Abstract
Quantifying seed viability is required for seed bank maintenance. The classical methods for detecting seed viability are time consuming and frequently cause seed damage and unwanted germination. We have established a novel micro-optrode technique (MOT) to measure seed viability in a quick and non-invasive manner by measuring the oxygen influxes of intact seeds, approximately 10 seconds to screen one seed. Here, we used soybean, wheat, and oilseed rape as models to test our method. After 3-hour imbibition, oxygen influxes were recorded in real-time with the total measurement taking less than 5 minutes. The results indicated a significantly positive correlation between oxygen influxes and viability in all 3 seed types. We also established a linear equation between oxygen influxes and seed viability for each seed type. For measurements, seeds were kept in the early imbibition stage without germination. Thus, MOT is a reliable, quick, and low-cost seed viability detecting technique.
Similar content being viewed by others
Introduction
Seed viability is one of the most important parameters in seed germination and seedling establishment1. To properly maintain a large amount of seeds, measurement of seed viability is important and is the primary cost involved in long-term maintenance of a sustainable seed bank. To test this, many methods have been employed, including germination test, 2, 3, 5-triphenyltetrazolium hydrochloride dyeing, and electric conductivity measurement2. However, all of these methods result in seed damage or undesired germination following the test. In addition, the above methods require a long time for only a single measurement. To overcome these shortcomings, an infrared thermography approach has been developed to non-invasively detect seed viability3. However, this technique still requires approximately one hour to accurately test each sample3. Based on our routine seed bank work, we found it necessary to develop a low-cost, quick, and user-friendly operation system to improve measurement efficiency and limit unnecessary cost for seed banks.
Oxygen is an essential element for seed germination. Using Clarke-type oxygen microelectrodes, Baker et al4 measured the rate of oxygen consumption (ROC) during the early stage of seed imbibition. This technique involved measurement of oxygen concentration at the seed surface, and calculation of ROC based on the acquired time series. Clark sensors suffer from temporal drift, low signal to noise ratio, sensor fouling, and poor limit of detection5,6,7. In the last few decades, optical microelectrodes (or micro-optrodes) have been developed to remedy these problems.
ROC is correlated with oxygen fluxes in the micro-environment near the surface of seeds. ROC and oxygen flux are both important parameters to describe seed germination and seedling establishment4,8,9,10. Oxygen flux is the dynamic movement of oxygen in a localized field. The magnitude and direction of oxygen flux across the cell/tissue membrane are driven by a cascade of biological reactions (e.g., respiratory, stress signalling, ROS production). Therefore, oxygen flux is a key environmental factor that limits seed viability and germination, and may be utilized as an essential parameter to measure the viability of imbibed seeds. Techniques which monitor oxygen flux (rather than steady state concentration) have been shown to be much more accurate for describing physiological transport in the unstirred layer near living cells/tissues11,12. Here, we developed a real-time and non-invasive micro-optrode technique (MOT) for detecting seed viability by the measurement of oxygen fluxes during seed respiration.
The MOT is a highly sensitive and selective technique to measure oxygen concentrations and fluxes on the cell surface. Porterfield et al.13 invented this technique (known generally as self referencingoptrodic sensing) and applied the tool to basic research for the measurement of oxygen fluxes in animal and plant systems13. Since that time, MOT has been utilized in a number of systems, including those for monitoring the effect of water quality on fish embryos by oxygen flux14, detecting chemical toxicity to bacterial biofilms15, determining plant root oxygen flux profiles7, screening auxin transport mutants16 and a protocol has even been established for the Arabidopsis root system17. The MOT is an ultrasensitive tool with high temporal and spatial resolution for detecting the physiological activity of live cells/tissues11. Use of this methodology will provide novel insights into seed science research.
In our routine work, we successfully employ MOT to quantify seed viability, by measuring surface oxygen influxes of intact seeds. These seeds are from the National Seed Bank of China of the Institute of Crop Science, Chinese Academy of Agricultural Sciences. The seed bank was built in 1986. It is one of the four largest national seed banks worldwide18, and has collected and safeguarded over 390,000 crop seeds accessions. It is the centre for the long-term maintenance of crop seeds in China, and plays important roles in crop genetic resources conservation and distribution. Some of the tested seeds have been kept in storage for more than 10 years; the seed aging process resulted in the generation of a seed viability gradient. In this study, we utilized three models, the soybean (Glycine max L.), wheat (Triticum aestivum L.), and oilseed rape (Brassica napus L.) seeds. These three types of seeds contain protein, starch, and lipid as reserve substances respectively. Utilization of these seeds represents the typical oxygen requirements of the different seed groups in our seed bank. Our results indicated a significantly positive linear correlation between oxygen influxes and seed viability for the three kinds of seeds tested. The changes of oxygen influxes correlate with decreased or increased seed viability after seeds were treated with NaN3 and NADH. Moreover, we generated equations that represent the relationship between oxygen fluxes and seed viability during early imbibition but not germination. As such, this equation allows for the accurate prediction of seed viability. According to our results, this technique and method will improve the detection efficiency of seed viability and allows for the establishment of a digital classification of seeds. Importantly, determination of oxygen flux was completed in only 10 seconds per sample; where and a typical steady state oxygen concentration measurement requires 5 minutes. Taken together, MOT is a reliable tool for the advance study of the molecular mechanisms of seed aging, germination, and conservation. In the routine work at the seed bank, MOT provides a financial advantage and time-saving solution for the maintenance and upkeep of seed data.
Results
Optimization of the oxygen flux testing conditions
When soaked in solution, seeds gradually uptake water. To test whether the soaking time affects oxygen flux, soybean seeds soaked for 1 h to 5 h were subjected to the MOT test. Results indicated that there was no significant difference in oxygen flux for soybean seeds with 99.0% or 0% viability after soaking before the MOT test (Supplementary Table S1). Thus, soaking for 1 h to 5 h had no effect on MOT detection.
Seeds are composed of embryonic axis and cotyledons or endosperm, which have different functions during germination. Oxygen consumption of these parts during germination might also differ. To test whether the position of the seed during the MOT test affects the results, we selected three typical positions and performed the MOT test. For wheat seeds, the sites were located at the centre of the embryo (site1), the centre of the side with the embryo (site2), and the centre of the ventral groove (site3) (Fig. 1A). For soybean seeds, the sites were located at the top of the embryonic axis (site1), the centre of the embryonic axis (site2), and the centre of one cotyledon (site3) (Fig. 1A). For oilseed rape, site1, site2, and site3 represent the centre of the embryonic axis, the point opposite to the embryonic axis, and the centre of one cotyledon, respectively (Fig. 1A). Different oxygen fluxes were observed among the testing sites in three seeds; this difference was not dependent on whether the seed was live or dead. For wheat, soybean, and oilseed rape, the biggest oxygen fluxes were detected at site1, stie2, and site1, respectively, i.e. at the centre of the embryo/embryonic axis. The oxygen fluxes at the endosperm or cotyledon were much lower (Fig. 1B). Therefore, the following measurements were acquired at the centre of the embryos/embryonic axis.
(A) Three seeds and detect sites. For wheat seed, site1, site2 and site3 represents the center of the embryo, the center of side with embryo of the whole seed, and the center of the ventral groove, respectively. For soybean seeds, the sites were located at the top of the embryonic axis (site1), the centre of the embryonic axis (site2), and the centre of one cotyledon (site3). For oilseed rape, site1, site2 and site3 represents the center of the embryonic axis, the opposite side of the embryonic axis, and the center of one of the cotyledons, respectively. The bar represent is 5 mm. (B) Oxygen influx at different sites of wheat seeds (red) with 91% and 0% viability, soybean seeds (blue) with 99% and 0% viability, and oilseed rape (green) with 99% and 0% viability. Positive value indicates oxygen influx. Bars represent standard errors.
Oxygen flux signals in live and dead seeds
The background oxygen flux rate in the working solution without any seed was recorded. The signal varied around zero, indicating relatively stable oxygen concentrations in the testing solution. The oxygen flux rate in live wheat seeds (91.0% viability) and dead wheat seeds (0% viability) were then determined in the working solution. There was an obvious difference in the oxygen flux rate between the live and dead wheat, soybean, and oilseed rape (Fig. 2), demonstrating that oxygen consumption varied with seed viability. We also observed that, within a 10 min timeframe, the oxygen influxes in the live and dead seeds were relatively stable. Therefore, the testing time for the following measurements was reduced to 5 min.
 ) and 0% (
) and 0% ( ) viability, soybean seeds of 99% (
) viability, soybean seeds of 99% ( ) and 0% (
) and 0% ( ) viability, oilseed rape of 99% (
) viability, oilseed rape of 99% ( ) and 0% (
) and 0% ( ) viability.
) viability.Correlation between seed viability and oxygen fluxes
Using the MOT testing condition determined by this study, we tested the oxygen fluxes of seed types of various viabilities. The oxygen influxes increased with seed viability (Fig. 3, supplementary Table S2). For example, in wheat seeds, the oxygen influx of the dead seed was only 9.66 ± 0.80 pmol cm−2 s−1, but was 27.46 ± 0.99 pmol cm−2 s−1 in 91%-viability seeds. A notable linear regression was found between the oxygen influx and seed viability with a correlation reaching up to 0.9692 (P = 0.0004) by the regression analysis (Fig. 3). A similar relationship was observed in soybean and oilseed rape, and the correlation values were 0.9820 (P = 0.0001) and 0.9540 (P = 0.0043), respectively (Fig. 3). These results demonstrated that MOT can be used to assess seed viability. We altered the X and Y coordinates, then attained the predict equations of seed viability by oxygen influx:



where G is the germination percent or seed viability, JO2 is the oxygen influx of the seed.
Effects of inhibition and promotion of respiration on oxygen fluxes and seed viability
The effects of respiration inhibitor (NaN3) and promoter (NADH) on the oxygen fluxes in wheat seeds were tested. For live seeds (91% viability), the average oxygen influx reduced from the original 25.93 ± 0.91 pmol cm−2 s−1 to 22.74 ± 2.60 pmol cm−2 s−1, after being treated with 0.1 mM NaN3 for 30 min (Fig. 4, Supplementary Table S3). The average oxygen influx for seeds with 43% viability was 17.44 ± 1.50 pmol cm−2 s−1 and increased to 20.91 ± 1.57 pmol cm−2 s−1 after treatment with 10 mM NADH for 30 min (Fig. 4, Supplementary Table S3).
Seeds were subjected to germination tests to re-evaluate the seed viability after both treatments. We found that after NaN3 or NADH treatment, the viability of the 91% seeds reduced to 67%, while seeds with 43% viability increased to 50% viability, respectively. Meanwhile, the average length and weight of the seedlings varied with seed germination percentages and oxygen influxes (Supplementary Table S3). When the oxygen influx data after NaN3 or NADH treatment was included in equation (2), we achieved 66% and 57% viability, respectively. These values were very close to the viability results of the germinating test (Supplementary Table S3). These results confirmed that oxygen influx testing by MOT can be used to evaluate seed viability.
Discussion
Seed viability determines seed germination and seedling development in higher plants19. The accuracy and efficiency of seed viability test is not only important for seed conservation, but also for basic scientific studies. To obtain the data of seed viability with minimum time cost and seed damages, developing of fast and non-destructive detecting methods attracted main interesting from researchers. For instance, the infrared thermography and the Q2 technology3,20 were developed in current years. The Q2 technologies evaluated seed viability by detecting the reduction of oxygen concentration in a closed chamber20. However, continued attenuation of the oxygen concentration in seeds, causes hypoxia stress on seeds, and significantly reduces the rate of germination9,21. Furthermore, both the infrared thermography and the Q2 technology still cost too much time in routine seed bank maintaining works. In this study, we successfully employed the MOT method to assess seed viability. We found a linear correlation between seed viability and oxygen influx in all three kinds of tested seeds: protein seeds, starch seeds, and oil seeds. With the experimental data, we generated linear equations that predict seed viability using oxygen influxes. The slopes of equations (2), (3) and (4) indicated that wheat seeds exhibit higher oxygen consumption, oilseed rape weakly consumes oxygen, and soybean consumes moderate amounts of oxygen. We show that MOT test can evaluate seed viability at the early stage of imbibition before germination, which could minimize imbibition damage to seeds. The MOT system is accurately controlled promising the precision of the measurement. Using our system, we positioned the optrode to a very small region of about 30 μm in diameter on the surface of embryonic axis, and the distance between the optrode and the surface of seed was kept at 30 μm (Supplementary Fig. S1). Thus, we can directly detect the core oxygen flux signal in the micro-environment on the seed embryonic axis. This signal will reflect the seed viability. In conclusion, we confirmed that the MOT is a fast, non-invasive and real-time seed viability testing method, with considerable research significance and potential economic value in the agriculture sciences.
The oxygen fluxes in wheat and soybean seeds with 0% viability were much higher than the blank and the dead oilseed rap seeds (Fig. 1, Fig. 3, Supplementary Table S2). To address this, soybean seeds with 99.0% viability were killed with boiled water for 10 min, and then used for MOT test. We found that the signal of the killed seeds waved around zero, similar to the blank (supplementary Fig. S2). In this work, seeds producing abnormal seedlings were not regarded as viable ones. As shown in supplementary Table S4, some of the seeds with 0% viability did germinated but none of them could from normal seedlings. The rate of abnormal seedlings in dead wheat and soybean seed was 5% and 10%, respectively, which was much higher than that of oilseed rape (supplementary Table S4). Besides, TZ test (supplementary Table S5) showed that over 70% dead soybean seeds got stained, indicating live cells in non-germinating seeds. Seeds that producing abnormal seedlings, together with live cells in non-germinating seeds, should be responsible for the high background oxygen uptake in dead soybean and wheat seeds.
Oxygen is a necessary factor for seed function. To confirm whether oxygen influx tested by MOT is from seed respiration, we employed pharmacological treatments, by using NaN3 and NADH to inhibit and activate respiration in wheat seeds. The results indicated that oxygen influxes declined and increased after NaN3 and NADH treatment, respectively, confirming that oxygen influx was caused by respiration. The percent of seed germination, whether determined by the real germination test or via theoretical prediction by equation (2), is consistent with the results of our pharmacological experiment using NaN3 and NADH (Supplementary Table S3). These results further confirmed that seed viability is closely related to respiration. These equations also showed that wheat seeds exhibit higher oxygen consumption, oilseed rape weakly consumes oxygen, and soybean consumes moderate amounts of oxygen. Thus, the oxygen demand during seed early germination might differ among seed types, which was similar with the previous observations by Baker et al.4.
In summary, the MOT method has contributed to the study of seed aging, stress, conservation longevity mechanisms in root7, fish14, and fungi22. MOT allows for the detection of single seeds and provides a novel method to compare the difference of activating one seed by oxygen or ions fluxes. Further work is required in two major areas, one is determining the mechanisms of seed germination, viability, and aging in individual seeds; the other is designing an improved MOT device for high throughput detection.
Methods
Plant materials
Wheat (Triticum aestivum L.), soybean (Glycine max L.), and oilseed rape (Brassica napus L.) seeds were used in this study as models to investigate each group with respect to starch, protein, and lipid content as reserve substances. Seeds were packaged in aluminium foil bags and naturally aged in a room without an air-conditioner in Beijing, China. It took over ten years for the seeds to completely lose their viability. After aging, seed viability was detected by a 7-day germination test according to the rules published by ISTA2. Seeds with abnormal seedlings were not regard as germinated/viable in this research. The seed viabilities were as follows (for detailed information, please see Supplementary Table S4):
-
wheat seeds: 91.0, 79.5, 61.0, 43.0, 20.0, and 0%.
-
soybean seeds: 99.0, 80.0, 55.0, 31.0, 21.0, and 0%.
-
oilseed rape: 99.0, 82.0, 61.5, 42.0, and 0%.
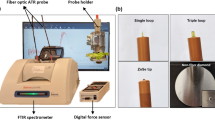
Oxygen flux measurement with MOT
Prior to testing, seeds were soaked in measuring solution (0.1 mM CaCl2, 0.1 mM KCl, 0.3 mM MES, pH 6.0) for three hours, and then transferred to a new solution to detect oxygen fluxes. Oxygen fluxes of seeds were measured in real-time and non-invasively using a micro-optrode (World Precision Instruments, Sarasota, USA) based on NMT (BIO-IM, YOUNGERUSA, LLC, Amherst, USA) by Xuyue (Beijing) Sci. & Tech Co. Ltd. The MOT was calibrated in measuring solution with known oxygen concentrations (0 and 21%) by nitrogen and air purging at 20°C. The overall setup was designed according to previous literature7,17 to insure proper measurement of the concentration gradients from seeds. The equation of oxygen flux based on the changes of phase angel (φ) was:

where JO2 is oxygen flux (pmol cm−2 s−1), D is molecular diffusion coefficient for oxygen in measuring solution (2.1 × 10−5 cm2 s−1 at 20°C), φ1 is phase angle at near pole, φ2 is phase angle at far pole, and m is linear slope of calibration plot within ambient conditions (0–21% O2). ΔX is the distance moving the optrode between two positions close to the seed surface in a preset excursion (30 μm in our experiment, Supplementary Fig. S1) at a programmable frequency of about 0.1 Hz.
A seed was fixed on the bottom of a Petri dish with a plastic colloidal cloth. Measuring solution was then added to the Petri dish to cover the entire seed. The measuring point (embryo/embryonic axis) was located by microscope and the micro-optrode was adjusted to the point using a three dimensional motor. The oxygen fluxes were recorded every 10 seconds and measured for at least 5 minutes. Finally, the data of oxygen fluxes and the images were acquired and recorded in real-time using the imFlux software (YOUNGERUSA).
Seed treatment with respiration inhibitor and promoter
To test the oxygen signal and the relationship between oxygen consumption and seed viability, oxygen flux changes in wheat seeds with 91% and 43% viability were determined before and after NaN3 treatment (a respiration inhibitor) and NADH (a respiration promoter). The wheat seed was soaked in the testing solution for 3 h and the basal oxygen flux was recorded. Then, the seed was treated with NaN3 (0.1 mM) or NADH (10 mM) for 30 min. After this treatment, seeds were washed with test solution to get rid of NaN3 or NADH and the oxygen flux was again tested. Seed viability after treatment with NaN3 or NADH was then detected using the germination test2. The seedling length (mm) and mean seedling weight (mg) after 7-day germination were also recorded.
Data analyses
Each measurement was repeated with 20 to 30 individual seeds (supplementary Table S2). The results were expressed as means ± standard errors (S.E.). The correlation between seed viability and oxygen flux was analysed using SigmaPlot (Version 10.0). Data were tested for significance by one-way ANOVA using the SPSS (Version 18.0) software. The figures were generated using SigmaPlot and Microsoft Excel 2003.
References
Rajjou, L. et al. Seed germination and vigor. Annu. Rev. Plant Biol. 63, 507–533 (2012).
ISTA. International Rules for Seed Testing. (International Seed Testing Association, 2009).
Kranner, I., Kastberger, G., Hartbauer, M. & Pritchard, H. W. Noninvasive diagnosis of seed viability using infrared thermography. PNAS 107, 3912–3917 (2010).
Baker, C. J., Roberts, D. P., Mock, N. M. & Blount, V. L. A novel open-system technique to monitor real-time oxygen consumption during early phases of seed germination. Seed Sci. Res. 14, 17–26 (2004).
Wolfbeis, O. S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 76, 3269–3284 (2004).
Chaturvedi, P. M. et al. Emerging technologies for non-invasive quantification of physiological oxygen transport. Planta In press (2013).
McLamore, E. S., Jaroch, D., Chatni, M. R. & Porterfield, D. M. Self-referencing optrodes for measuring spatially resolved, real-time metabolic oxygen flux in plant systems. Planta 232, 1087–1099 (2010).
Bewley, J. D. Seed germination and dormancy. Plant Cell 9, 1055–1066 (1997).
Bradford, K. J., Côme, D. & Corbinear, F. Quantifying the oxygen sensitivity of seed germination using a population-based threshold model. Seed Sci. Res. 17, 33–43 (2007).
Bradford, K. J., Benech-Arnold, R. L., Côme, D. & Corbinear, F. Quantifying the sensitivity of barley seed germination to oxygen, abscisic acid, and gibberellin using a population-based threshold model. J. Exp. Bot. 59, 335–347 (2008).
McLamore, E. S. & Porterfield, D. M. Non-invasive tools for measuring metabolism and biophysical analyte transport: self-referencing physiological sensing. Chem. Soc. Rev. 40, 5308–5320 (2011).
Newman, I., Chen, S. L., Porterfield, D. M. & Sun, J. Non-invasive flux measurements using microsensors: theory, limitations, and systems. Methods MolBiol. 913, 101–117 (2012).
Porterfield, D. M., Rickus, J. L. & Kopelman, R. Non-invasive approaches to measuring respiratory patterns using a PtTFPP based, phase-lifetime, self-referencing oxygen optrode. Proc. SPIE. 6380, 63800S.1–63800S.8 (2006).
Sanchez, B. C., Ochoa-Acuña, H., Porterfield, D. M. & Sepúlveda, M. S. Oxygen flux as an indicator of physiological stress in fathead minnow (Pimephales promelas) embryos: a real-time biomonitoring system of water quality. Environ. Sci. & Technol. 42, 7010–7017 (2008).
McLamore, E. S., Zhang, W., Porterfield, D. M. & Banks, M. K. Membrane-aerated biofilm proton and oxygen flux during chemical toxin exposure. Environ. Sci. & Technol. 44, 7050–7057 (2010).
McLamore, E. S. et al. Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J. 63, 1004–1016 (2010).
Wan, Y. L. et al. Non-invasive measurement of real-time oxygen flux in plant systems with a self-referencing optrode. Protocol Exch. 10.1038/protex.c2011.266. (2011).
FAO. Draft Second Report on the State of the World's Plant Genetic Resources for Food and Agriculture. 58 (Food and Agriculture Organization, 2009).
Bewley, J. D., Bradford, K. J., Hilhorst, H. W. M. & Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd Edition. 133–181 (Springer, 2013).
Van Asbrouck, J. & Taridno, P. Using the single seed oxygen consumption measurement as a method of determination of different seed quality parameters for commercial tomato seed samples. Asian J. Food & Agro-Industry 2, Special Issue S88–S95 (2009).
Armstrong, W., Webb, T., Darwent, M. & Beckett, P. Measuring and interpreting respiration critical oxygen pressures in roots. Ann. Bot. 103, 281–293 (2009).
Lew, R. R. & Levina, N. Oxygen flux magnitude and location along growing hyphae of Neurospora crassa. FEMS Microbiol. Lett. 233, 125–130 (2004).
Acknowledgements
This work was supported by the National Key Technology R&D Program (2013BAD01B01), and the Core Research Budget of the Non-profit Governmental Research Institution (ICS, CAAS, 2011006). We would like to thank Editage for providing editorial assistance.
Author information
Authors and Affiliations
Contributions
X.X., W.J.W. and X.X.L. designed the research, X.X., Y.L.W. and W.J.W. performed calculations and data analysis, X.X., W.J.W. and G.K.Y., prepared figures and tables, X.X., Y.L.W. and W.J.W. wrote the manuscript, E.S.M. revised the manuscript, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (DOC 267 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Xin, X., Wan, Y., Wang, W. et al. A real-time, non-invasive, micro-optrode technique for detecting seed viability by using oxygen influx. Sci Rep 3, 3057 (2013). https://doi.org/10.1038/srep03057
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03057
This article is cited by
-
Gaseous environment modulates volatile emission and viability loss during seed artificial ageing
Planta (2021)
-
Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: implications on seed technology traits
Plant Cell Reports (2017)
-
The fluxes of H2O2 and O2 can be used to evaluate seed germination and vigor of Caragana korshinskii
Planta (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.





 ). Positive value indicates oxygen influx.
). Positive value indicates oxygen influx.
