. W.W. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, W., Du, M., Gu, D. et al. MDM2 SNP309 polymorphism is associated with colorectal cancer risk. Sci Rep 4, 4851 (2014). https://doi.org/10.1038/srep04851
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04851
This article is cited by
-
Common genetic variation in ETV6 is associated with colorectal cancer susceptibility
Nature Communications (2016)
-
BRCA1 and MDM2 as independent blood-based biomarkers of head and neck cancer
Tumor Biology (2016)
-
Impact of the Mdm2SNP309-G allele on a murine model of colorectal cancer
Oncogene (2015)
-
The association between the rs6495309 polymorphism in CHRNA3 gene and lung cancer risk in Chinese: a meta-analysis
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.