Abstract
Thrombospondin-1 is a matricellular protein with potent antitumour activities, the levels of which determine the fate of many different tumours, including renal carcinomas. However, the factors that regulate this protein remain unclear. In renal carcinomas, hypoxic conditions enhance the expression of angiogenic factors that help adapt tumour cells to their hostile environment. Therefore, we hypothesized that anti-angiogenic factors should correspondingly be dampened. Indeed, we found that hypoxia decreased the thrombospondin-1 protein in several clear cell renal carcinoma cell lines (ccRCC), although no transcriptional regulation was observed. Furthermore, we proved that hypoxia stimulates multiple signals that independently contribute to diminish thrombospondin-1 in ccRCC, which include a decrease in the activity of oxygen-dependent prolylhydroxylases (PHDs) and activation of the PI3K/Akt signalling pathway. In addition, thrombospondin-1 regulation in hypoxia proved to be important for ccRCC cell migration and invasion.
Similar content being viewed by others
Introduction
Clear cell renal cell carcinomas (ccRCC) are highly vascularized tumours, in part due to the increase in the levels of angiogenic factors associated with these tumours, such as the vascular endothelial growth factor (VEGF)1. The angiogenic factors associated with these carcinomas are mostly regulated by the hypoxia-inducible transcription factors, HIFs (reviewed in2). These factors are stabilized in ccRCC in which the von Hippel-Lindau (vhl) tumour suppressor gene is lost or mutated, or when oxygen availability is decreased. Diminished oxygen availability is a common feature of malignant tumours and in fact, it is regarded as trigger for enhanced proliferation and metastasis. The hypoxia generated during tumour growth involves a cell adaptive response aimed at restoring the oxygen supply and maintaining cell viability2. Most responses to hypoxia are mediated through the specific gene expression programs regulated by HIFs, heterodimeric transcription factors composed of a constitutively expressed beta subunit (HIFβ) and an oxygen-regulated alpha subunit (HIFα). In normoxic conditions HIFα subunits are extremely unstable and their integrity is dependent on hydroxylation by oxygen-dependent prolyl hydroxylases (PHD1, PHD2 and PHD3)3,4. These enzymes require molecular oxygen as co-substrate for their catalytic activity and they are therefore believed to act as oxygen sensors that directly control HIF stability/activity in response to variations in oxygenation. Under normoxic conditions, HIFα proteins are hydroxylated on specific residues and then polyubiquitinated by the VHL-E3 ubiquitin ligase complex, provoking their degradation by the proteasome5,6. However, while HIFs control most hypoxic responses, other HIF-independent responses to hypoxia have been described7,8.
Hypoxia alters the balance between pro- and anti-angiogenic factors, favoring the stimulation of angiogenesis9. Therefore, enhanced angiogenesis in response to hypoxia is part of an adaptive response aimed at achieving increased oxygen and nutrient delivery to growing tissues. Although the regulation of genes that inhibit angiogenesis is also important in order to maintain tumour homeostasis, little attention has been paid to the effects of hypoxia in the regulation of anti-angiogenic factors. One of the best known inhibitors of angiogenesis is the matricellular protein thrombospondin-1 (TSP-1)10,11. TSP-1 is a multifunctional protein that belongs to a family of five extracellular matrix proteins with similar structures12. TSP-1 was the first endogenous angiogenesis inhibitor identified and its expression is considered critical for the maintenance of an anti-angiogenic microenvironment. Indeed, TSP-1 loss is associated with tumour metastasis and poor outcome (Reviewed by13), although in other tumour types TSP-1 can promote tumour growth and metastasis14,15,16. TSP-1 modulates biological processes such as cell adhesion, motility, proliferation, cell-cell and cell-matrix interactions12,17,18. In addition to its anti-angiogenic effects, other activities fulfilled by TSP-1 are also important in tumour development, including the positive modulation of innate anti-tumour immunity19 and the regulation of tumour blood flow20. TSP-1 expression is frequently lost during malignant transformation due to the inactivation of tumour suppressor genes such as p53 and PTEN21,22, while TSP-1 expression may be repressed by the activation of oncogenes like Myc, Id1, src, c-Jun, ras and HER223,24,25,26,27,28. Additionally, DNA hypermethylation can also diminish TSP-1 levels29.
Positive and negative regulation of TSP-1 by hypoxia has been described in several publications, indicating that hypoxia effects on TSP-1 levels are clearly cell type dependent30,31,32,33,34,35,36,37. Here, we have examined the effects of hypoxia on TSP-1 regulation in ccRCC cell lines, demonstrating that hypoxia stimulates multiple signals that contribute independently to decrease TSP1 levels, affecting ccRCC migration and invasion.
Results
Oxygen availability regulates TSP-1 protein levels in ccRCC cells
Previous studies have indicated that TSP-1 levels are associated with advanced tumour stage in patients with ccRCC, its expression having a significant impact on survival38. On the other hand TSP-1 levels are generally lower in renal carcinoma cells than in normal renal cells39. Since tumour hypoxia alters the balance between pro- and anti-angiogenic factors, favouring the expression of the former, we hypothesized that it might also reduce the expression of angiogenic inhibitors. Thus, we examined the effect of hypoxia on the expression of TSP-1 in several ccRCC cell lines. TSP-1 is a matricellular protein, mostly secreted to the extracellular space, thus we analyzed the soluble TSP-1 secreted into the conditioned media, as well as total lysates of monolayer cultures containing the membrane and matrix bound together with the intracellular protein. We found that TSP-1 protein levels from concentrated conditioned media (100x) were very low and they were not significantly affected when cells were cultured under hypoxic conditions (Fig. 1a). The same results were observed when secreted soluble protein was analyzed by ELISA (see Supplementary Fig. S1 online). However, we found that TSP-1 protein levels were significantly lower in response to hypoxia in the total lysates of all the cell lines analyzed (Fig. 1a). To analyze the amount of intracellular TSP-1 in our total lysate, the cells were recovered with EDTA or trypsin before preparing the lysate, such that extracellular bound TSP-1 did not contribute to the lysate. Surprisingly, negligible amounts of TSP-1 were observed in these intracellular cell lysates (see Supplementary Fig. S2 online), indicating that most of the TSP-1 analyzed in our total lysates corresponded to membrane and matrix bound TSP-1 protein.
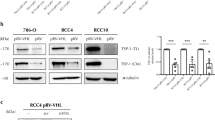
Effect of hypoxia on TSP-1 levels in ccRCC cell lines.
a) 786-O and RCC4 cells were cultured under normoxic (Nx) or hypoxic (Hp) conditions for 24 h and TSP-1 protein levels in total lysates (TL) or conditioned media (CM) were analyzed in Western blots b) TSP-1 and VEGF mRNA levels were determined by quantitative real time PCR in 786-O and RCC4 cell lines cultured for 24 h under normoxic or hypoxic conditions. Gene expression was represented as the fold induction against the levels in normoxic conditions after mRNA levels were normalized to β-actin. The values represent the average from at least three different experiments. c) Analysis of TSP-1 protein in RCC4 cells exposed to normoxia, hypoxia or anoxia (Ax, 0.1% O2) for 24 h (left) and then reoxygenated for the times indicated (right). A representative experiment of n = 4 performed is shown. As a loading control α-tubulin was used in all panels.
Since hypoxia did not significantly change the levels of TSP-1 mRNA (Fig. 1b left), the loss of this protein under these conditions did not appear to occur at the transcriptional level. By contrast, the mRNA levels of VEGF, a known hypoxia-regulated gene, were up-regulated as expected (Fig. 1b right). In addition, TSP-1 protein was regulated by oxygen availability in a concentration-dependent manner, since lowering the oxygen concentration from 1% O2 (hypoxia) to 0.1% O2 (considered as anoxia) produced a greater decrease in protein levels (Fig. 1c left). To confirm the specific effects of hypoxia on the TSP-1 protein and given that most of hypoxic responses are reverted after reoxygenation, the cells were reoxygenated for 12, 24 and 48 h after an initial 24 h period of hypoxia. There was an increase of TSP-1 protein levels 12 h after reoxygenation, which rose close to the normoxic levels (Fig. 1c right), indicating that the hypoxic effects on TSP-1 protein levels are specific and reversible.
Hypoxia decreases TSP-1 protein levels independently of HIF
The HIF family of transcription factors are considered to be the master regulators of hypoxia-regulated genes. Because the above results indicated no transcriptional regulation of TSP-1 in hypoxia, HIF was not expected to directly influence this protein. However, we assessed the possibility that HIF might indirectly down-regulate TSP-1 protein levels during hypoxia by analyzing the effects of specific siRNAs to knock down the two most known HIF isoforms, HIF-2α and HIF-1α in the 786-O and RCC4 cell lines. Silencing these factors did not prevent the decrease in TSP-1 protein associated with hypoxia (Fig. 2). Therefore, from these results we concluded that the loss of TSP-1 in ccRCC mediated by hypoxia occurs independently of HIF.
TSP-1 regulation in RCC cell lines is independent of HIF.
TSP-1 and HIF2α protein levels in 786-O cells transfected with a scrambled siRNA (scr), or siRNAs specific for HIF2α (siHIF2α) were analyzed after 24 h under normoxic or hypoxic conditions (left panel). TSP-1, HIF2α and HIF-1α protein levels in RCC4 cells transfected with scrambled (scr) or siRNA to HIF-1α (siHIF1α) or HIF-2α (siHIF2α) were analyzed after 24 h in normoxic or hypoxic conditions (right), with α-tubulin as a loading control. The results are representative of at least three experiments performed.
Akt signalling contributes to the hypoxia-induced down-regulation of TSP-1 protein
Activation of the PI3K/Akt signalling pathway is responsible for the diminished TSP-1 levels in tumour-associated endothelial cells from RCC40. On the other hand, activation of Akt by hypoxia has been reported by other authors41,42. Hence, we investigated whether Akt signalling regulated TSP-1 in our ccRCC cell lines exposed to hypoxia. Initially, we evaluated the effect of the PI3K inhibitor LY294002 on TSP-1 protein levels in RCC4 cells cultured under normoxic or hypoxic conditions. Hypoxia increased Akt phosphorylation in these cell lines, an effect that was inhibited in the presence of LY294002. In parallel to this Akt activation, TSP-1 protein levels decreased, although they recovered significantly when hypoxia was induced in the presence of LY294002 (Fig. 3a). To further assess the role of this signalling pathway in the regulation of TSP-1 by hypoxia, RCC4 cells were transfected with a plasmid encoding a constitutively active form of Akt (gagAkt). Akt phosphorylation was enhanced in cells transfected with the constitutively active Akt, yet more importantly, this active Akt induced a robust decrease in TSP-1 protein when compared to cells transfected with the empty vector (pCMV6) (Fig. 3c). These effects were only evident at the protein level and TSP-1 mRNA was not affected by the presence of LY294002 (Fig. 3b) or when cells were transfected with gagAkt (Fig. 3d).
Role of Akt signalling in the regulation of TSP-1 protein during hypoxia.
a) TSP-1 protein levels were analyzed in total lysates from RCC4 cells cultured for 24 h under normoxic or hypoxic conditions in the presence of vehicle alone (−) or with 6-12 μM LY294002 (LY). Total Akt and phosphorylated Akt (pAkt) were analyzed in the same samples. TSP1 protein was quantified by densitometry and the change in TSP-1 in LY-treated cells was calculated. b) TSP-1 mRNA expression in RCC4 cells cultured under normoxic or hypoxic conditions in the presence or absence of LY for 24 h was quantified by quantitative RT-PCR. The mRNA levels were normalized to beta-actin and gene expression was represented as the fold induction during hypoxia against the levels during normoxia. c) TSP-1, total Akt and phosphorylated Akt levels were analyzed in total lysates from RCC4 cells transfected with the indicated amounts of a plasmid encoding the empty vector (pCMV6) or a constitutive active form of Akt (gagAkt). TSP-1 protein levels were quantified as in a) and normalized to α-tubulin. d) TSP-1 mRNA expression in RCC4 cells transfected with 1 μg of pCMV6 or gagAkt. Gene expression was represented as the fold change of gagAkt against pCMV6 transfected cells and normalized to β-actin . In all panels a representative experiment or average ± SEM of several performed is shown. n = 4* p≤0.05, Students-T test.
The decrease of TSP-1 provoked by hypoxia is modulated by PHDs
Other components of the oxygen signalling pathway are the prolylhydroxylases (PHDs), considered to be the main oxygen sensors that respond to hypoxia. Since the activity of these enzymes significantly dampened in hypoxia43, RCC4 cells in normoxia were treated with dimethyloxaloylglycine (DMOG), a widely used inhibitor of these enzymes. As during hypoxia, TSP-1 mRNA levels did not change significantly in the presence of DMOG, while Glut-1 mRNA, a well-known target of HIF, showed the expected up-regulation (Fig. 4a left). Interestingly, exposure to DMOG diminished the amount of TSP-1 protein in a concentration-dependent fashion (Fig. 4a right). Furthermore, we could not detect TSP-1 in the conditioned media from cells treated with DMOG (see Supplementary Fig. S3 online). To further demonstrate the role of PHDs in the effects of hypoxia on TSP-1, we knocked down the expression of all three known PHDs (PHD1, PHD2 and PHD3) with specific siRNAs. Silencing of the three PHDs did not affect the amount of TSP-1 mRNA transcripts (Fig. 4b left) but it did diminish the amount of TSP-1 protein (Fig. 4b right). Furthermore, lack of changes on HIF1α or HIF2α levels under these conditions support our conclusion that TSP-1 is regulated during hypoxia independently of HIF. By contrast, overexpression of PHDs in RCC4 VHL positive cells (see Supplementary Fig. S4 online), which express lower levels of PHDs than RCC4, recovered the levels of TSP-1 protein during hypoxia (Fig. 4c). Together, these results indicated that the hypoxia-mediated decrease in PHD activity also contributes to the decrease in TSP-1 protein during hypoxia.
Role of PHDs in the regulation of TSP-1 protein levels during hypoxia.
a) TSP-1 and Glut-1 mRNA levels in RCC4 cells cultured for 24 h in the absence (control) or presence of 1 mM DMOG (left). mRNA levels were normalized to β-actin and gene expression was represented as the fold induction of DMOG treated against the control. TSP-1 and HIF-2α protein levels analyzed in total lysates from RCC4 cells cultured for 24 h in the absence (−) or presence of DMOG (right). b) PHD1, 2, 3 and TSP-1 mRNA levels were quantified by quantitative RT-PCR and normalized to β-actin, representing gene expression in relation to that observed with the scr (left panel). TSP-1, PHD1, PHD2, PHD3, HIF1α, HIF-2α and α-tubulin protein was analyzed in total lysates from RCC4 cells transfected with a non-specific (scr) or siRNA specific to PHD1, PHD2 and PHD3 (right panel). c) TSP-1 protein levels were analyzed and quantified in total lysates from RCC4 VHL+ cells transfected with vectors encoding for PHD1, PHD2 and PHD3 (PHDs), or the empty vector (vector) and cultured under normoxic or hypoxic conditions for 24 h. A representative experiment or average ± SEM of several performed is shown. n = 3 * p≤0.05, Students-T test.
The levels of TSP-1 regulate ccRCC cell migration and invasion
It has been shown that angiogenic factors stimulate ccRCC cell migration, although the role of endogenous angiogenic inhibitors, like TSP-1, in autocrine regulation of ccRCC cells remains unexplored. Taking this into account, we asked whether the decreased TSP-1 protein levels during hypoxia play a functional role in ccRCC cell migration and invasion. Accordingly, we first performed RCC4 cell migration assays under normoxic or hypoxic conditions, observing an increase in cell migration under hypoxic conditions (Fig. 5a). To determine whether the loss of TSP-1 during hypoxia influenced cell migration, purified TSP-1 was added to the RCC4 cells and migration was assessed under normoxic or hypoxic conditions. When the cells were exposed to exogenously added TSP-1 (66 nM), FBS or LPA-stimulated migration was significantly inhibited in both normoxia and hypoxia (Fig. 5b). Furthermore, the RCC4 cells were transfected with a specific siRNA directed against TSP-1 (Fig. 5c left) and migration assays were performed by stimulating with FBS. TSP-1 silencing produced a significant increase in RCC4 cell migration compared to the cells transfected with scrambled RNA (Fig. 5c right). This increased migration was also inhibited by adding exogenous TSP-1 into the upper chamber (Fig. 5d). Moreover, similar results were observed when migration was analyzed in wound healing assays (see Supplementary Fig. S5 online). In addition cells transfected with TSP-1 siRNA were also analyzed for their ability to invade through a type I collagen matrix, demonstrating a significant increase in cell invasion associated with TSP-1 silencing (Fig. 5e). Taken together, these results indicate that TSP-1 produced by ccRCC cells can inhibit migration in response to various chemoattractants. Subsequently, hypoxia-mediated decrease in TSP-1 protein levels stimulates ccRCC cell migration and invasion.
Effect of TSP-1 levels on RCC cell migration and invasion.
a) RCC4 cells were cultured under normoxic or hypoxic conditions for 48 h, after which migration was assessed in transwell assays. Cells (20 × 103 cells/well) were seeded on top of the transwells and allowed to migrate for 16 h under normoxic or hypoxic conditions. As a chemoattractant LPA (10 μM) was added to the lower chamber. b) RCC4 cells were cultured under normoxic or hypoxic conditions for 48 h. Afterwards cell migration stimulated with 5% FBS or 10 μM LPA in the lower chamber was assessed for 16 h under normoxia (Nx) or hypoxia (Hp) in the absence (−) or presence (+) of 66 nM TSP-1. c) Cells were transfected with TSP-1 or scrambled (scr) siRNA and the efficiency of the interference on TSP-1 protein levels was analyzed in Western blotts (left panel). Cell migration stimulated by 5% FBS was analyzed after transfection. The proportion of migrating cells (% over scr) and a representative photomicrograph is shown. d) Cells transfected as in c) were allowed to migrate in the absence or presence of 66 nM TSP-1 using LPA (10 μM) as a chemoattractant. The average number of cells that migrated in three random fields was calculated and cell migration was represented as the percentage of cells that migrated (± SEM). n≥3 in all panels, *p≤0.05, **p≤0.01, Students-T test. e) Cells transfected with TSP-1 siRNA or scramble (scr) were allowed to invade through type I collagen coated inserts. The values in the graph represent the cells that invaded (average number of cells ± SEM). *p≤0.05, Students-T test.
Discussion
RCC is the most common malignant tumour of the adult kidney and despite recent advances in diagnostic imaging, patients are typically diagnosed at an advanced stage. Therefore, studies of the molecular biology of these tumours can only benefit their management. Inactivation of tumour suppressor genes directly influences the angiogenic phenotype of these tumours, altering the relative amounts of inducers and inhibitors they secrete. In addition, the tumour microenvironment exerts selective pressure that favours the regulation of specific genes involved in tumour progression, for example when conditions of hypoxia arise. While such pressure may affect genes involved in angiogenesis, cell proliferation, cell-cell and cell-matrix interaction proteins, it may also involve downregulation of genes that inhibit tumour growth and angiogenesis2. Our studies in ccRCC cell lines indicated that a hypoxic microenvironment diminishes the levels of TSP-1, an inhibitor of angiogenesis, which proved to influence ccRCC behaviour, enhancing the migratory and invasive potential of these cells in in vitro assays.
The importance of TSP-1 in renal cancer has been demonstrated previously38,39,40. In these reports it is shown that TSP-1 is important in maintaining normal kidney angiostasis and its expression inversely correlates with microvascular density, proliferation and RCC progression. However, the effect of the hypoxic microenvironment on these tumours has not previously been assessed, nor whether it may regulate TSP-1 levels. The increase in angiogenesis in response to hypoxia is part of the adaptive response that aims to achieve enhanced oxygen and nutrient delivery to growing tissues. Hence, a hypoxia-mediated decrease in TSP-1 protein in ccRCC cell lines could be part of the hypoxic metastatic programme. The decrease in TSP-1 expression during hypoxia has been shown previously in other immortalized and tumour cells31,36. By contrast, hypoxia augments the TSP-1 in endothelial cells, coronary artery smooth muscle cells and in human dermal fibroblasts30,32,34,35. These results indicate that TSP-1 affects tumourigenesis by inducing different biological effects in distinct cell types. In hypoxic endothelial cells, the induction of TSP-1 might be a negative feedback mechanism to counterbalance the effect of pro-angiogenic factors such as VEGF. Conversely, tumour cells decrease the levels of anti-angiogenic factors in hypoxic conditions in order to favour their own survival and growth.
The mechanisms that regulate TSP-1 in hypoxia may differ in distinct cell types as hypoxia may induce TSP-1 by enhancing mRNA stability34 or through transcriptional regulation mediated by HIF-1α30,32. However, hypoxia decreases TSP-1 through post-transcriptional mechanisms31,36. While there are only a few known examples of genes repressed by HIF44,45, the expression of many genes is repressed during hypoxia independently of HIF. In this respect, recent genome-wide bioinformatics or experimental studies7,8 have demonstrated that HIF-binding is mainly associated with the upregulation of gene expression. Transcriptional repression does not seem to be directly mediated by HIF, which is consistent with our observations that the loss of TSP-1 during hypoxia is HIF-independent. Therefore, in our tumour cells the mechanisms that may account for the decrease in TSP-1 associated with hypoxia may include post-transcriptional, translational or post-translational mechanisms.
It was previously shown that 3′ and 5′-untranslated region (UTR) of the TSP-1 mRNA transcript are involved in post-transcriptional regulation, either affecting mRNA stability or mRNA translation46,47. However we found no significant differences in the activity of a reporter fused to the TSP-1 UTR in compared normoxic and hypoxic conditions (unpublished results). Regulation by miRNAs was also considered since TSP-1 is a target for repression by the miR-17-92 microRNA cluster in human adenocarcinoma48. Indeed, miR-21 may be upregulated in ccRCC tissue and this miRNA can also be induced by hypoxia49,50. Surprisingly, in hypoxic conditions we did not detect induction of this miRNA in our cell lines (unpublished results) and therefore, the decrease of TSP-1 levels during hypoxia is not likely to be mediated by the microRNAs reported to regulate TSP-1 production. TSP-1 mRNA may also be regulated by uncoupling from polysomes, such as in response to high glucose47. However, we found no significant differences when the TSP-1 mRNA in the polysomal and non-polysomal fractions were analyzed (unpublished results).
It was previously shown that Akt signalling can both positively and negatively affect TSP-1 levels in endothelial cells from different sources40,51,52, yet our results proved that Akt signalling diminished the amount of TSP-1 protein in RCC cells. In a recent report by Kaur S. et al.53 the authors found that TSP-1, through its binding to CD47, potently inhibits VEGFR2 signalling and the downstream activation of Akt. Based on these results, it is possible that TSP-1 might also inhibit Akt phosphorylation in RCC cells and therefore, the decreased TSP1 expression under hypoxia could provoke, rather than only represent a consequence of the increased levels of phosphorylated Akt. However, the knockdown of TSP-1 in our RCC cells under normoxic conditions did not affect Akt phosphorylation . Another possibility is that PHDs may be responsible for the changes in Akt phosphorylation and the decrease in TSP-1. In this respect, it was previously shown that inhibiting PHDs with deferroxamine augments Akt phosphorylation42 and on the other hand the activity of PHDs is also regulated by hypoxia (reviewed in2). The silencing of PHDs with specific siRNA, or their inhibition, produces a consistent decrease in TSP1 protein in ccRCC. By contrast, over-expression of PHDs in hypoxia partially recovered the decrease in TSP-1. However and despite the fact that in other cell lines we observed diminished Akt phosphorylation when PHDs were over-expressed during hypoxia (unpublished results), PHDs did not appear to affect Akt phosphorylation levels in ccRCC cell lines. However, our results indicated that PHDs can at least partially counteract the negative effect of phosphorylated Akt on TSP-1 levels in hypoxia. While PHDs have targets other than HIF, the regulatory influence of the hydroxylation of these targets is not completely clear54. These results are very interesting given that TSP1 could be another target of PHDs and on the other hand, PHDs could regulate tumour behaviour independently of HIF by acting as a tumour suppressor. Further studies will be necessary to understand the mechanisms by which PHDs regulate TSP-1 and how the PHD pathway interacts with the Akt signalling to regulate the levels of TSP-1 during hypoxia.
The effects of anti-angiogenic inhibitors may be bypassed by tumour cells, either by increasing the expression of pro-angiogenic factors or by decreasing the expression of those factors that inhibit it. Here, we show that hypoxia can stimulate multiple signals which contribute to the decrease in TSP-1 levels in ccRCC, influencing ccRCC behaviour (Fig. 6). Several reports have demonstrated that TSP-1 induces tumour cell migration, however in our model, we found that TSP-1 inhibits ccRCC cell migration in response to different stimuli. These results reinforce the pleiotropic nature of TSP-1 and depending on the environmental conditions, the presence of its different receptors may have different, even opposite, effects on cell behaviour and biological process. TSP-1 can promote or inhibit angiogenesis and promote or inhibit tumour growth. Our results demonstrate that in addition to its previously reported anti-angiogenic activity on renal carcinomas, TSP1 suppression by hypoxia can elicit autocrine stimulation of ccRCC migration that might be important for the tumour outcome. Further studies aimed at understanding the exact mechanisms regulating TSP-1 in ccRCC will be beneficial to the design of new therapies against these tumours.
Methods
Cell culture
The 786-O and RCC4 cell lines55 were cultured in RPMI 1640 with GLUTAMAX-I (Invitrogen, San Diego, CA) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin and they were maintained at 37°C in the presence of 5% CO2 and 21% O2 (normoxia). For hypoxic or anoxic experiments, cells were incubated at 37°C in an Invivo2 400 hypoxia workstation (Ruskinn Technology, West Yorkshire) in the presence of 5% CO2 and 1% or 0.1% oxygen, respectively, for the times indicated. When hypoxia and reoxygenation cycles were performed, the cells were seeded at 10–20% confluence and then subjected to hypoxia (1% O2) or normoxia for up to 24 h. Subsequently, the cells were maintained in normoxia for 6–48 h.
Antibodies and reagents
The monoclonal Ab-11 anti-TSP-1 antibody, (clones D4.6, AG.1, MBC 200.1) was obtained from Neomarkers Lab Vision (Freemont, CA) and the polyclonal anti-HIF2α antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Heidelberg, GE). The monoclonal anti-tubulin antibody was purchased from Sigma (Madrid, Spain) and the monoclonal anti-HIF-1α antibody was from BD Transduction Laboratories (San Jose, CA). The PHD1, PHD2 and PHD3 polyclonal antibodies were from Bethyl laboratories (Montgomery, TX) while the polyclonal anti-pAkt and anti-Akt were from Cell Signalling (Danvers, MA). The antibodies were used in western blots at the concentration suggested by the manufacturer. Secondary polyclonal (GE Healthcare, Pittsburgh, PA) or monoclonal (Dako, Carpinteria, CA) HRP-conjugated antibodies were used and detected by Immun-StarTM WesternCTM kit (BioRad, Hertfordshire, UK). LY294002 was from Merck and was used at the concentrations indicated. The Oleoyl-L-alpha lysophosphatidic acid (LPA) was from Sigma and the Dimethyloxaloylglycine (DMOG) was from Enzo Life Sciences (Farmingale, NY).
Conditioned media preparation
Soluble secreted TSP-1 was analyzed in concentrated cell conditioned medium. Cells were cultured in the absence of FBS for 24 h under normoxia or hypoxia and afterwards, the conditioned medium (CM) was collected, centrifuged and treated with a protease inhibitor cocktail (Roche). The CM was then concentrated 100x times using Amicon Ultra (Millipore, Billerica, MA, USA) devices with 50 kDa cut-off.
siRNA-mediated gene silencing
siRNA experiments were carried out with specific pools of siRNAs directed against human TSP-1, HIF1α, HIF2α, PHD1, PHD2 or PHD3 (Santa Cruz) or with a non-targeted pool of control siRNAs (scr). Cells were transfected with Lipofectamine 2000 (Invitrogen), according to the manufactureŕs instruction.
Real time PCR analysis
To determine the changes in gene expression induced by hypoxia or the effects of gene silencing, quantitative RNA analysis was performed by Real Time PCR with StepOne Plus (Applied Biosystems, Carlsbad, CA). Cells were grown to 95% confluence in 60 mm culture dishes and the total RNA was isolated from cells using the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston, TX). RNA (1 μg/sample) was reverse-transcribed to cDNA with Improm II RT (Promega, Madison, WI) in a final volume of 20 μl. For Q-RT-PCR 1 μl of cDNA was amplified with the specific primers pairs using the following temperature cycles: 10 min initial denaturation at 95°C; 15 s denaturation at 95°C, 1 min annealing at 60°C and extension over 40 cycles. Melting curves were obtained at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s, labelling samples with power SYBR green (Applied Biosystems). The primer pairs used were: TSP-1 (F: ACT GGG TTG TAC GCC ATC AGG; R: CTA CAG CGA GTC CAG GAT CAC), HPRT (F: ATT GTA ATG ACC AGT CAA CAG GG; R: GCA TTG TTT TGC CAG TGT CAA), VEGF (F: TGCCAAGTGGT CCCAG; R: GTGAGGTCT TGATCCG), PHD1 (F: GCGCTGCATCACCTGTATCTAT; R: CCGC CATGCACCT TAACG, PHD2 (F: CCCTCATGAAGTACAACCAGCAT; R: CATCTGCATCAA AATACCAAACAGT) PHD3 (F:TGCATCACCTGCATCTACT ATCTG; R:TACATGGTGGGATCCTGCG) and GLUT-1 (F: TCAACCGCAACGA GGAGAA; R: CTGTCCCGCGCAGCTT).
DNA cell transfection
RCC4 cells were transfected with a plasmid encoding a constitutive active form of Akt (gagAkt) or with the empty vector (pCMV6: plasmids kindly provided by Dr Ana Clara Carrera, CNB, Madrid, Spain). The cells were also transfected with plasmids encoding PHD1, PHD2 and PHD3 (PCDNA3-PHD1:flag, PHD2:flag and PHD3:flag) or the empty PCDNA3 vector as a control. Briefly, cells were seeded and grown to 50–70% confluence and then transfected using the jetPEI reagent (Genycell Biotech, Spain). For the gagAkt or PCMV6 plasmids, 1 or 2 μg of DNA/p60 was diluted in 48 μl of 150 mM NaCl. Separately, 4 μl of jetPEI reagent was diluted in 46 μl of 150 mM NaCl and mixed with the DNA solution. This mixture was vortexed, incubated for 20 min at room temperature and then added to the cells. The cell media was completed to 2 ml final volume containing 10% of FBS and 1% of penicillin and streptomycin and after transfection the cells were cultured for 36 h before they were used in the experiments. When transfected with PCDNA3-PHD1, PHD2 and PHD3:flag, or the empty vector PCDNA3, 10 μg of each plasmid or vector was used and 48 h post-transfection, the cells were subjected to serum deprivation. The cells were then processed 24 h post-deprivation and protein or mRNA levels were analyzed in western blots (total lysate) or by Q-RT-PCR.
Cell migration and invasion assays
Migration and invasion assays were evaluated in Transwell filters (6.5-mm diameter, 8 μm pore size: from Costar Corning, NY). The ccRCC cells serum starved for 24 h were seeded in the upper transwell (20 × 103 cells/well in 160 μl RPMI without FBS) in the presence or absence of 60 nM TSP-1 (Athens Research & Technology, GA) and they were allowed to migrate for 16 h at 37°C. As chemoattractants, RPMI with 5% FBS or 10 μM LPA were added in the lower chamber, using basal media as a negative control. The cells in the upper surface of the membrane that did not migrate were gently removed with Q-tips, while the cells on the lower surface were fixed, stained with Diff-Quick (International Reagent, Kobe, Japan) and counted under the microscope (20X). An average of three random fields per condition were analysed. For invasion assays, the cells were loaded on top of Transwell filters previously coated with Collagen type I (20 μg/ml) and the experiment was carried out over 16–18 h.
Statistical analysis
Each experiment was repeated at least three times and the results were expressed as the mean ± SEM. Statistical significance was determined with the Students-T test and a p ≤ 0.05 was considered significant.
References
Rioux-Leclercq, N. et al. Plasma level and tissue expression of vascular endothelial growth factor in renal cell carcinoma: a prospective study of 50 cases. Hum Pathol 38, 1489–1495 (2007).
Calzada, M. J. & del Peso, L. Hypoxia-inducible factors and cancer. Clin Transl Oncol 9, 278–289 (2007).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Ivan, M. et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 292, 468–472 (2001).
Mole, D. R. et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 284, 16767–16775 (2009).
Ortiz-Barahona, A., Villar, D., Pescador, N., Amigo, J. & del Peso, L. Genome-wide identification of hypoxia-inducible factor binding sites and target genes by a probabilistic model integrating transcription-profiling data and in silico binding site prediction. Nucleic Acids Res 38, 2332–2345 (2010).
Messmer-Blust, A., An, X. & Li, J. Hypoxia-regulated angiogenic inhibitors. Trends Cardiovasc Med 19, 252–256 (2009).
Good, D. J. et al. A tumour suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A 87, 6624–6628 (1990).
Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumour growth. J Cell Mol Med 6, 1–12 (2002).
Bornstein, P. Thrombospondins as matricellular modulators of cell function. J Clin Invest 107, 929–934 (2001).
Henkin, J. & Volpert, O. V. in Expert Opin Ther Targets, Vol. 15, Edn. 2011 1369–1386 (2011).
Castle, V., Varani, J., Fligiel, S., Prochownik, E. V. & Dixit, V. Antisense-mediated reduction in thrombospondin reverses the malignant phenotype of a human squamous carcinoma. J Clin Invest 87, 1883–1888 (1991).
Tuszynski, G. P., Gasic, T. B., Rothman, V. L., Knudsen, K. A. & Gasic, G. J. Thrombospondin, a potentiator of tumour cell metastasis. Cancer Res 47, 4130–4133 (1987).
Tuszynski, G. P., Rothman, V. L., Deutch, A. H., Hamilton, B. K. & Eyal, J. Biological activities of peptides and peptide analogues derived from common sequences present in thrombospondin, properdin and malarial proteins. J Cell Biol 116, 209–217 (1992).
Adams, J. C. & Lawler, J. The thrombospondins. Int J Biochem Cell Biol. 36, 961–968 (2004
Tucker, R. P. The thrombospondin type 1 repeat superfamily. Int J Biochem Cell Biol 36, 969–974 (2004).
Martin-Manso, G. et al. Thrombospondin 1 promotes tumour macrophage recruitment and enhances tumour cell cytotoxicity of differentiated U937 cells. Cancer Res 68, 7090–7099 (2008).
Isenberg, J. S. et al. Thrombospondin 1 and vasoactive agents indirectly alter tumour blood flow. Neoplasia 10, 886–896 (2008).
Dameron, K. M., Volpert, O. V., Tainsky, M. A. & Bouck, N. The p53 tumour suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb Symp Quant Biol 59, 483–489 (1994).
Wen, S. et al. PTEN controls tumour-induced angiogenesis. Proc Natl Acad Sci U S A 98, 4622–4627 (2001).
Janz, A., Sevignani, C., Kenyon, K., Ngo, C. V. & Thomas-Tikhonenko, A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res 28, 2268–2275 (2000).
Mettouchi, A. et al. SPARC and thrombospondin genes are repressed by the c-jun oncogene in rat embryo fibroblasts. EMBO J 13, 5668–5678 (1994).
Slack, J. L. & Bornstein, P. Transformation by v-src causes transient induction followed by repression of mouse thrombospondin-1. Cell Growth Differ 5, 1373–1380 (1994).
Volpert, O. V. et al. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell. 2, 473–483 (2002).
Wen, X. F. et al. HER2 signalling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 25, 6986–6996 (2006).
Watnick, R. S., Cheng, Y. N., Rangarajan, A., Ince, T. A. & Weinberg, R. A. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumour angiogenesis. Cancer Cell 3, 219–231 (2003).
Li, Q., Ahuja, N., Burger, P. C. & Issa, J. P. Methylation and silencing of the Thrombospondin-1 promoter in human cancer. Oncogene 18, 3284–3289 (1999).
Distler, J. H. et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum 56, 4203–4215 (2007).
Laderoute, K. R. et al. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clin Cancer Res 6, 2941–2950 (2000).
Osada-Oka, M., Ikeda, T., Akiba, S. & Sato, T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem 104, 1918–1926 (2008).
Park, J. E. et al. Hypoxic tumour cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics 9, 1085–1099 (2010).
Phelan, M. W., Forman, L. W., Perrine, S. P. & Faller, D. V. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med 132, 519–529 (1998).
Polotsky, V. Y. et al. Intermittent and Sustained Hypoxia Induce a Similar Gene Expression Profile in the Human Aortic Endothelial Cells. Physiol Genomics (2010).
Tenan, M. et al. Thrombospondin-1 is downregulated by anoxia and suppresses tumourigenicity of human glioblastoma cells. J Exp Med 191, 1789–1798 (2000).
Hu, C. J. et al. Promoter region methylation and reduced expression of thrombospondin-1 after oxygen-glucose deprivation in murine cerebral endothelial cells. J Cereb Blood Flow Metab 26, 1519–1526 (2006).
Zubac, D. P. et al. The expression of thrombospondin-1 and p53 in clear cell renal cell carcinoma: its relationship to angiogenesis, cell proliferation and cancer specific survival. J Urol 182, 2144–2149 (2009).
Veliceasa, D. et al. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J 274, 6365–6377 (2007).
Bussolati, B., Assenzio, B., Deregibus, M. C. & Camussi, G. The proangiogenic phenotype of human tumour-derived endothelial cells depends on thrombospondin-1 downregulation via phosphatidylinositol 3-kinase/Akt pathway. J Mol Med 84, 852–863 (2006).
Kwon, D. S. et al. Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol 85, 1189–1199 (2006).
Alvarez-Tejado, M. et al. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem 276, 22368–22374 (2001).
Semenza, G. L. HIF-1, O(2) and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107, 1–3 (2001).
Chen, K. F., Lai, Y. Y., Sun, H. S. & Tsai, S. J. Transcriptional repression of human cad gene by hypoxia inducible factor-1alpha. Nucleic Acids Res 33, 5190–5198 (2005).
Wen, W. et al. Suppression of cyclin D1 by hypoxia-inducible factor-1 via direct mechanism inhibits the proliferation and 5-fluorouracil-induced apoptosis of A549 cells. Cancer Res 70, 2010–2019 (2010).
McGray, A. J., Gingerich, T., Petrik, J. J. & Lamarre, J. Regulation of thrombospondin-1 expression through AU-rich elements in the 3′UTR of the mRNA. Cell Mol Biol Lett 16, 55–68 (2011).
Bhattacharyya, S., Marinic, T. E., Krukovets, I., Hoppe, G. & Stenina, O. I. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem 283, 5699–5707 (2008).
Dews, M. et al. Augmentation of tumour angiogenesis by a Myc-activated microRNA cluster. Nat Genet 38, 1060–1065 (2006).
Liu, L. Z. et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One 6, e19139 (2011).
Polytarchou, C. et al. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res 71, 4720–4731 (2011).
Niu, Q. et al. Inhibition of Tie-2 signalling induces endothelial cell apoptosis, decreases Akt signalling and induces endothelial cell expression of the endogenous anti-angiogenic molecule, thrombospondin-1. Cancer Biol Ther 3, 402–405 (2004).
Chen, J. et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 11, 1188–1196 (2005).
Kaur, S. et al. Thrombospondin-1 inhibits VEGF receptor-2 signalling by disrupting its association with CD47. J Biol Chem 285, 38923–38932 (2010).
Jokilehto, T. & Jaakkola, P. M. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med 14, 758–770 (2010).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Acknowledgements
This work was supported by the Ministerio de Ciencia e Innovacion (MCINN) SAF2009-11113 and METOXIA, a Collaborative Project under the 7th Research Framework Programme of the European Union FP7-HEALTH-2007B (ref. HEALTH-F2-2009-222741).
Author information
Authors and Affiliations
Contributions
RB-M, AO, MF-C and GM performed the experiments. RB-M, AO, MF-C, MC-E and MJC analyzed and interpreted the data. MJC conceived and designed experiments and wrote the paper. BJ, OS contributed to the interpretation of the data and critical reviewed the manuscript
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bienes-Martínez, R., Ordóñez, A., Feijoo-Cuaresma, M. et al. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIF-independent suppression of thrombospondin-1. Sci Rep 2, 788 (2012). https://doi.org/10.1038/srep00788
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00788
This article is cited by
-
Differential action of pro-angiogenic and anti-angiogenic components of Danhong injection in ischemic vascular disease or tumor models
Chinese Medicine (2022)
-
pVHL-mediated regulation of the anti-angiogenic protein thrombospondin-1 decreases migration of Clear Cell Renal Carcinoma Cell Lines
Scientific Reports (2020)
-
Blocking the autocrine regulatory loop of Gankyrin/STAT3/CCL24/CCR3 impairs the progression and pazopanib resistance of clear cell renal cell carcinoma
Cell Death & Disease (2020)
-
THBS1 facilitates colorectal liver metastasis through enhancing epithelial–mesenchymal transition
Clinical and Translational Oncology (2020)
-
Breast Cancer Dormancy in Bone
Current Osteoporosis Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.