Abstract
In this study, an assay that combines the ease and simplicity of the qualitative approach for measuring catalase activity was developed. The assay reagents comprised only hydrogen peroxide and Triton X-100. The enzyme-generated oxygen bubbles trapped by Triton X-100 were visualized as foam, whose height was estimated. A calibration plot using the defined unit of catalase activity yielded the best linear fit over a range of 20–300 units (U) (y = 0.3794x − 2.0909, r2 = 0.993). The assay precision and reproducibility at 100 U were 4.6% and 4.8%, respectively. The applicability of the assay for measuring the catalase activity of various samples was assessed using laboratory strains of Escherichia coli, catalase-deficient isogenic mutants, clinically isolated Shiga toxin-producing E. coli, and human cells. The assay generated reproducible results. In conclusion, this new assay can be used to measure the catalase activity of bacterial isolates and human cells.
Similar content being viewed by others
Introduction
Catalase is a ubiquitous antioxidant enzyme that degrades hydrogen peroxide into water and oxygen1. Several pathogens produce catalase in order to defend themselves against attacks by hydrogen peroxide, a weapon commonly used by the host's immune system, in addition to oxidative stress. A previous report has in fact demonstrated that a catalase-deficient mutant pathogen was more susceptible than its wild-type strain to the oxidative stress induced by hydrogen peroxide and immune cell attacks (which involve hydrogen peroxide)2. It is thus useful to measure the catalase activity of pathogens in order to gain a better understanding of the underlying mechanisms of their pathogenicity, including their resistance towards oxidative stress.
Escherichia coli has 2 catalase enzymes, hydroperoxidase I (HPI) and HPII, which catalyze the dismutation of hydrogen peroxide to water and oxygen1. The katG gene product, HPI, is transcriptionally induced during logarithmic growth in response to low concentrations of hydrogen peroxide3. However, HPII, encoded by katE and positively regulated by rpoS, is not peroxide inducible and is a key player in survival during the stationary phase and other stresses4,5. Several studies have focused on these 2 catalases, as they play pivotal roles in protecting cells against the effects of oxidative stress6. Because HPII activity varies among clinical isolates, it is necessary to distinguish between the activities of HPI and HPII in order to investigate the activity of the RpoS-encoded stress response gene rpoS.
To date, several methods have been described for measuring catalase activity in bacteria. One of the simplest qualitative procedures involves determination of the enzyme's presence in the test bacterial isolate by using hydrogen peroxide, which is broken down to bubble-producing O2 by catalase-positive bacteria7,8. On the other hand, quantitative approaches focusing on careful measurements include colorimetric and spectrophotometric assays9,10,11,12,13,14. There are several limitations inherent in these methods, including cumbersome procedures and high cost, despite the availability of convenient kits. Furthermore, during quantitative analyses, although the reaction occurs spontaneously, the enzyme increases the reaction rate considerably. Therefore, while processing a large number of samples, the initial and final reaction rates differ among different samples, thus producing ambiguous results. This necessitates the development of a simple and cost-effective method for measuring catalase activity.
In order to address these challenges, in this study, we developed an assay that combines the ease and simplicity of the qualitative approach for measuring catalase activity. The assay uses simple and readily available reagents, namely hydrogen peroxide, Triton X-100, and catalase. We applied this assay to clinical isolates and laboratory strains of E. coli and its derivatives carrying mutations in the catalase genes or in their regulatory factors, and human cells.
The underlying principle of this approach is that the oxygen bubbles generated from the decomposition of hydrogen peroxide by catalase are trapped by the surfactant Triton X-100. The trapped oxygen bubbles are then visualized as foam, the test-tube height of which is measured to quantify the catalase activity.
Results
In the present study, by employing our novel approach, we measured the catalase activity by quantifying the trapped oxygen gas, which is visualized as foam. The oxygen gas generated by the catalase–hydrogen peroxide reaction in test tubes is shown in Fig. 1A, where the height of foam developed in the tubes was measured. The calibration curve plotted using the defined unit of catalase activity is shown in Fig. 1B. A linear regression on the pooled data yielded the best linear fit over a range of 20–300 units (U) of catalase activity (y = 0.3794x − 2.0909, r2 = 0.993); hence, all the measurements were carried out in that range. The assay precision (intraassay coefficient of variation) and reproducibility (interassay coefficient of variation) at 100 U were determined to be 4.6% and 4.8%, respectively. The assay generated reproducible results (Fig. 1B and 1C).
(A) Image of test tubes showing foam developed as a result of catalase activity. Each solution of concentration of catalase (100-μL) was added in a Pyrex tube (13 mm diameter × 100 mm height, borosilicate glass; Corning, USA). Subsequently, 100 μL of 1% Triton X-100 and 100 μL of undiluted hydrogen peroxide (30%) were added to the solutions and mixed thoroughly and were then incubated at room temperature. Following completion of the reaction, the height of O2-forming foam in the test tube was measured using a ruler. (B) The best linear fit between the foam heights and catalase activity was observed over a range of 20–200 U (y = 0.3794x − 2.0909, r2 = 0.993). Mean values are shown (n = 3). Error bars represent the standard deviation. (C) The height of foam generated after mixing catalase, Triton X-100, and H2O2.
No reagents were used to stop the reaction, as the generation of oxygen stops naturally within 5 min. After the reaction, the generation of bubbles ceased completely, despite an additional 100 μL of hydrogen peroxide being added to all the test tubes. To verify the specificity of the assay for measuring catalase activity, the catalase inhibitor azide was used and, as expected, foam formation was inhibited.
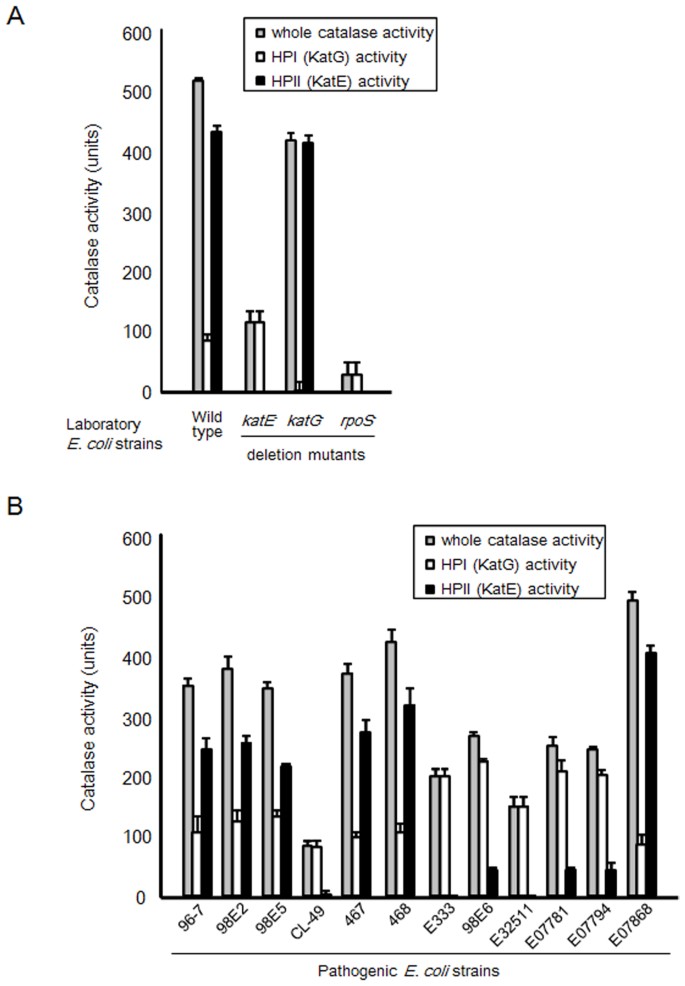
By applying the developed method to E. coli strains carrying mutations in the catalase genes or in their regulatory factors, we were able to determine the catalase activity in the katE, katG, and rpoS deletion mutants, as well as in the wild type (Fig. 2A), and to demonstrate the ability of the assay to accurately discriminate between the activities of HPI and HPII. Only HPI or HPII activity was observed in the katE and katG deletion mutants, respectively, and the rpoS deletion mutant showed only HPI activity. In contrast, both HPI and HPII activities were noted in the wild-type strain.
Furthermore, different clinical isolates were analysed for their catalase activity (Fig. 2B). HPI activity was detected in all the isolates tested in the study. However, the activity of HPII varied among the isolates.
In addition, we applied the developed method to assay the catalase activity of LNCaP and PC-3 cells, which are human prostate cancer cell lines commonly used in the field of oncology. A linear regression on the pooled data yielded the best linear fit over a range of 2.5 × 106 to 1 × 107 LNCaP and PC-3 cells (Fig. 3A and B). The catalase activity of LNCaP cells was higher than that of PC-3 cells (Fig. 3A and B).
Discussion
Using the method, the limit of detection for the assay was higher than that of other previous methods9,10,11,12,13,14, but the linearity, intraassay precision, and interassay precision were similar. In terms of handling, the method was simpler than other quantitative methods.
In this study, the catalase activity of various samples was measured by using the new method. When the assay was applied to E. coli strains carrying mutations in the catalase genes or in their regulatory factors, the expected results were obtained. When the assay was also applied to various clinical isolates of Shiga toxin-producing E. coli, the activity of HPII (which is encoded by katE and is positively regulated by rpoS) was found to vary among the isolates. These findings are consistent with previous reports7,15,16,17,18. In addition, the developed method could measure the catalase activity in human cells, where the activity in LNCaP was higher than that in PC-3. This result is also in accord with a past report19. Our method will be useful for easy measurement of catalase activity in various samples.
With regard to the technical aspects, the choice of surfactant was a key point for measuring the catalase activity in this assay. We tested 3 common surfactants, namely Triton X-100, Tween-20, and sodium dodecyl sulphate. Three surfactants produced similar results. Triton X-100 formed fine foam, however, other surfactants, especially SDS, formed rough foam. Therefore, we chose Triton X-100 in the assay. Next, we confirmed that the use of clean (unstained) new test tubes ensured reproducible results. Finally, this assay comprises simple procedures, in that it involves only the mixing of the bacterial suspension, Triton X-100, and hydrogen peroxide solution, without any other procedures such as the preparation of bacterial extract. In terms of costs, the cost per test of reagents used in this method is close to being gratis, and no machinery or equipment is required for the assay.
In conclusion, we have developed a simple and cost-effective assay and demonstrated its feasibility in determining the catalase status of bacterial and human cells.
Methods
Bacterial strains and culture conditions
Various pathogenic Escherichia coli strains20 were employed: 96-7 (STEC), 98E2 (STEC), 98E5 (STEC), CL-49 (STEC), 467 (STEC), 468 (STEC), E333 (STEC), 98E6 (STEC), E32511 (STEC), E07781 (VT2+), E07794 (VT2+), and E07868 (VT1+ and VT2+). In addition, deletion mutants of katE (which encodes heat-stable HPII), katG (which encodes heat-labile HPI), and rpoS (which positively regulates the expression of katE catalase), and the wild-type (BW25113) strain were also studied. All strains were obtained from the National Institute of Genetics (NIG, Mishima, Japan). The bacteria were propagated in Luria-Bertani (LB; BD Biosciences, USA) medium at 37°C, with shaking under aerobic conditions. The bacterial culture was inoculated into 10 mL of LB medium and cultured for 16 h at 37°C with shaking.
Cell lines
The LNCaP and PC-3 cell lines used in this study were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 containing 10% foetal bovine serum and Antibio-Antimyco (Life Technologies, Carlsbad, CA, USA). The cells were collected using trypsin, washed with PBS, and then used for the assay.
Reagents and sample preparations
The reagents used were hydrogen peroxide solution (30% (w/w) in H2O; Sigma-Aldrich, USA), 1% Triton X-100 (Sigma-Aldrich), catalase from bovine liver (2950 units/mg solid; 65% protein; 4540 units/mg protein, the product number C1345, Sigma-Aldrich), and 10 μM azide (an inhibitor of catalase activity21; Sigma-Aldrich).
Catalase powder, which was purchased from Sigma-Aldrich, was solved in 100-μL distilled pure water for preparing each concentration of catalase standards. Bacteria samples were prepared using overnight-cultured bacteria. Bacterial cells (10 mg) were suspended in 100-μL of physiological saline (diluted, if necessary). Human cells (105–107 cells) were suspended in 100-μL of phosphate buffered saline.
Quantification of catalase activities
In the present method, to quantify the catalase activity, a calibration curve was plotted with the defined unit of catalase activity. Each catalase solution (100-μL) or bacterial suspension (100-μL) was added in a Pyrex tube (13 mm diameter × 100 mm height, borosilicate glass; Corning, USA). Subsequently, 100 μL of 1% Triton X-100 and 100 μL of undiluted hydrogen peroxide (30%) were added to the solutions and mixed thoroughly and were then incubated at room temperature. Following completion of the reaction, the height of O2-forming foam that remained constant for 15 min in the test tube was finally measured using a ruler.
Discrimination of HPI and HPII activities
In order to accurately distinguish between the activities of the heat-labile catalase HPI and heat-stable catalase HPII in E. coli, the bacterial suspension was divided into 2 identical aliquots. One aliquot of the bacterial suspension was subjected to heat treatment (55°C, 15 min). Finally, the residual catalase activity following heat treatment was subtracted from the total catalase activity to determine the activity of the heat-labile catalase.
Inhibition of catalase activity
To test the specificity of the assay for catalase, the 100 μL of physiological saline solution in the enzyme assay was replaced with 100 μL of 10 μM azide to inhibit catalase activity21.
References
Loewen, P. C., Switala, J. & Triggs-Raine, B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch. Biochem. Biophys. 243, 144–149 (1985).
Day, W. A., Jr, Sajecki, J. L., Pitts, T. M. & Joens, L. A. Role of catalase in Campylobacter jejuni intracellular survival. Infect. Immun. 68, 6337–6345 (2000).
Triggs-Raine, B. L. & Loewen, P. C. Physical characterization of katG, encoding catalase HPI of Escherichia coli. Gene 52, 121–128 (1987).
Loewen, P. C. & Switala, J. Purification and characterization of catalase HPII from Escherichia coli K12. Biochem. Cell Biol. 64, 638–646 (1986).
Schellhorn, H. E. Regulation of hydroperoxidase (catalase) expression in Escherichia coli. FEMS Microbiol. Lett. 131, 113–119 (1995).
Farr, S. B. & Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55, 561–585 (1991).
Subbarayan, P. R. & Sarkar, M. A comparative study of variation in codon 33 of the rpoS gene in Escherichia coli K12 stocks: implications for the synthesis of sigma(s). Mol. Genet. Genomics 270, 533–538 (2004).
Schellhorn, H. E. & Stones, V. L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174, 4769–4776 (1992).
Goldblith, S. A. & Proctor, B. E. Photometric determination of catalase activity. J. Biol. Chem. 187, 705–709 (1950).
Lemberg, R. & Foulkes, E. C. Reaction between catalase and hydrogen peroxide. Nature 161, 131 (1948).
Chance, B. The reaction of catalase and cyanide. J. Biol. Chem. 179, 1299–1309 (1949).
Chance, B. The reactions of catalase in the presence of the notatin system. Biochem. J. 46, 387–402 (1950).
Beers, R. F., Jr & Sizer, I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952).
Li, Y. & Schellhorn, H. E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. 18, 185–187 (2007).
Jishage, M. & Ishihama, A. Variation in RNA polymerase sigma subunit composition within different stocks of Escherichia coli W3110. J. Bacteriol. 179, 959–963 (1997).
Visick, J. E. & Clarke, S. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J. Bacteriol. 179, 4158–4163 (1997).
Ferenci, T. The spread of a beneficial mutation in experimental bacterial populations: the influence of the environment and genotype on the fixation of rpoS mutations. Heredity 100, 446–452 (2007).
Chiang, S. M., Dong, T., Edge, T. A. & Schellhorn, H. E. Phenotypic diversity caused by differential RpoS activity among environmental Escherichia coli isolates. Appl. Environ. Microbiol. 77, 7915–7923 (2011).
Jung, K. et al. Antioxidant enzymes in malignant prostate cell lines and in primary cultured prostatic cells. Free Radical Biol. Med. 23, 127–133 (1997).
Iida, K., Mizunoe, Y., Wai, S. N. & Yoshida, S. Type 1 fimbriation and its phase switching in diarrheagenic Escherichia coli strains. Clin. Diagn. Lab. Immunol. 8, 489–495 (2001).
Klots, M. G., Kim, Y. C., Katsuwon, J. & Anderson, A. J. Cloning, characterization and phenotypic expression in Escherichia coli of catF, which encodes the catalytic subunit of catalase isozyme CatF of Pseudomonas syringae. Appl. Microbiol. Biotechnol. 43, 656–666 (1995).
Acknowledgements
We thank our laboratory colleagues for their comments and help regarding our study. A part of the study was supported by The Jikei University Research Fund, The Jikei University Graduate Research Fund, Grant-in-Aid for Young Scientists (A), The Uehara Memorial Foundation, The MEXT-supported Program for the Strategic Research Foundation at Private Universities, Science Research Promotion Fund of The Promotion and Mutual Aid Corporation for Private Schools of Japan (non-profit organization).
Author information
Authors and Affiliations
Contributions
T.I. designed the study. T.I., Y.K., A.T., S.S., K.O., I.H., K.T., Y.M. carried out experiments and discussed in detail about the obtained results. T.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Iwase, T., Tajima, A., Sugimoto, S. et al. A Simple Assay for Measuring Catalase Activity: A Visual Approach. Sci Rep 3, 3081 (2013). https://doi.org/10.1038/srep03081
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03081
This article is cited by
-
Cell phenotype changes and oxidative stress response in Vibrio spp. induced into viable but non-culturable (VBNC) state
Annals of Microbiology (2023)
-
The isolation of rumen enterococci strains along with high potential utilizing cyanide
Scientific Reports (2023)
-
Paper-based sensors for bacteria detection
Nature Reviews Bioengineering (2023)
-
Genomics assisted characterization of plant growth-promoting and metabolite producing psychrotolerant Himalayan Chryseobacterium cucumeris PCH239
Archives of Microbiology (2023)
-
Immobilization of catalase on functionalized magnetic nanoparticles: a statistical approach
3 Biotech (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.