Abstract
Predicting infertility is central to reproductive biology, medicine and evolutionary biology. In-vitro studies suggest that oxidative sperm damage causes infertility. Oxidative sperm damage can be reduced via two fundamental pathways: the removal of oxygen radicals by antioxidants, or the interference with cell metabolism to reduce the formation of oxygen radicals. Oxidative damage protection of spermatozoa should evolve frequently, especially during female sperm storage. However, in-vivo evidence linking oxidative protection and fertility is rare. We show that the intra-sperm production rate of oxygen radicals and the sperm metabolic rate were reduced in female bedbugs, Cimex lectularius, compared to males and females laid fertile eggs. Females became infertile when sperm oxygen radicals and sperm metabolic rate increased to male levels. Our results link female fitness to sublethal sperm damage, imply adaptive benefits of interfering with sperm metabolism and offer the hypothesis that polyandry may serve to replace low-quality sperm.
Similar content being viewed by others
Introduction
Predicting infertility is a significant current challenge in several biological disciplines1. In reproductive medicine, substantial efforts are devoted to research of why one sixth of couples in the western world suffer from infertility problems1. In animal health research and conservation genetics, breeding programmes are designed to reduce infertility between specific male and female genotypes. In evolutionary biology, infertility is a main component of fitness, the central predictor of directional evolutionary change. Across these disciplines, efforts to explain infertility are largely devoted to sperm counts or to male genotypic traits that are correlated with ejaculate characteristics1. This genetic approach, however, requires the additional consideration of environmental effects on sperm function, many of which are known as lifestyle effects on male fertility2 and include the effects of male diet, smoking habits or sexually transmitted microbes on sperm function2,3.
Damaging effects by such environmental effects are not restricted to sperm while in the male but may also happen when the sperm is stored in the female. This notion is important because females of all internally fertilizing species store sperm, some for very long periods4,5,6,7,8, thus achieving reproductive independence from the presence of males, or promoting sperm competition4. The question how females prevent such damaging effects to sperm during storage has received little attention. To date, female sperm storage has been mainly viewed as a numerical process where female become infertile simply because they receive too few sperm, or lose, or use up too many before the full complement of eggs is fertilized9,10,11. Sexual selection research has therefore suggested that one function of multiple mating by females is to receive a sufficient amount of sperm12,13.
This focus on the number of sperm, or living sperm, is insufficient when considering the above-mentioned environmental factors, or lifestyle effects. The damaging effects on sperm caused by the environmental factors accumulate in the sperm cell and lead to sperm cell ageing14,15,16, which can restrict female fertility or fitness14,15,16. However, disentangling whether female infertility is caused by sperm quantity or by sperm quality is important because numerical sperm limitation only limits the number of zygotes produced. By contrast, stored sperm that is aged or otherwise sublethally damaged can fertilize eggs and result in offspring with disease symptoms and reduced fitness14,15,16. In this paper we, therefore, aim to disentangle effects of sperm quantity and sperm quality, i.e. sperm age, on female infertility.
Damage accumulation during sperm ageing encompasses a suite of biochemical and physiological mechanisms14,15,16 but oxidative damage by oxygen radicals are major players14,15,16,17,18,19,20,21,22,23,24,25,26. Oxidative damage can be reduced and hence cellular ageing delayed, by two fundamental pathways: the antioxidant pathway and the interference pathway. The majority of studies considered the former, i.e. consider how antioxidants capture the oxygen radicals produced by the cell7,8,21,27. The effectiveness of the antioxidant pathway in improving sperm performance is currently debated25,27,28. The second pathway concerns the extracellular (i.e. extra-sperm) interference with cell metabolic pathways in order to lower the formation of oxygen radicals in the first place. This possibility has received much less theoretical7,14 and empirical attention29, but may be important. A previous study found sperm metabolism in female insects was reduced over several weeks of sperm storage with the corresponding intra-sperm oxygen radicals production also being low29. The reduction was proposed to be adaptive because it may delay sperm ageing and so reduce negative fitness effects on females29, but was not tested. Here we now attempt to examine this significance. Using bedbugs, Cimex lectularius, a long-lived, sperm-storing insect30, we tested whether female infertility is lower during periods when oxygen radicals production are suppressed in sperm29 compared to times when radicals are not suppressed.
Sperm cells are morphologically the most diverse cell type in the animal kingdom31. It is possible that sperm physiology is also very variable between species. An additional goal of this study was, therefore, to establish whether the relationship between sperm metabolism and oxygen radicals is similar to that observed in another insect species29.
To summarise, in a long-lived, re-emerging pest species30 we test whether female reproduction is limited by the depletion of sperm quantity or sperm quality, we probe the plausibility of the interference hypothesis of antioxidant defence and, as a first step as to its general applicability, compare sperm metabolism to that of another species.
Results
Weekly recording any infertile eggs (Methods) in the clutches of sexually isolated females, we established that females became infertile after long-term sperm storage, at 9.5 ± 1.6 (SE) weeks (Fig. 1). Most (89.5%, N = 19) infertile females recovered full fertility when they were re-mated, excluding female age as the primary cause of infertility.
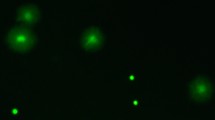
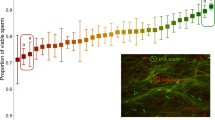
(A) Live sperm cells (stained green), surrounding unspecified female cells (stained red) of bedbugs extracted from the female sperm storage organ. The white circle illustrates the < 40 μm diameter of the laser beam that was used to excite an oxygen probe within sperm cells. (B) The intracellular production of oxygen radicals of sperm extracted from the male (filled diamonds, N = 13) and female storage organs (empty diamonds) (upper graph) in relation to the time course that sexually isolated females (N = 54) become infertile (lower graph). The intra-sperm radicals production was sampled in females after short-term storage (6 h, N = 6), intermediate-term storage (3.5 weeks, N = 11) and long-term storage (10 weeks, N = 7). Arrows denote means per storage period. Grey lines connecting data points denote sperm form the same male examined from the male and the female sperm storage.
Next, we excluded that sperm quantity depletion (lack of sperm numbers) was the primary cause of infertility in bedbugs: In 54 females we manipulated the number of sperm transferred to the female by interrupting copulations and the number of sperm used by altering her egg laying rate (Methods). Time-failure analysis showed that the number of sperm transferred (z = 1.25, P = 0.211) did not explain variation in the time females started to become infertile. The number of sperm used by females did (z = 3.67, P = 0.0002) but females that laid more eggs became infertile later (Fig. S1), i.e. the opposite direction of what were predicted if more fertilization events would lead to higher sperm usage. The non-significant interaction of the two factors (z = 0.087, P = 0.931) was removed from the model (Table S1). The results did not change if a different infertility indicator was used (Fig. S1, Table S1). When infertility started, as well as two weeks later when females laid all-infertile clutches, most stored sperm was alive (mean 85.6%, range 50–100%, n = 11) (see also Methods), which excludes sperm mortality as the cause of infertility and leaves sperm quality as a predictor of female infertility.
In order to test whether the accumulation of oxidative damage is a candidate trait of sperm-quality mediated female infertility, we measured the rate of intracellular oxygen radicals production in sperm cells using time-resolved microfluorimetry17,29. This parameter is independent of protein antioxidants, of sperm density and of most other ejaculate parameters17,29. In sperm samples taken from males and from females after short (6 h post mating), intermediate (3.5 weeks post mating) and long (10 weeks post mating) duration of storage, we recorded the fluorescence lifetime of an intra-sperm probe (1-pyrene butyric acid, PBA)29. The fluorescence lifetime of this probe decreases with increasing concentration of oxygen radicals17,32,33,34. If females would adaptively reduce oxidative damage in the stored sperm cells, we expected higher fluorescence lifetimes in sperm cells that were stored for short or intermediate duration (before the start of infertility) compared to males. This prediction was confirmed: Paired sperm samples of the same male that were taken from both the male and the female showed that fluorescence lifetime is higher, i.e. oxygen radicals production is lower, in the female (paired t6 = −3.373, P = 0.015, mean difference: 6.10 ± 0.83 (SE) nanoseconds (range −0.63 to 13.3 nanoseconds) (Fig. 1B). If eventually oxidative damage would accumulate to cause female infertility, we predicted that fluorescence lifetimes of sperm after long-term storage would be increased compared to short and intermediate duration. This prediction was also upheld. Sampling at short, intermediate and long-term storage, the fluorescence lifetimes were 150.96 ± 0.73 (SE), 153.09 ± 0.49 (SE) and 148.26 ± 0.76 (SE) nanoseconds, respectively (Fig. 1B) and in males 149.31 ± 0.98 (SE) nanoseconds. Thus, the rate of oxygen radicals production in sperm was 17.7% lower after 6 h (Mixed effect model, t-value = 1.98, d.f. = 20, P = 0.0621) and 31.3% lower after 3.5 weeks of storage (Fig. 1B; Mixed effect model, t-value = 3.68, d.f. = 20, P = 0.0016; Table S2) compared to 10 weeks of storage (i.e. the mean start of infertility).
One way that oxygen radicals can be reduced is by an alteration of the sperm metabolic rate, but it is not clear whether a reduced or an increased sperm metabolic rate is related to oxygen radicals production (discussed in ref. 29). We, therefore, measured the sperm metabolic rate using the autofluorescence of free and protein-bound NAD(P)H molecules17,29 and compared it with the oxygen radicals production from the same samples. In bedbugs, unlike in crickets29, the rate of intracellular oxygen radicals production and the metabolic rate of sperm were positively correlated in both sexes (Fig. S2). This correlation is a necessary pre-condition for females29 (and in our case also males) to decrease sperm oxygen radicals production by a reduction in sperm metabolic rate. In support, after 3.5 weeks of storage, the sperm metabolic rate was 14.3% lower than after 10 weeks (Mixed effect model, t-value = 2.600, d.f. = 20, P = 0.009, Table S3) and not significantly different from after 6 h (Mixed effect model, t-value = 0.714, d.f. = 20, P = 0.241). Again using only paired samples, we found that sperm metabolic rate at short-term storage was 19.7% (range –5.55 to 41.4) lower than in males (paired t6 = 2.681, P = 0.036). Statistically modelling sperm oxygen radicals production as a function of sperm metabolic rate revealed that metabolic rate significantly decreased with decreasing oxygen radicals production (Mixed effect model, t-value = −6.87, P < 0.001, Table S4).
Together, our results are consistent with the hypothesis that sperm-storing females incur fitness costs when the rates of oxygen radicals production and metabolism of the sperm are not suppressed compared to the sperm they receive from males. In other words, females have an adaptive benefit from a reduced metabolic rate and oxygen radicals production in stored sperm. Our results also suggest that in terms of the relationship between sperm metabolic rate and oxygen radicals production rate not all species are equal.
Discussion
Our study yielded results that are significant for a number of research areas. For evolutionary and reproductive biology, our result show that a numerical perspective of sperm storage9,10,11,12,13 is insufficient to explain female infertility and hence, insufficient to explain multiple mating by females35. Female infertility of sexually isolated females should not automatically be assigned to a lack of sperm number13,36. In our study, infertility was caused by a decrease in sperm quality. That sperm quality decreases over storage time, rather than representing a fixed genotypic quality of a male as assumed by sexual selection models, reinforces the suggestion14,29 that the predictive power of sperm competition theory31 will be improved by accounting for temporal variation in sperm function. Our method can be used to examine the nature of the decline in sperm quality during sperm ageing. Different decline curves yield fundamentally different predictions about the costs and benefits of sperm ageing14.
Cell senescence is a thermodynamic necessity14,15,16,17,18,19. The fact that healthy offspring are being produced even after relatively long sperm storage in many animals4,5,6,7,8 suggests that oxidative damage to sperm is reduced over extended periods. We have demonstrated here (see also29) that two components of sperm function are indeed reduced: sperm oxygen radicals production and sperm metabolic rate. Here we additionally showed that if both parameters return to male levels, females suffer infertility. While in some species, the antioxidant pathway of sperm protection has demonstrated fitness benefits for males and females27,28, antioxidants only have small effects on human fertility25. It is, therefore, possible that the other fundamental pathway of oxidative stress prevention (the one confirmed here) - a suppression of the formation of oxygen radicals (and other reactive oxygen species) - is important. Future studies (see also29) should test whether interfering with cell metabolism is a general candidate mechanism to reduce oxidative stress in sperm. Our finding that sperm metabolic rate was positively correlated with oxygen radicals production is a necessary pre-condition of the hypothesis7,8,14,29 that it is the females that reduce oxygen radicals production by interfering with sperm metabolism and so down-regulate it. The reverse is less plausible, that reduced oxygen radicals production in the cell causes reduced metabolic rate, because the established biochemical pathways of cellular energy production do not seem to require oxygen radicals.
We observed an increased radicals production in sperm cells towards the end of the sperm storage period in bedbug females. It remains to be tested whether this increase is a maladaptive side effect of the unavoidable ageing process or the result of an adaptive female adjustment of oxygen radicals suppression. For example, if reduced radicals suppression by females would increase the mortality of only those sperm that have accumulated more damage than others (e.g., older sperm), one could speculate that the reduced radicals suppression may adaptively prevent the fertilization of eggs with aged sperm and so avoid female investment into embryos with reduced fitness14,17. Alternatively, the vicious circle idea of the free radical theory of ageing37, but see38,39,40, predicts that the oxygen radicals suppression has to constantly increase to keep pace with the constantly accumulating oxidative damage. Under this hypothesis, therefore, the costs of investing into radicals suppression will constantly increase and reach a point where they outweigh the benefits of fertilizing the eggs with non-damaged sperm. Although this sounds unlikely, in an insect related to bedbugs, females upheld investment into sperm storage even though this resulted in fewer eggs being produced11. However, in this species, females naturally had no opportunity to re-mate11 and so have to maintain storage of non-damaged sperm. If, by contrast, re-mating opportunities for females are predictable in time, reducing the investment into radicals suppression after the average re-mating period is beneficial to females and so may evolve. Given that we found that infertility started, on average, 9.5 weeks (at 25°C) after sexual isolation, is there any reason to assume that 9.5 weeks is a predictable, evolved average period of re-mating? We believe there is: In the wild, bedbug populations are highly inbred as they are usually founded by single, i.e. sexually isolated, females41. For sexually isolated females, it takes 8–9 weeks (at 25°C) to produce sexually mature sons, a figure close to 9.5 weeks. Consequently, it may be worth testing the idea that sperm radicals suppression in bedbug females has evolved to be maintained only for the average time it takes a mother to receive new sperm from their own sons.
In conclusion, our examination of the sperm storage period in bedbugs coupled with a fluorescence-based survey of sperm metabolism revealed that sublethal sperm damage is a previously little recognized source of fitness variation. That reducing sperm metabolic rate and oxygen radicals production during sperm storage is adaptive augments the numerical view of female sperm limitation, represents a novel hypothesis why females mate multiple times and offers a potential mechanism how females of many taxa can store sperm for extended periods. Our results extend the current view of sperm quality in postcopulatory sexual selection studies that is restricted to a dead-live dichotomy, or a genetically determined, temporally invariable, sperm genotype. Finally, by characterizing the relationship between oxygen radicals production and cell metabolic rate we present insect sperm cells as a convenient ex-vivo model system of cell metabolism and mitochondrial energetics.
Methods
The study species
Mating
Common bedbug males, Cimex lectularius L. 1758, exclusively mate by traumatic insemination. The male pierces the female cuticle and ejaculates the sperm into the spermalege, a secondary copulatory organ situated in the open haemocoel. C. lectularius males transfer sperm at a constant rate during mating42 and across three matings17 (Supplementary Fig. S3). Therefore, copulation duration was used to manipulate the number of sperm that females received. Interrupting copulations does not appear to lead to a differential allocation of sperm and seminal fluid in males of the population used here43.
Sperm storage
Within 4 hours of mating, sperm leave the spermalege, move through the female haemolymph and enter the female oviduct. From the oviduct, sperm move to the sperm storage organ, the paired seminal conceptacles, where most sperm arrive within 12 hours. From there, the sperm move to and fertilise the eggs in, the ovaries or are stored in storage organs.
Female fertility
Females can live and lay eggs for almost a year, provided food and sperm are available30,44,45. If females are sexually isolated, offspring are being produced for 6–9 weeks. Eggs are easy recovered and their fertilization status scored45,46,47. Fertile eggs are greyish white, firm and taut, with embryo eyespots visible. They are easily distinguished from infertile eggs, which are darkish grey or brown, shrunk and shrivelled (e.g. ref. 48). Except for the first clutch, all eggs in a batch are usually fertile.
Effect of sperm availability on the onset of infertility
Altering sperm number transferred and used
Fifty-four females were randomly assigned to either of three sperm treatment levels. Females either received one ‘ejaculate unit’45, i.e. were mated once for 60 s (S1, n = 18), four units (four 60 s matings in succession (S4, n = 18), or left to copulate four times for ad libitum duration (n = 17, one female had escaped) (Fig. S1). The latter treatment resulted in a mean copulation duration of 400 s (range 270 to 780 s), equivalent to 6.5 units. The same male was used for successive matings within a given pair and there were 2–3 h between matings. The transfer of sperm was confirmed in a non-invasive way after the first mating by the visible occurrence of a white mass just underneath the female cuticle.
Mated females within each sperm treatment level were randomly assigned to be either fed once per week, or once every two weeks. This treatment manipulated the number of eggs laid and fertilized and hence, the number of sperm used by the female (see below).
Females were kept in individual vials equipped with a strip of filter paper as oviposition substrate. Starting at 14:00 h every Tuesday, the filter paper with attached eggs was removed (female order randomized at each week). Then, females were fed according to treatment and placed into the same tube with a new piece of filter paper. All eggs were counted and their fertilization status examined. A linear mixed effects model of feeding rate as an explanatory variable, the number of eggs in consecutive weeks as the dependent variable, considered within female identity, i.e. random effect confirmed that feeding treatment was effective over the entire duration of the feeding treatment (until week 9): higher feeding rate resulted in ca. 46% more fertilized eggs being laid [mean: 65.7 ± 0.96 (SE)] compared to lower feeding rate [mean: 45.0 ± 0.74 (SE) (feeding rate × week interaction: slope 0.885 ± 0.231, t1,369 = 3.827, P = 0.0002).].
Infertility measurement
We used the occurrence of the second infertile egg as an indicator of the onset of infertility. After the first infertile egg, 18 out of 54 (33%) females laid one or more fully fertile clutches but after their second infertile egg, only 6 out of 54 (11%) females did so. This suggests that the first infertile egg may be related to a chance event, whereas the second infertile egg denotes the beginning of infertility. However, using the first infertile egg did not alter the results (Fig. S1, Table S1). The occurrence of the third infertile egg was related to that of the second (Supplementary Fig. S1). When sperm is limited, females start to lay clutches with both fertile and infertile eggs, thereafter clutches with only infertile eggs and finally cease to lay any eggs (Fig. S1).
Sperm physiology
Sperm storage periods
We set up mating trials48 to have available females for sperm physiology measurement that had stored sperm for intermediate sperm storage duration (20–26 days, henceforth 3.5 weeks) and 66 to 72 days (henceforth 10 weeks). Females allocated to investigation after short-term sperm storage were mated 6 hours before the analysis. Note that at this time, sperm have not yet entered the female sperm storage organ but are still situated in the haemolymph.
Estimating cell metabolic rate using NAD(P)H fluorescence
NAD(P)H is found either free or bound to protein. Total NAD(P)H decreases with increasing ATP production. Because free NAD(P)H is used up first49, the proportion of bound NAD(P)H relative to total NAD(P)H increases with increasing metabolic rate. Free NAD(P)H is fluorescent with an emission peak at 460 nm and a lifetime τ1 of less than 1 nanosecond. The emission maximum of protein-bound NAD(P)H has a longer wavelength and lifetime τ2 (2–10 nanoseconds)49,50,51,52,53. If the relative contributions of the amplitudes of τ1 and τ2, are a1 for free NAD(P)H and a2 for bound-NAD(P)H, a2/(a1 + a2) gives an estimate of the metabolic rate51,52,53.
Intra-sperm ROS production
1-pyrene butyric acid (PBA) is a cell permeable oxygen probe sensitive to free radicals in solution33 and living cells32,34. Upon collision with PBA, oxygen free radicals change PBA fluorescence intensity and decrease fluorescence lifetime. The method is particularly sensitive to small, mobile radical molecules such as superoxide radical (O2−) and nitric oxide (NO°) but does not respond to the hydroxyl radical (which has too short a half-life) and non-radical ROS, such as hydrogen peroxide and peroxynitrite33. By measuring the concentration of free radicals with a short half-life (< 20 min) but not the concentration of hydrogen peroxide to which these radicals dismute, we obtain an estimate of the in situ (i.e. < 20 min half-life) production of ROS. Membrane crossing by external oxidants or antioxidants is several orders of magnitude slower than PBA fluorescence changes. Therefore, any chemical reactions between oxygen radicals and antioxidants are unlikely to affect the results. PBA preferentially binds to dissolved proteins32 rather than entering cellular membranes. Any PBA signal obtained from sperm indicates, therefore, that extracellular antioxidant proteins are absent and that we do not measure passive ROS removal.
Additional advantages of measuring ROS production by PBA fluorescence changes have been summarized earlier29: it is independent of intracellular probe concentration and the cell density. Unlike the routinely used lucigenin-based ROS measurements that produce ROS themselves54, our method does not generate ROS. PBA is insensitive to alterations in the lipid concentration of the membrane55 suggesting that if such changes would occur, they are unlikely to affect the fluorescence lifetime of PBA.
Sample preparation and staining procedure
The sperm-containing vesicles were removed from the male using forceps and placed into a drop of 20 μl buffer on a microscope slide. Sperm were collected with a pipette and placed into a 20 μl drop of 1 μM PBA, incubated for 4 minutes and washed three times in three different drops of 20 μl of buffer on a microscope slide by drawing the sperm solution ten times up and down a pipette. The sperm were then pipetted into 10 μl buffer in the centre of a Sykes-Moore chamber. ROS concentration was measured in this chamber.
To collect sperm from the female, females were dissected in a dissection bowl filled with buffer. The sperm storage organ was removed, rinsed on the outside in 20 μl buffer and placed into a new 20 μl drop. The organ was opened by tearing its ends carefully apart, which released one or a few sun-shaped aggregations of sperm (Fig. 1A). These were transferred into 20 μl 1 μM PBA.
Dead sperm with intact membranes were needed to calculate lifetime τ0 (see below), which is terminated when ROS production stops. These sperm were handled as described above, but after rinsing in buffer they were fixed in Baker solution (10% paraformaldehyde in 1% aqueous calcium chloride) for 15–20 minutes32. The time from dissection to the fluorescence measurement was recorded for each sample. There was no difference in handling time between male and female samples (males: 21.5 min; females: 28.2 min; t30.77 = −1.204, P = 0.238), even when one outlier was removed of a female sperm sample measured after 120 minutes (females: 23.5 min) (t25.33 = −0.660, P = 0.515).
References
World Health Organization. WHO laboratory manual for the Examination and processing of human sperm. World Health Organiz, 5th ed. (2010).
Wogatzky, J. et al. The combination matters--distinct impact of lifestyle factors on sperm quality: a study on semen analysis of 1683 patients according to MSOME criteria. Reprod Biol Endocrinol 10, 115 (2012).
Otti, O., McTighe, A. & Reinhardt, K. In vitro antimicrobial sperm protection by an ejaculate-like substance. Funct Ecol 27, 219–226 (2013).
Birkhead, T. R. & Møller, A. P. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol J Linn Soc 50, 295–312 (1993).
Keller, L. Queen lifespan and colony characteristics in ants and termites. Insectes Soc 45, 235–246 (1998).
den Boer, S. P. A., Baer, B., Dreier, S., Aron, S., Nash, D. R. & Boomsma, J. J. Prudent sperm use by leaf-cutter ant queens. Proc Biol Sci 276, 3945–3953 (2009).
Heifetz, Y. & Rivlin, P. K. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology 73, 723–739 (2010).
Holt, W. V. & Lloyd, R. E. Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73, 713–722 (2010).
Wedell, N., Gage, M. J. G. & Parker, G. A. Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol 17, 313–320 (2002).
Gromko, M. H. in Sperm competition and the evolution of animal mating systems, Smith, R. L. Ed. (Acad Press, London), pp 331–338 (1984).
Roth, S. & Reinhardt, K. Facultative sperm storage in response to nutritional status in a female insect. Proc Biol Sci 270, S54–S56 (2003).
Simmons, L. The evolution of polyandry: Sperm competition, sperm selection and offspring viability. Annu Rev Ecol Evol Syst 36, 125–146 (2005).
Gowaty, P. A., Kimd, Y.-K., Rawlings, J. & Anderson, W. W. Polyandry increases offspring viability and mother productivity but does not decrease mother survival in Drosophila pseudoobscura. Proc Natl Acad Sci USA 107, 13771–13776 (2010).
Reinhardt, K. 2007 Evolutionary consequences of sperm cell aging. Q Rev Biol 82, 375–393 (2007).
Pizzari, T., Dean, R., Pacey, A., Moore, H. & Bonsall, M. B. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol Evol 23, 131–140 (2008).
White, J. et al. Multiple deleterious effects of experimentally aged sperm in a monogamous bird. Proc Natl Acad Sci USA 105, 13947–13952 (2008).
Siva-Jothy, M. T. The young sperm gambit. Ecol Lett 3, 172–174 (2000).
Tarin, J. J., Perez-Albala, S. & Cano, A. Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Update 6, 532–549 (2000).
Halliwell, B. & Gutteridge, J. Free Radicals in Biology and Medicine, Oxford Univ Press, 4th ed. (2007).
Wallace, D. C., Fei, W. & Procaccio, V. Mitochondrial energetics and therapeutics. Annu Rev Pathol Mech Dis 5, 297–348 (2011).
Blount, J. D., Møller, A. P. & Houston, D. C. Antioxidants, showy males and sperm quality. Ecol Lett 4, 393–396 (2001).
Aitken, R. J., de Iuliis, G. N. & McLachlan, R. I. Biological and clinical significance of DNA damage in the male germ line. Int J Androl 32, 46–56 (2009).
de Lamirande, E. & Lamothe, G. Reactive oxygen-induced reactive oxygen formation during human sperm activation. Free Radic Biol Med 46, 502–510 (2009).
Ramalho-Santos, J. et al. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 15, 553–572 (2009).
Zini, A. & Al-Hathal, N. Antioxidant therapy in male infertility: fact or fiction? Asian J Androl 13, 374–381 (2011).
Koppers, A. J., De Iuliis, G. N., Finnie, J. M., McLaughlin, E. A. & Aitken, R. J. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J Clin Endocrinol Metab 93, 3199–3207 (2008).
Almbro, M., Dowling, D. K. & Simmons, L. W. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol Lett 14, 891–895 (2011).
Poland, V. et al. Stored sperm differs from ejaculated sperm by proteome alterations associated with energy metabolism in the honeybee Apis mellifera. Molec. Ecol 20, 2643–2654 (2011).
Ribou, A.-C. & Reinhardt, K. Reduced metabolic rate and oxygen radicals production in stored insect sperm. Proc Biol Sci 279, 2196–2203 (2012).
Reinhardt, K. & Siva-Jothy, M. T. Biology of bed bugs (Cimicidae). Annu Rev Entomol 52, 351–374 (2007).
Birkhead, T. R., Hosken, D. J. & Pitnick, S. S. Sperm Biology: An Evolutionary Perspective. Academic press, 1st ed. (2009).
Ribou, A.-C., Vigo, J. & Salmon, J. M. Lifetime of fluorescent pyrene butyric acid probe in single living cells for measurement of oxygen fluctuation. Photochem Photobiol 80, 274–280 (2004).
Oter, O. & Ribou, A.-C. Quenching of long lifetime emitting fluorophores with paramagnetic molecules. J Fluoresc 19, 389–397 (2009).
Rharass, T., Vigo, J., Salmon, J. M. & Ribou, A.-C. New method for the detection of reactive oxygen species in anti-tumoral activity of adriamycin: A comparison between hypoxic and normoxic cells. Free Radical Res 42, 124–134 (2008).
Pizzari, T., Worley, K., Burke, T. & Fromann, D. P. Sperm competition dynamics: ejaculate fertilizing efficiency changes differentially with time. BMC Evol Biol 8, 332 (2008).
Loyau, A., Blanchet, S., van Laere, P., Clobert, J. & Danchin, E. When not to copy: female fruit flies use sophisticated public information to avoid mated males. Sci. Rep. 2, 768 (2012).
Harman, D. Aging: overview. Ann. N.Y. Acad. Sci. 928, 1–21 (2001).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants and aging. Cell 120, 483–495 (2005).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem J 417, 1–13 (2009).
Lapointe, J. & Hekimi, S. When a theory of aging ages badly. Cell Mol Life Sci 67, 1–8 (2010).
Saenz, V. L. Booth, W. Schal, C. & Vargo, E. L. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 49, 865–875 (2012).
Siva-Jothy, M. T. & Stutt, A. A matter of taste: direct detection of female mating status in the bedbug. Proc Biol Sci 270, 649–652 (2003).
Reinhardt, K., Naylor, R. & Siva-Jothy, M. T. Male mating rate is constrained by seminal fluid availability in bed bugs, Cimex lectularius. PLoS ONE 6, 7e22082 (2011).
Usinger, R. L. Monograph of the Cimicidae. Thomas Say Foundation. Entomol. Soc. Am., Philadelphia (1966).
Reinhardt, K., Naylor, R. & Siva-Jothy, M. T. Ejaculate components delay reproductive senescence while elevating female reproductive rate in an insect. Proc Natl Acad Sci USA 106, 21743–21747 (2009).
Reinhardt, K., Naylor, R. & Siva-Jothy, M. T. Reducing a cost of traumatic insemination: female bed bugs evolve a unique organ. Proc Biol Sci 270, 2371–2375 (2003).
Hosokawa, T., Koga, R., Kikuchi, Y., Meng, X.-Y. & Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107, 769–774 (2011).
Reinhardt, K., Naylor, R. & Siva-Jothy, M. T. Situation exploitation: higher male mating success when female resistance is reduced by feeding. Evolution 63, 29–39 (2009).
Blinova, K. et al. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 47, 9636–9645 (2008).
Lakowicz, J. R., Szmacinski, H., Nowaczyk, K. & Johnson, M. L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci USA 89, 1271–1275 (1992).
Chorvat Jr, D. & Chorvatova, A. Multi-wavelength fluorescence lifetime spectroscopy: A new approach to the study of endogenous fluorescence in living cells and tissues. Laser Physics Lett. 6, 175–193 (2009).
Skala, M. C. et al. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes and cellular morphology in precancerous epithelia. Proc Natl Acad Sci USA 104, 19494–19499 (2007).
Ghukasyan, V. V. & Kao, F.-J. Monitoring cellular metabolism with fluorescence lifetime of reduced Nicotinamide Adenine Dinucleotide. J Phys Chem C 113, 11532–11540 (2009).
Fridovich, I. Superoxide Anion Radical (O2.-), Superoxide Dismutases and Related Matters. J Biol Chem 272, 18515–18517 (1997).
Dumas, D. et al. Membrane fluidity and oxygen diffusion in cholesterol-enriched erythrocyte membrane. Arch Biochem Biophys 341, 34–39 (1997).
Acknowledgements
We thank A. Dobson, D. Dowling, A. McTighe, R. Naylor, O. Otti and J. Rolff for comments on the manuscript and technical help. Financial support was provided the VolkswagenFoundation (84–780), NERC (NE/D009634/1) and a visitors' support scheme by the Languedoc-Roussillon regional government to K.R.
Author information
Authors and Affiliations
Contributions
K.R. and A.C.R. conceived the idea, K.R. and A.C.R. carried out the research, K.R. and A.C.R. analysed the data, K.R. and A.C.R. wrote the manuscript. K.R. prepared the figures. K.R. and A.C.R. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplement
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Reinhardt, K., Ribou, AC. Females become infertile as the stored sperm's oxygen radicals increase. Sci Rep 3, 2888 (2013). https://doi.org/10.1038/srep02888
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02888
This article is cited by
-
Ranking parameters driving siring success during sperm competition in the North African houbara bustard
Communications Biology (2023)
-
Morphology of testis, sperm, and spermatheca in two capable hybridized termite species indicates no interspecific reproductive isolation
International Journal of Tropical Insect Science (2022)
-
Production and scavenging of reactive oxygen species both affect reproductive success in male and female Drosophila melanogaster
Biogerontology (2021)
-
Distinct metabolic profiles in Drosophila sperm and somatic tissues revealed by two-photon NAD(P)H and FAD autofluorescence lifetime imaging
Scientific Reports (2019)
-
Complex interactions between sperm viability and female fertility
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.