Abstract
Hydrogenation of iron nanoparticles was performed both computationally and experimentally where previously chemically-bonded iron hydride is considered to be unachievable under ordinary conditions. Density functional theory (DFT) calculations predict that hydrogenated iron nanoparticles are stabilized on a single-layer graphene/Cu substrate. Experimentally, iron nanoparticles were deposited onto a graphene/Cu substrate by vacuum deposition. Hydrogenation was done at 1atm of hydrogen gas and under liquid nitrogen. Mass spectrometry peak confirmed the hydrogen release from hydrogenated iron nanoparticles while a scanning transmission electron microscopy is used in order to link a geometrical shape of iron hydride nanoparticles between experimental and theoretical treatments. The hydrogenated iron nanoparticles were successfully synthesized where hydrogenated iron nanoparticles are stable under ordinary conditions.
Similar content being viewed by others
Introduction
Synthesis of iron hydrides is challenging due to the extreme conditions under which they generally exist1,2. Thus far, iron hydrides have been reported to exist within the atmospheres of stars such as the Sun and red dwarfs in a gas state as FeH and FeH2 molecules and within the earth's core in a bulk state, requiring very low temperatures such as −243°C or high pressure such as 3.5 GPa2,3,4,5,6,7,8,9. Iron hydride molecules have been synthesized by evaporating the iron source within a hydrogen-argon gas mixture at −263°C10,11,12. Theoretical studies predict that small iron nanoparticles have the ability to be hydrogenated in a gas phase13,14. However, synthesis and deposition of hydrogenated iron nanoparticles on a surface has not yet been achieved under ordinary conditions. Here we theoretically screened for optimal substrates that support iron hydrides by using density functional theory (DFT) and experimentally tested hydrogenation of iron nanoparticles on the most optimal substrate, single layer graphene/Cu. DFT calculations indicate that a single layer graphene preserved the hydrogenation properties of iron clusters. Iron nanoparticles were then experimentally deposited on a single-layer graphene/Cu subtrate via vaccuum deposition where transmission electron microscopy (TEM) revealed that iron nanoparticles formed planar shapes on graphene/Cu. Hydrogenation of the iron nanoparticles was performed with 1 atm of hydrogen gas and hydrogenated under liquid nitrogen. Mass spectrometry captured the peak of hydrogen release from the hydrogenated iron particles. Hydrogenated iron nanoparticles were successfully synthesized on the single layer graphene/Cu substrate which is stable under ordinary conditions.
Graphene is a two dimensional crystal which has shown remarkable electronic and mechanical properties, yet it still has undiscovered effects15,16. Graphene is commonly synthesized on a Cu substrate by chemical vapor deposition as a Cu substrate is proven to have advantages for commercial applications due to industrial scalability and efficient production17,18,19. Recent research has discovered that graphene is also considered to be an effective substrate for supporting nanoparticles20,21. The properties of nanoparticles are strongly dependent on their size and structure22,23,24. In particular, small gas-phase iron nanoparticles are calculated to have the capacity to absorb large amounts of hydrogen and are known to have large magnetic moments where the magnetic moment can be controlled upon hydrogenation13. However, the properties of nanoparticles can be affected by the surface upon the depositon of nanoparticles, making it imperative to find a way to preserve the desired properties of the nanoparticles without interferance from other nanoparticles or surfaces. Therefore, optimal surface planes for iron nanoparticle were computationally explored.
Computational Result
Density functional theory (DFT) calculations were performed within a real space grid of the projector augmented wave method25,26. The exchange-correlation of the vdW-DF is implemented27. Linear combination of atomic orbitals mode with GPAW is used. Grid spacing is set to 0.20 Å and 4×4×1 special k points of the Brillouin zone sampling is used for surface calculations where the 15Å of vacuum was applied to the z-axis for surface calculations.
DFT calculations were applied in order to find the surface planes that can preserve the hydrogenation properties of Fe clusters. A FeH cluster was deposited on the surface planes of commonly-used substrates including Al2O3, SiO2, MgO and a single layer graphene sheet. Calculations indicated that the hydrogenation properties of the FeH cluster are preserved most on a single layer graphene compared to the other surfaces where the properties of the FeH cluster are strongly affected by the surface. However, a single layer graphene sheet must be deposited on a substrate in order to hold a single graphene sheet during the experiment. A Cu substrate was chosen as it is widely used as a supporting substrate for graphene synthesis18,19. In particular, the binding energy and the distance between single layer graphene and Cu are calculated to be −0.15 eV and 3.60 Å, respectively. The single layer graphene is weakly bonding with the Cu substrate through physisorption and as a result the properties of the single layer graphene are not affected by the Cu substrate.
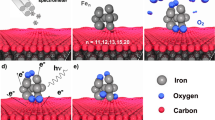
An atomic model of the FeH cluster on a single layer graphene/Cu(111) is shown in Figure 1. Calculations indicate that the FeH cluster is weakly bonded with graphene/Cu(111) where the binding energy is calculated to be −1.15 eV. The binding energy of H on Fe/graphene/Cu(111) is calculated to be 0.51 eV. The magnetic moments of the Fe atom within FeH on a single layer graphene/Cu(111) and the Fe atom within a gas-phase FeH cluster are both calculated to be 3.00 µB. The bond length of a gas-phase FeH is calculated to be 1.56 Å where the FeH cluster on a single layer graphene/Cu(111) is calculated to be 1.60 Å. The electronic structure of the FeH cluster on the single layer graphene/Cu(111) suggests that it may be possible to hydrogenate the iron nanoparticles on a single layer graphene/Cu substrate.
Further DFT calculations were applied in order to understand the geometrical shape of bare Fe clusters on graphene/Cu. Calculations show that Fe4 cluster forms a regular tetrahedron structure on graphene/Cu(111) as shown in Figure 1 (c) while previous theoretical work reports that the ground state structure of gas phase Fe4 has a tetragonal disphenoid structure13. The Fe7 cluster shown in Figure 1 (d) has a hexagonal shape while gas-phase Fe7 has a pentagon structure13. The theoretical data suggests that small iron clusters grow towards regular planar shapes. Bare Fe clusters on graphene was previously observed by transmission electron microscopy where Fe clusters form regular planar shapes from a top view against a two dimensional graphene plane, confirming the planar-forming behavior of the bare Fe clusters28. DFT calculations were further performed in order to understand the geometrical shape of the hydrogenated Fe4 and Fe7 clusters on graphene/Cu(111) as shown in Figure 1 (e) and (f). Calculations show that the structures of Fe4 and Fe7 are elongated by hydrogenation while the geometrical shape still remains same as bare Fe4 and Fe7.
Experimental Result
Experimentally, a single layer graphene sheet was grown on a Cu surface by chemical vapor deposition where a single layer of graphene was confirmed by Raman spectroscopy. Fe nanoparticles were deposited by using vacuum deposition. Fe nanoparticles on graphene/Cu is then observed by using STEM (FEI, Tecnai Osiris) 1 atm of pressure of hydrogen gas was then applied to the Fe nanoparticles on graphene/Cu, followed by hydrogenation of Fe nanoparticles under liquid nitrogen (−196°C) for 3 hours. The dehydrogenation of hydrogenated Fe nanoparticles were analyzed by mass spectrometry.
Deposited Fe nanoparticles on the graphene/Cu sample were observed by using a STEM as shown in Figure 2 (a). Figure 2 (a) provides that small Fe nanoparticles are spread over the graphene/Cu substrate where dark areas are confirmed to be Fe by energy dispersive X-ray spectrometry (EDS) shown in Figure 2 (b). Thus, Fe nanoparticles were successfully deposited by vacuum deposition. Figure 2 (a) also shows that the sizes of the Fe nanoparticles range from 1 nm to 3 nm. In particular, each Fe nanoparticle has the possibility to form planar shapes as DFT calculations suggest Fe clusters form a planar shape on graphene shown in Figure 1 (c) and (d).
Further DFT calculations were performed in order to fill the size of nanoparticles between the calculation and experiment. Figure 2 (c) and (d) shows the Fe25 and Fe25H16 on graphene/Cu(111), respectively. The size of Fe25 and Fe25H16 on graphene/Cu(111) is calculated to be 1.12 nm and 1.15 nm, respectively. The structure of Fe25 on graphene/Cu(111) shown in Figure 2 (c) have triangle like structure which similar structure is seen in Figure 1 (a). Figure 2 (d) shows that Fe25 can be hydrogenated on graphene/Cu(111) where the structure is slightly elongated upon hydrogenation.
Dehydrogenation of the hydrogenated Fe nanoparticles on a graphene/Cu sample was investigated by using mass spectrometry in order to confirm if the Fe nanoparticles were hydrogenated. Two type of samples were prepared: i) hydrogenated Fe on graphene/Cu and ii) hydrogenated graphene/Cu for comparison where hydrogenation for both samples was performed under the same conditions. Both hydrogenated samples were taken from the sample holder after hydrogenation and switched to a new sample holder under an argon-filled glovebox. Sample holders with each sample were then vacuumed for 1 hour in order to remove gas impurities.
Each sample was then inserted into a furnace which was pre-heated to 400°C. Figure 3 indicates that hydrogen gas is released from the hydrogenated Fe on the graphene/Cu sample (Figure 3 i) at 110 seconds, whereas the hydrogen release was not observed in the hydrogenated graphene/Cu case (Figure 3 ii). This confirmed that hydrogen is released from the Fe nanoparticles. Note that the temperature of sample holder indicates 190°C at the peak of hydrogen release, 110 seconds.
Conclusion
In conclusion, hydrogenation of Fe nanoparticles was explored from both computational and experimental approaches. DFT calculations revealed that hydrogenation properties of iron nanoparticles were well preserved on a single layer graphene/Cu substrate. Iron nanoparticles were then experimentally deposited on the graphene/Cu substrate by using a vacuum deposition. Low temperature hydrogenation of iron nanoparticles on the graphene/Cu substrate successfully created hydrogenated iron nanoparticles on graphene/Cu which is stable under ordinary conditions.
References
Greenwoord, N. N. & Earnshaw, A. Chemistry of the Elements (Pergamon Press, Oxford, 1984).
Badding, J. V., Hemley, R. J. & Mao, H. K. High-pressure chemistry of hydrogen in metals: In situ study of iron hydride,. Science 253, 421–424 (1991).
Antonov, V. E. et al. Crystal structure and magnetic properties of high-pressure phases in the feh and fecrh systems,. Int. J. Hydrogen Energy 14, 371–377 (1989).
Stevenson, D. J. Hydrogen in the earth's core,. Nature 268, 130–131 (1977).
Okuchi, T. Hydrogen partitioning into molten iron at high pressure: Implications for earth's core,. Science 278, 1781–1784 (1997).
Narygina, O. X-ray diffraction and mössbauer spectroscopy study of fcc iron hydride feh at high pressures and implications for the composition of the earth's core,. Earth Planet. Sci. Lett. 307, 409–414 (2011).
Whetten, R. L., Cox, D. M., Trevor, D. J. & Kaldor, A. Correspondence between electron binding energy and chemisorption reactivity of iron clusters,. Phys. Rev. Lett. 54, 1494–1497 (1985).
Carroll, P. K. & McCormack, P. The spectrum of feh: Laboratory and solar identification,. Astrophys. J. 177, 33 (1972).
Phillips, J. G., Davis, S. P., Lindgren, B. & Balfour, W. J. The near-infrared spectrum of the feh molecule,. Astrophys. J. 65, 721–778 (1987).
Andrews, L. Matrix infrared spectra and density functional calculations of transition metal hydrides and dihydrogen complexes,. Chem. Soc. Rev. 33, 123–132 (2004).
Chertihin, G. V. & Andrews, L. Infrared spectra of feh, feh2 and feh3 in solid argon,. J. Phys. Chem. 99, 12131–12134 (1995).
Wang, X. & Andrews, L. Infrared spectra and theoretical calculations for fe, ru and os metal hydrides and dihydrogen complexes,. J. Phys. Chem. A 113, 551–563 (2009).
Takahashi, K., Isobe, S. & Ohnuki, S. Chemisorption of hydrogen on fe clusters through hybrid bonding mechanisms,. Appl. Phys. Lett. 102, 113108 (2013).
Bessarab, P. F., Uzdin, V. M. & Jónsson, H. Effect of hydrogen adsorption on the magnetic properties of a surface nanocluster of iron,. Phys. Rev. B 88, 214407 (2013).
Geim, A. K. & Novoselov, K. S. The rise of graphene,. Nature Mater. 6, 183–191 (2007).
Schedin, F. et al. Detection of individual gas molecules adsorbed on graphene,. Nature Mater. 6, 652–655 (2007).
Reina, A. et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition,. Nano Lett. 9, 30–35 (2009).
Xuesong, L. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils,. Science 324, 1312–1314 (2009).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes, . Nat. Nanotechnol. 5, 574–578 (2010).
Mashoff, T. et al. Hydrogen storage with titanium-functionalized graphene,. Appl. Phys. Lett. 103, 013903 (2013).
Sun, S. et al. Single-atom catalysis using pt/graphene achieved through atomic layer deposition,. Sci. Rep. 3 (2013).
Jena, P. & Castleman, A. W. Clusters: A bridge across the disciplines of physics and chemistry,. Proc. Natl. Acad. Sci. USA 103, 10560–10569 (2006).
Yacamán, M. J., Ascencio, J. A., Liu, H. B. & Gardea-Torresdey, L. Structure shape and stability of nanometric sized particles,. J. Vac. Sci. Technol., B 19, 1091–1103 (2001).
Baletto, F. & Ferrando, R. Structural properties of nanoclusters: Energetic, thermodynamic and kinetic effects,. Rev. Mod. Phys. 77, 371–423 (2005).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas,. Phys. Rev. 136, B864–B871 (1964).
Mortensen, J. J., Hansen, L. B. & Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method,. Phys. Rev. B 71, 035109 (2005).
Dion, M. et al. Van der waals density functional for general geometries,. Phys. Rev. Lett. 92, 246401 (2005).
Wang, H. et al. Unraveling the atomic structure of ultrafine iron clusters,. Sci. Rep. 2 (2012).
Acknowledgements
We thank Lauren Takahashi for valuable discussions. CPU time is supported in part by Hokkaido university academic cloud, information initiative center, Hokkaido university, Sapporo, Japan. Experimental work was conducted at Hokkaido University, supported by “Nanotechnology Platform” Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Contributions
K.T. coordinated the project, designed, ran and analyzed the density functional theory calculation and experiment and wrote paper. Y.W. designed and ran the TEM and sample preparation. S.C. and Y.N. ran the sample preparation and mass spectrometry analysis. S.I. organized the discussions and S.O. sponsored the project. All authors discussed about the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Takahashi, K., Wang, Y., Chiba, S. et al. Low temperature hydrogenation of iron nanoparticles on graphene. Sci Rep 4, 4598 (2014). https://doi.org/10.1038/srep04598
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04598
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.