Abstract
Malaria threatens millions of people annually and is a burden to human health and economic development. Unfortunately in terms of disease control, no effective vaccines are available and the efficacy of treatment is limited by drug resistance. Genetic manipulation in Plasmodium falciparum is hampered due to the absence of robust methods for genetic analyses. Electroporation-based transfection methods have allowed the study of gene function in P. falciparum, with low efficiency. A lipid nanoparticle was developed that allowed nuclear targeting of pDNA with increased efficiency in reporter assay, compared to traditional electroporation method. This method has for the first time, facilitated transfection using both circular and linear DNA in P. falciparum thereby serving as an alternative to electroporation with an increase in transfection efficiency. Availability of a robust method for functional genomic studies in these organisms may be a catalyst for discovery of novel targets for developing drugs and vaccines.
Similar content being viewed by others
Introduction
Functional genomic approaches are critical to understand the biological role(s) of particular gene sequence and determinants of parasite virulence and pathogenicity. Although the availability of P. falciparum genome sequence has made real strides in our understanding of the biology of the organism, genetic manipulation of malaria parasites still poses problems due to the lack of robust methods for genetic manipulation and analyses in P. falciparum. Such methods are especially critical to understand the function of nearly the 50% hypothetical genes in Plasmodium.
Currently, electroporation (EP) during the intraerythrocytic stage of the parasites is the only method that is used to introduce DNA into P. falciparum. Efficient transfection of DNA into this stage has been a major road block as the DNA has to traffic through four lipid bilayers: the plasma membrane of the erythrocyte, parasite's parasitophorous vacuole membrane, parasite's plasma membrane and finally the parasite's nuclear membrane. The first successful transfection in P. falciparum was reported in 19951, where upstream and downstream regulatory elements were the key to drive reporter gene expression. Later, other modifications were developed to improve transfection techniques and achieve stable transfections2,3,4. A PubMed search for P. falciparum transfection resulted in 270 published references, of which only a handful discuss improved transfection efficiency methods for testing gene sequences in the malaria parasite. Introduction of exogenous plasmid DNA (pDNA) or reverse genetic manipulation thus are feasible in P. falciparum, although the efficiency of transfection has remained extremely low5 (~1 × 10−6). Further improvements are needed considering the size of the parasite genome (23.3 Mb in size, with 5300 genes) and nearly 50% unannotated genes.
To improve the transfection efficiency and to ease the process of transferring DNA into the malaria parasites without generating any cytotoxicity, we investigated the use of lipid based nanoparticles (nanosomes) using the plasmid pHLH-1 containing firefly luciferase reporter gene. To date, lipid based nanoparticles have been widely used for the delivery of foreign materials including drugs, proteins, genes (i.e. pDNA, siRNA, miRNA etc.) in vitro6,7 and in vivo8,9. Recently, we have designed and developed a novel lipid based nanoparticle system for the delivery of pDNA and siRNA in liver and cancer cell10,11,12. These nanosomes were prepared using a mixture of cholesterol and 1,2 dioleoyl-3-trymethylammonium-propane (DOTAP). Though those formulations could significantly deliver genes to either virus infected hepatocytes or cancer cells, they were not effective to deliver pDNA to the parasite-infected RBC (unpublished). We therefore conducted a series of systematic optimization experiments to develop nanosome formulations that efficiently delivered pDNA to parasite-infected RBC in both transient and stable transfection modes.
Results
K4 formulation has highest reporter activity with transient transfection
Table 1 describes the compositions of various formulations (K1, K2, K3 and K4) developed for this study. These formulations (K1–K4) were combined with 25 μg of pHLH-1 plasmid and evaluated in transient transfection using P. falciparum infected RBC using method D (described below). All the experiments were performed in triplicates and reporter plasmid was tested by electroporation in parallel for comparison1. Formulations K1 and K2 did not perform any better than EP, while the highest reporter activity was observed with K4 (Fig. 1b). We also optimized various protocols of transfection conditions with our best performing formulation, K4. As seen in Fig. 1a, method D (i.e., K4 formulation added to the parasite pellet resuspended in 0.5 ml of incomplete serum-free RPMI media, incubated for 30 min at 37°C and then diluted to 5 ml culture medium) revealed the highest reporter activity.
Luciferase activity in P. falciparum- infected erythrocytes transfected by electroporation (EP) and nanosome delivery.
(a) Optimization of K4-DNA mediated P. falciparum transfection. In [A]: K4-DNA was added to parasite pellet and resuspended in culture media. In [B]: K4-DNA was incubated with parasite pellet for 30 min at 37°C and then diluted with culture media. In [C]: K4-DNA was added to parasite pellet, resuspended in 0.5 ml incomplete medium and immediately diluted with culture media. In [D]: K4-DNA was mixed with parasite pellet resuspended in 0.5 ml incomplete media, incubated for 30 min at 37°C and then diluted with culture media. In [E]: K4-DNA was mixed with parasite pellet resuspended in 1 ml incomplete media and diluted with culture media and in [F]: K4-DNA was mixed with parasite pellet resuspended in 1 ml incomplete media, incubated for 30 min at 37°C and diluted with culture media. (b) Luciferase activity was measured 48 h after transfection for EP and four different nanosome formulations K1-K4 using plasmid pHLH-1. Formulations (K1-K4) were added to parasite pellet incubated as described in method [D] above. (c) Luciferase activity was measured 48 h after transfection for EP (50 μg) and K4 using 2.5 μg, 10 μg, 25 μg and 50 μg plasmid pHLH-1, respectively. (d) Luciferase activity was measured 48 h after transfection for EP and K4, (100% to 25% proportional reduction of K4 formulation) with plasmid pHLH-1 using method D. (e) Luciferase activity was measured after transfection by EP and K4 formulation method using 25 μg circular and linear pHLH-1. Aliquots of parasites were withdrawn every 48 hrs for luciferase activity detection and continued for up to 192 h (Day 8). (f) Luciferase activity was measured 48 h after transfection by EP and K4 formulation method using 25 μg of pHLH-1 and pLH plasmid using method D. All experiments (a–f) were performed in triplicates and vertical bars indicate standard deviation. P values were calculated using unpaired t test and P < 0.05 indicates that there was a statistically significant difference between the formulation and electroporation luciferase activity.
Next, we optimized the ratio of plasmid DNA and K4 formulation. As seen in Fig. 1c, increasing pDNA concentration from 2.5 to 50 μg showed a dose-dependent increase in luciferase expression. Conversely, we also varied the total amount of K4 formulation, keeping pDNA constant (25 μg). As seen in Fig. 1d, reducing the total formulation amount by 25% caused ~34% overall reduction of luciferase activity and any further decrease completely abolished luciferase activity indicating that the overall ratio of K4 to DNA is critical for effective delivery and that 75% of K4 formulation is the minimum effective amount required to deliver DNA across the various layers in Plasmodium infected RBCs.
Linear DNA can be transfected with K4 formulation
Previous studies in P. falciparum have shown that linear DNA is not the preferred substrate for transfection by standard EP method. While reasons are not known, it has been suggested that linear DNA probably undergoes degradation as it traverses across the four membranes to reach parasite nucleus2. We were curious to reevaluate linearized DNA using K4 formulation. Linearized and circular plasmids were transfected through EP and K4 formulation methods. As seen in Fig. 1e, parasites that were transfected with linear DNA through EP method had no reporter expression from day 1 to day 8. In contrast, we observed, for the first time, reporter expression from parasites transfected with linear DNA through nanosome formulation K4. We do not know why EP method does not facilitate transfection with linear pDNA. In any case the transfection method presented here can be adopted with PCR amplified reporter gene without the need for cloning into a plasmid vector.
We also tested luciferase reporter activity using the pLH plasmid that contains the full length firefly luciferase gene, but lacks the hrp3 promoter. As seen in Fig 1f, there was no reporter activity with pLH plasmid 48 hours after transfection using either K4 or EP methods. These results also established that the reporter activity seen with pHLH-1 was due to parasite specific promoter driven gene expression upon transfection and not due to any nonspecific Luc signal from spurious expression or any artifact from the methods used.
Biophysical characterization of various formulations favors K4 formulation for gene delivery
We next undertook biophysical characterization (particle size, zeta potential and DNA encapsulation efficiency) of formulated pDNA with nanosomes to understand the basis for gene delivery differences for various formulations tested. Table 2 shows the particle size distribution of various formulations. K4-cir (25 μg pDNA), generated the smallest particles (262 ± 57; 70th percentile), suggesting that success of an efficient P. falciparum transfection using formulation may require particles to be of optimal size range to pass through various membrane layers and reach the parasite nucleus. K4 also showed a moderate range of positive zeta potential (26 ± 4), which was due to the presence of cationic lipid and protamine sulfate; however no adverse side effect or cell toxicity were noticed during the transfection with K4 formulation. A comparison of the efficiency of encapsulation of all four formulations (i.e., K1–K4) prepared with the same amount of pDNA (i.e., 25 μg) showed that the efficiency of encapsulation of K1 was significantly lower (p < 0.05) than the other three formulations (i.e., K2–K4); 26% vs. >95%). The K4-pDNA encapsulation efficiency was the maximum (98%), which indicates the higher availability of the pDNA in this formulation.
GFP expression visualized using K4 formulation
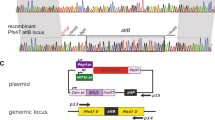
Finally, we evaluated the application of nanosome mediated DNA delivery to P. falciparum for effecting stable transfection, which involves homologous recombination events involving exogenous pDNA and the genome locus. For these studies we employed a targeting plasmid construct, p16FAGFP13, designed to disrupt the Pfs16 gene locus via homologous recombination in P. falciparum. Fig. 2a (upper panel) shows the genomic organization of the disrupted Pfs16 locus13 post the recombination event between the targeting plasmid p16FAGFP and Pfs16 genomic locus. We examined K4 and EP transfected parasite cultures on different days by Giemsa staining for parasitemia and by fluorescence microscopy for GFP positive parasites. Fig. 2b shows GFP expression in trophozoite stage infected RBCs obtained upon transfection and drug selection (panels A and B, 43 days post K4 transfection and panels C and D, 51 days post EP). Although stably transfected parasites in both the cases showed comparable growth kinetics, the percentage of GFP positive parasite in the K4 group was 75% (range 64 to 86%) as compared to 60% (range 59 to 61%) in the EP group, suggesting not only faster appearance of stably transfected parasites but also at higher frequency.
GFP expression and PCR analysis from P. falciparum- infected erythrocytes by electroporation (EP) and K4 formulation methods.
(a) Schematic representation of genomic organization of a disrupted Pfs16 locus. Arrows indicate the location and orientation of various primers used in PCR analyses. Table below indicates the expected size of the various PCR products. (b) K4 (panels A and B) and EP (panels C and D) and parasites transfected with p16FAGFP showing expression of GFP when viewed under fluorescence microscope using 100× magnifications. Bright field (BF) image of the same is shown on the left for each image. The percentage of GFP positive parasites in the K4 group was 75% (range 64 to 86%) as compared to 60% (range 59 to 61%) in the EP group (c) PCR genotype analysis using primer pair A, B and C for wild type and transfected parasites. Numbers on left indicate molecular weight standards (size in base pairs).
We used diagnostic PCR to confirm the integration of targeting plasmid in the genomic locus of the parasites transfected by EP and K4 nanosome formulation. DNA was extracted from transfected parasites (day 50 for K4 and day 58 for EP) and evaluated by PCR analyses using primers specific for various regions of disrupted Pfs16 locus. Fig. 2a arrowheads show the positions of these primers and the lower panel in Fig. 2a shows the expected size of the PCR products using these primer pairs. As seen in Fig. 2c, primer pair A amplified a 600 bp product resulting from a recombinational crossover at the genomic locus in parasites transfected using EP or K4 formulation, whereas, as expected, it was absent in wild type genomic DNA(lanes A). Another diagnostic PCR, using primer pair B amplified a 342 bp fragment in transfected parasites and not in wild type, further confirming integration of transfected plasmid at the Pfs16 locus (lanes B). Finally, primer pair C amplified a 273 bp fragment in wild type and transfected parasites, detecting the presence of the target sequence.
Discussion
Understanding malaria parasite's biology, including gene function has been severely hindered by the lack of means to identify essential genes in various life cycle stages of P. falciparum, a lethal form of malaria parasite. Progress has been limited due to poor transfection technologies and efficiency5. In addition to the requirement for exogenous DNA to cross 4 membrane lipid bilayers, is the added complication that parasites maintain circular plasmids used for transfections as stable episomes14. Direct electroporation of erythrocytic ring stage parasites1 or purification of matured parasite blood stages that are allowed to invade plasmid loaded red blood cells2 are routinely used for transfecting P. falciparum. As only blood stages of the parasites have been shown to be transformed successfully, the limitations mentioned above leads to inability to identify genes essential for blood stage development15.
There have been significant advances in our ability to genetically manipulate Plasmodium since the introduction of transfection technology. However, due to low efficiency of transfections, it has not been possible to evaluate functions of nearly 50% of total genes and improved and easier transfection protocol are needed if large scale genetic manipulation of parasite is to be accomplished. In this study, nanosomes that are cationic lipid (DOTAP)-based nanometer sized lipid nanoparticles were formulated. A novel nanoparticle formulation was prepared in a simple two-steps process using cholesterol, DOTAP, protamine sulfate (PS) and high mobility group protein (HMG-1). Although, cholesterol, DOTAP and protamine sulfate have been used either previously16, individually or in combination10,17 for transfecting various cells, this is the first time we are reporting a unique composition and method for transfecting P. falciparum infected red blood cells. The lipid nanoparticles allowed the DNA to efficiently cross the lipid bilayers and enabled nuclear targeting of both linear as well as circular plasmid DNA molecules.
The success of an efficient P. falciparum transfection requires the nanosome complex particle size to be small enough to cross several membrane bilayers. Natural cationic peptides such as protamine sulfate (PS) have been shown to increase gene transfection efficiency of both liposomal and polymeric nanoparticles16,18. In addition, it is also known that sialylated glycoproteins of the RBC membrane contribute to its negatively charged surface, creating a repulsive electric zeta potential19,20. There is hence a likelihood that positively charged nanosomes entrapping pDNA increase the chances of pDNA uptake through the RBC membrane. The positive charges to the particles in our studies come from cationic lipids (DOTAP) and cationic peptides (PS). To test if the peptide- lipid proportion improves the transfection efficiency, we also determined the optimum ratio of lipids and peptides to pDNA by varying the K4 formulation amount to a constant pDNA concentration. We determined that 75% of K4 formulation was critical for efficient gene delivery.
As compared to P. falciparum, higher transfection efficiency has been observed in other spp. of Plasmodium (rodent species such as P. berghei and P. yoelii); probably due to the fact the ability to transfect free form, bypasses the need for the pDNA to cross extra lipid bilayers. Additionally, linear DNA is preferred substrate for transfection in P. berghei, thereby reducing the time required for selection of integrants through homologous recombination (as circular plasmids easily replicate episomally lowering chances of genome integration). Also, another edge is that P. berghei transfection depends on double crossover recombination for gene targeting. In P. falciparum, circular plasmids preferentially integrate via single crossover unless employing double crossover vectors21. Using the nanosome based protocol for transfection allowed for linear DNA to be transfected into P. falciparum for the very first time. Although, the overall efficiency of the reporter gene expression was higher with circular than linear DNA, there was a significantly higher reporter gene expression with linear DNA through nanosome formulation when compared to electroporation, indicating that PCR generated sequences can be used directly for transfection, reducing the time required for cloning into a plasmid vector.
In summary, these results obtained from transient and stable transfection studies demonstrate that nanosome formulation method can be successfully used as an easier alternative to EP method for transfection and genetic manipulation in P. falciparum. As parasites are not exposed to electric field in this method, it was observed to cause only minimal and unavoidable cell lysis and thereby improved viability of parasites post transfection. The K4 nanosome formulation not only resulted in higher levels of reporter gene expression, but also faster selection of stably transfected parasites. Equally important is the observation on the expression of reporter gene with parasites transfected with linear DNA transfected using K4 nanosomes. Linear target sequences generated by PCR can be employed in large scale for high throughput studies to investigate parasite stage-specific gene expression and their role in parasite development, virulence and host-parasite adaptation.
Methods
Plasmid constructs and DNA preparation
The plasmid pHLH-1 containing a luciferase reporter gene driven by P. falciparum ring stage specific histidine- rich protein 3 (hrp3) promoter1 was prepared using plasmid maxi prep kit from Qiagen. Plasmid pLH is similar to pHLH-1 except that it lacked the hrp3 promoter. 25 μg of pHLH-1 plasmid was used in most experiments, unless specified otherwise. Circular pHLH-1 was linearized by sequentially digesting with BamH1 and Sma1 restriction enzymes2 and verified by gel electrophoresis and complete lack of bacterial transformation activity. Pfs16 targeting plasmid has been described earlier13 and was used in transfection with formulation and electroporation methods.
Chemicals
DOTAP and cholesterol were purchased from Avanti Polar-lipids Inc. (Birmingham, AL, USA). Human HMG-1 (high mobility group protein-1), protamine sulfate (PS) salt Grade X (PS), trehalose dihydrate and HPLC grade chloroform were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The Pico green assay kit used to measure DNA encapsulation was supplied by Molecular Probes (Eugene, OR, USA). All other reagents were of analytical grade and were supplied by either Sigma Chemical Co. (St. Louis, MO, USA) or VWR Int. LLC (Suwanee, GA, USA).
Preparation of liposomes
The liposomes were prepared by using solvent evaporation method10. Briefly, cholesterol and DOTAP, at the molar ratio of 1:1.1 were mixed in a high pressure homogenizer, (EmulsiFlex-B3). The lipids (10.47 mg DOTAP and 5.81 mg cholesterol) were dissolved in 10 ml HPLC-grade chloroform in a round bottom flask and dried overnight under nitrogen gas. The resulting films of the lipids were hydrated in 10 ml of de-ionized water to give a final concentration of 1.5 mM of each lipid. The lipid dispersions were warmed and mixed by rotation at 50°C for 45 minutes, followed by 35°C for 10 minutes and 3 hours at room temperature. Then 2 ml of the lipid dispersion was warmed at 50°C for 10 minutes and homogenized at 20,000 psi for 5 cycles. The resultant liposomes were collected in a new glass vial and stored at 4°C.
Preparation of nanosomes
Four different nanosome formulations were prepared using the above liposomes, protamine sulfate (PS), high mobility group protein (HMG-1), pDNA (pHLH-1) and trehalose. Trehalose was used as diluent and added in each of the four formulations. These formulations were prepared to evaluate the effect of each of the other excipients, i.e., protamine sulfate, HMG-1 protein and liposome. The composition of different formulations is listed in Table 1. The formulation K4 was composed of liposome, PS, HMG-1, pDNA and trehalose, whereas, K1 was lacking PS; K2 was lacking HMG-1; and K3 was lacking liposome. The following is a brief summary of the preparation technique. Twenty-five microgram of pDNA (60 μl) was taken in an eppendorf tube followed by addition of 30 μl of HMG-1 (0.2 μg/μl prepared in DNase/RNase free water). The mixture was mixed and incubated at RT for 10 min. Freshly prepared PS solution in de-ionized water (25 μg in 10.90 μl) was added gently to the mixture and the condensation of pDNA with PS and HMG-1 was performed by incubating the mixture at room temperature for 40 minutes. The liposome suspension was sonicated in ice cold water for 1 min. The liposome (DOTAP + cholesterol, 18.88 μg in 11.60 μl) were added to the above pDNA mixture and mixed rapidly by pipetting up and down thirty times. Finally, freshly prepared trehalose (250 μg) solution (37 mg/ml) was added to the pDNA transfection formulation followed by gentle vortexing 5 times to ensure thorough mixing of the formulations with trehalose. Prior to transfection experiments, the formulation was sonicated in ice cold water for 2 minutes followed by mixing by pipetting up and down for another 20 times.
Parasite cultivation
P. falciparum clone 3D7 was grown at 4% hematocrit in RPMI-1640 medium supplemented with 10% O+ Rh+ serum, using the candle jar method22. Sorbitol was used to synchronize parasite cultures and visualized by Giemsa stain23,24.
Parasite transfections by direct electroporation method
P. falciparum transfections were performed as described1. Briefly, 25 μg of plasmid DNA was precipitated in 70% ethanol a day before transfection. Parasite cultures at ~10% ring stage parasitemia were collected and washed once in cytomix (120 mM KCl, 0.15 mM CaCl2, 2 mM EGTA, 5 mM MgCl2, 10 mM K2HPO4/KH2PO4, 25 mM Hepes, pH 7.6). For each transfection, 2 × 109 total erythrocytes from synchronized culture were diluted to a total volume of 800 μl in cytomix, mixed with 25 μg of plasmid DNA and transferred to 0.4 cm gap electroporation cuvette. Parasites were electroporated with Gene Pulser II (Bio-Rad, Hercules, CA, USA) using the low voltage (0.31 kV) and high capacitance setting (960 μF)3. Electroporated cells were immediately mixed in 5 ml of culture medium and maintained under standard conditions. Culture media was changed 4 hours post transfection to remove any lysed cells.
Parasite transfection by formulation method
Parasite cultures at ~10% ring stage parasitemia were pelleted and 200 μl were used for transfections as described in Fig. 1 legend. The formulations were mixed with the parasite and various incubation methods as described in Fig. 1b were tested. Finally, method D, where formulation and parasite pellet were mixed in 0.5 ml incomplete media, incubated for 30 mins at 37°C followed by mixing in 4.5 ml of complete RPMI-1640 medium was carried out as it gave highest reporter expression. Culture media was changed 4 hours post transfection and maintained under standard conditions.
Stable transfections
3D7 parasites (~10% rings) were transfected using standard EP or K4 formulation procedures (above) in triplicates using 50 μg of p16FAGFP plasmid DNA. Transfected parasites were maintained under 5 nM WR99210, media was changed every second day and fresh red blood cells were supplemented weekly. After 43 and 51 days, Giemsa stain detectable parasites reemerged in the K4 formulation and electroporation transfected parasites, respectively and drug resistant parasites continued to grow normally in the presence of drug. Parasites were examined for GFP expression (Olympus BX41) and parasite DNA was used for PCR analysis to demonstrate genomic integration of plasmid DNA.
Firefly luciferase assays
Parasites were collected 48 hrs post transfection for luciferase assays. The parasites were released from red blood cells by 0.1% saponin lysis and washed twice in cold PBS. The released parasites were then lysed with 50 μl of lysis buffer (Promega- E397A) and frozen at −20°C. Luciferase assay reagent was prepared as per the manufacturer (Promega E1483). 50 μl of cell lysate was mixed with 100 μl of Luciferase Assay Reagent and mixed by vortexing briefly and quantified used a single tube luminometer (TD-20/20 Luminometer- Turner Designs). Firefly luciferase activity was measured as arbitrary light units with a read time of 10 s and delay of 2 s. Experiments were performed in triplicates and the average luciferase activity was normalized per 1000 million parasites for each assay.
Measurement of particle size and zeta potential
Delsa Nano C Particle Analyzer (Beckman Coulter Inc., Fullerton, CA, USA) was used to measure the particle size of different formulations at room temperature by employing dynamic laser light scattering method. The average particle size was reported as the mean ± standard deviation (n = 4). It was also used to measure the zeta potential of different formulations. The system was initially calibrated with standards and experimental samples were diluted in 1 mM KCl prior to the measurement of zeta potential.
Measurement of pDNA encapsulation efficiency in different formulations
The pDNA encapsulation efficiency was measured by following the procedure reported earlier10 with minor modification. The free and encapsulated pDNA in different formulations was measured by Pico green assay following the manufacturer's protocol. Briefly, pDNA nanosome formulations were centrifuged at 10,000 rpm (Allegra Centrifuge, Beckman Coulter Inc., Fullerton, CA) for 15 minutes at 4°C to separate the encapsulated pDNA particles present in the pellets from the free pDNA in the supernatant. 1% sodium dodecyl sulfate (SDS) (500 μl) was added to the pellets and the samples were then incubated at 37°C for 18 hours with gentle agitation (50 rpm). Blank nanosomes were also prepared without pDNA and their fluorescence reading was subtracted from pDNA formulations. The pDNA encapsulation efficiency was calculated by comparing pDNA amount present in the pellets to the total amount of pDNA that had been added during the formulation preparation. The results are reported as the mean ± standard deviation (n = 4).
Statistical analysis
The results were expressed as mean ± standard deviation. Statistical significance of luciferase activity among different formulation and electroporation was done using unpaired t test. Statistical significance of particle size and pDNA encapsulation efficiency among different formulations was compared by the Student-Newman-Keul's nonparametric test, using the Graph Pad Prism 5 software. p value of < 0.05 was considered significant.
References
Wu, Y., Sifri, C. D., Lei, H. H., Su, X. Z. & Wellems, T. E. Transfection of Plasmodium falciparum within human red blood cells. Proc Natl Acad Sci U S A 92, 973–977 (1995).
Deitsch, K., Driskill, C. & Wellems, T. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res 29, 850–853 (2001).
Fidock, D. A. & Wellems, T. E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A 94, 10931–10936 (1997).
Mamoun, C. B. et al. Transfer of genes into Plasmodium falciparum by polyamidoamine dendrimers. Mol Biochem Parasitol 103, 117–121 (1999).
O'Donnell, R. A. et al. A genetic screen for improved plasmid segregation reveals a role for Rep20 in the interaction of Plasmodium falciparum chromosomes. EMBO J 21, 1231–1239 (2002).
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A 84, 7413–7417 (1987).
Simoes, S. et al. Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides. Gene Ther 6, 1798–1807 (1999).
Sorensen, D. R., Leirdal, M. & Sioud, M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol 327, 761–766 (2003).
Liu, Y. et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 15, 167–173 (1997).
Kundu, A. K. et al. Development of nanosomes using high-pressure homogenization for gene therapy. J Pharm Pharmacol 62, 1103–1111 [pii] 10.1111/j.2042-7158.2010.01140.x (2010).
Chandra, P. K. et al. Inhibition of Hepatitis C Virus Replication by Intracellular Delivery of Multiple siRNAs by Nanosomes. Mol Ther 20, 1724–1736 (2012).
Kundu, A. K. et al. Development and optimization of nanosomal formulations for siRNA delivery to the liver. Eur J Pharm Biopharm 80, 257–267 (2012).
Kongkasuriyachai, D., Fujioka, H. & Kumar, N. Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (Pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption. Mol Biochem Parasitol 133, 275–285 (2004).
O'Donnell, R. A. et al. An alteration in concatameric structure is associated with efficient segregation of plasmids in transfected Plasmodium falciparum parasites. Nucleic Acids Res 29, 716–724 (2001).
Balu, B. & Adams, J. H. Advancements in transfection technologies for Plasmodium. Int J Parasitol 37, 1–10 (2007).
Dunne, M., Bibby, D. C., Jones, J. C. & Cudmore, S. Encapsulation of protamine sulphate compacted DNA in polylactide and polylactide-co-glycolide microparticles. J Control Release 92, 209–219 (2003).
Templeton, N. S. et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 15, 647–652 (1997).
Li, S., Rizzo, M. A., Bhattacharya, S. & Huang, L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther 5, 930–937 (1998).
Pollack, W. & Reckel, R. P. A reappraisal of the forces involved in hemagglutination. Int Arch Allergy Appl Immunol 54, 29–42 (1977).
Eylar, E. H., Madoff, M. A., Brody, O. V. & Oncley, J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem 237, 1992–2000 (1962).
Duraisingh, M. T., Triglia, T. & Cowman, A. F. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int J Parasitol 32, 81–89 (2002).
Jensen, J. B. & Trager, W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol 63, 883–886 (1977).
Trager, W. & Jensen, J. B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976).
Walliker, D. & Beale, G. Synchronization and cloning of malaria parasites. Methods Mol Biol 21, 57–66 (1993).
Acknowledgements
This work was funded in part by the NIH Grants R01 AI 04089-08S1, NIH R56 grant AI68052, NIH Grants 1G12RR026260-01, Louisiana Board of Regents RC/EEP (2007-11), LEQSF(2007-12)-ENH-PKSFI-PRS-02 and Military Infectious Disease Research Program Grant # W81XWH-07-1-0136. Authors also thank David Jacobs (Jacobus Pharmaceutical Co. Inc.) for the kind gift of WR99210.
Author information
Authors and Affiliations
Contributions
A.G., A.K., T.M. and N.K. designed the study. A.G. and A.K. performed experiments. A.G., A.K., T.M. and N.K. wrote the manuscript. T.M. and N.K. supervised the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Gopalakrishnan, A., Kundu, A., Mandal, T. et al. Novel Nanosomes for Gene Delivery to Plasmodium falciparum-infected Red Blood Cells. Sci Rep 3, 1534 (2013). https://doi.org/10.1038/srep01534
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01534
This article is cited by
-
Lyse-Reseal Erythrocytes for Transfection of Plasmodium falciparum
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.