Key Points
-
Discusses the highly variable way in which mouth cancer can present clinically and as such any persistent mucosal abnormality should be viewed with suspicion.
-
Emphasises that the detection of mouth cancer while the tumour is less than 2 cm in diameter is the single most important factor that can improve patient outcome.
-
Provides information on diagnostic aids for mouth cancer and potentially malignant mucosal disorders.
-
Outlines the guidance on the appropriate referral of urgent suspected cancer.
Abstract
Mouth cancer can present as a variety of abnormalities and visible changes affecting the oral mucosa, including ulceration, swelling and areas of erythema. The five-year survival from mouth cancer is poor at approximately 50%. Detection of the cancer while less than 2 cm in diameter with no metastasis greatly improves the outcome for the patient. Although many cancers in the mouth develop from what was previously an apparently normal mucosa, some arise in pre-existing conditions that are therefore regarded as potentially malignant. Regular assessment of the soft tissues within the mouth and the neck for the presence of abnormalities is an essential component of primary dental care. Any persistent and unexplained abnormality requires referral for definitive diagnosis and specialist management.
Introduction
Certain conditions that affect the oral mucosa have characteristic clinical signs and symptoms that allow diagnosis on the basis of clinical presentation alone without the need for special investigations. Examples of these would be the distinctive history and appearance of minor recurrent aphthous stomatitis and geographic tongue. However, squamous cell carcinoma (mouth cancer) contrasts with this markedly in that it can present with a range of mucosal changes and symptoms. On this basis any persistent solitary mucosal abnormality should be regarded as potentially sinister until proven otherwise.
One of the main reasons that that mouth cancer is given such high importance in dentistry and has been recommended as a topic for continuing professional development is the poor five-year survival, which overall is approximately 55%.1 The single most important factor that can improve five-year survival is detection of the tumour whilst small, specifically 2 cm or less in diameter with no regional node involvement or distant metastases (Stage I). Patients with a tumour detected at Stage I are associated with an 85% five-year survival compared to those with Stage IV (greater than 4 cm in diameter with regional node involvement and possible distant metastasis) for whom the five-year survival is only 10%.1,2 The importance of detection while small was also highlighted in a recent retrospective study of survival after aggressive surgery which revealed that a patient with a tumour that is detected with a maximum diameter 2 cm (tumour stage T1) had a mean post-treatment survival of 24.48 years (95% CI 22.45–26.50) while a patient with a tumour greater than 4 cm (tumour stage T4) had a mean survival of only 13.03 years (95% CI 11.56–14.49).3 These figures for survival are longer than those reported in studies and in part it must be appreciated that the research involved only one centre. In reality the five-year survival from advanced cancer is usually much shorter than 13 years.
It is important to appreciate that velocity of cancer growth in the mouth is not uniform. Some tumours may slowly increase in size over many months while others enlarge rapidly over a few weeks. On this basis the concept of 'early' diagnosis is slightly misleading since it is not actually the time that the cancer has been present that is most important but the size and presence of regional metastasis when the tumour is first detected that is key to an improved chance of survival for the patient.
It is useful to recognise that oral squamous cell carcinoma essentially represents epithelial cell replication within the lining of the mouth that is 'out of control' and as such is a surface event which should be visible on clinical examination. This feature contrasts with some other tumours that are not 'visible', such as ovarian cancer or pancreatic cancer. It is not unreasonable to expect a dental care professional to be able to detect a surface mucosal abnormality that is 2 cm in diameter since this size is approximately that of a human finger nail.
Clinical presentation
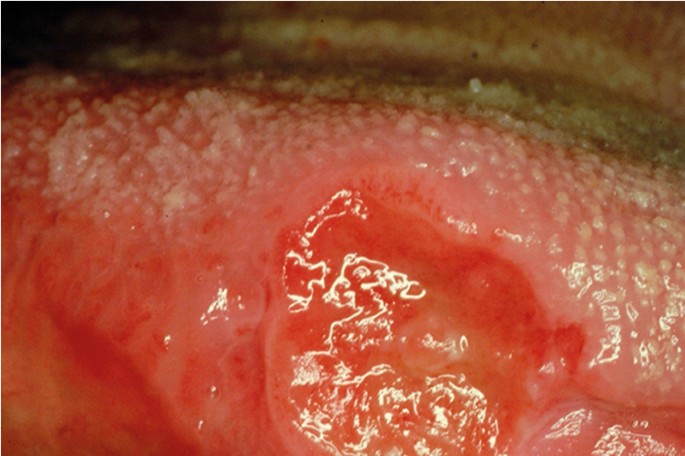
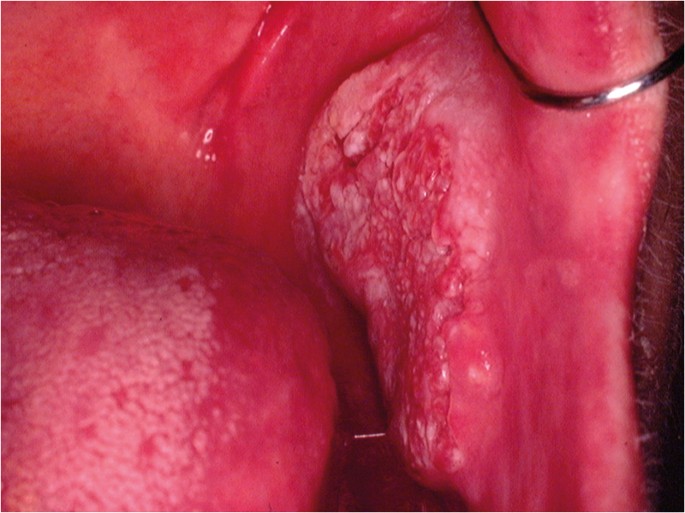
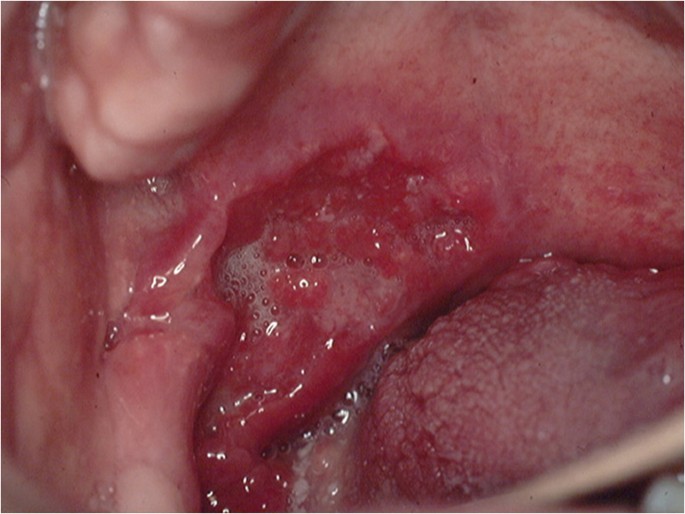
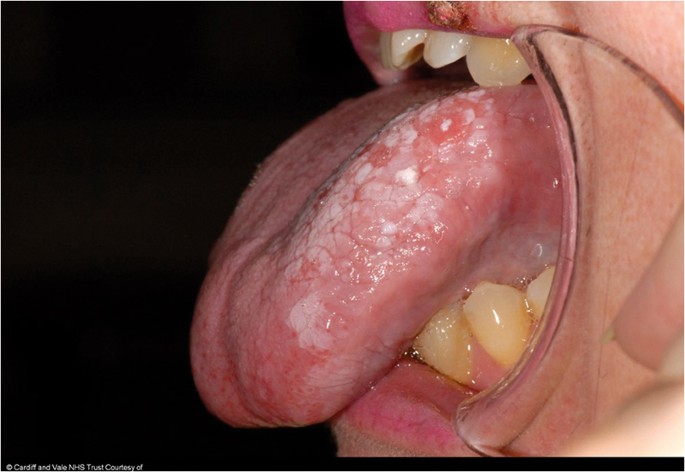
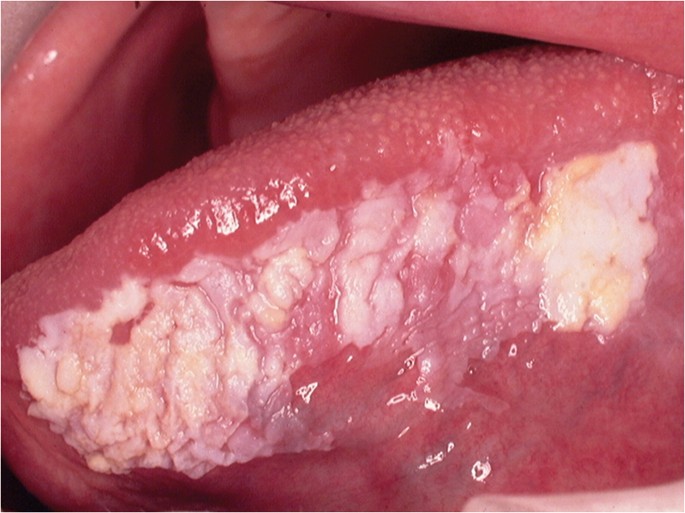
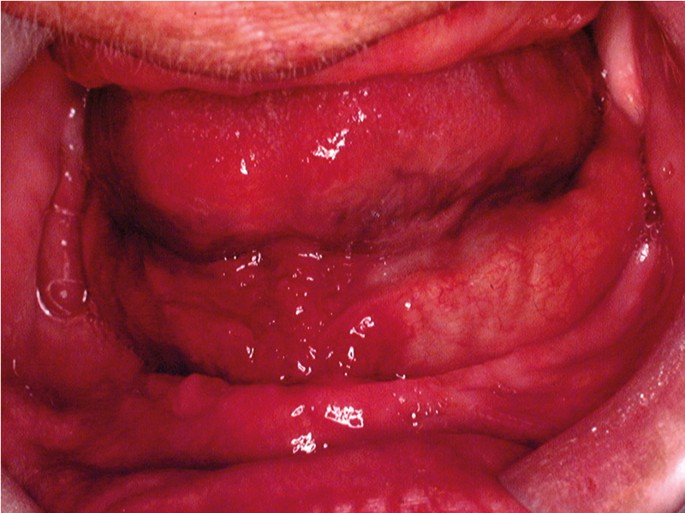
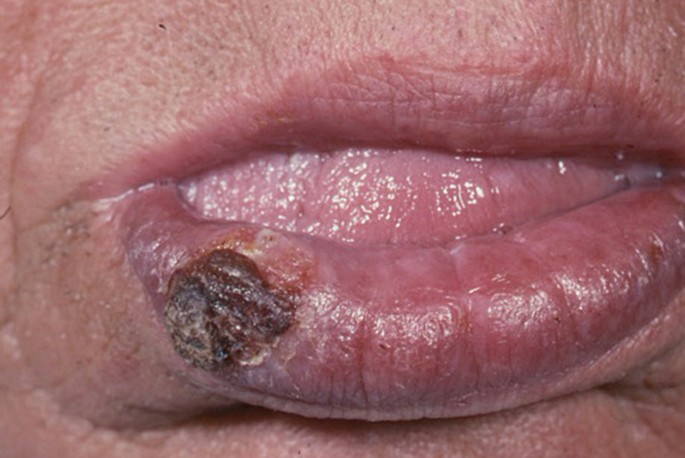
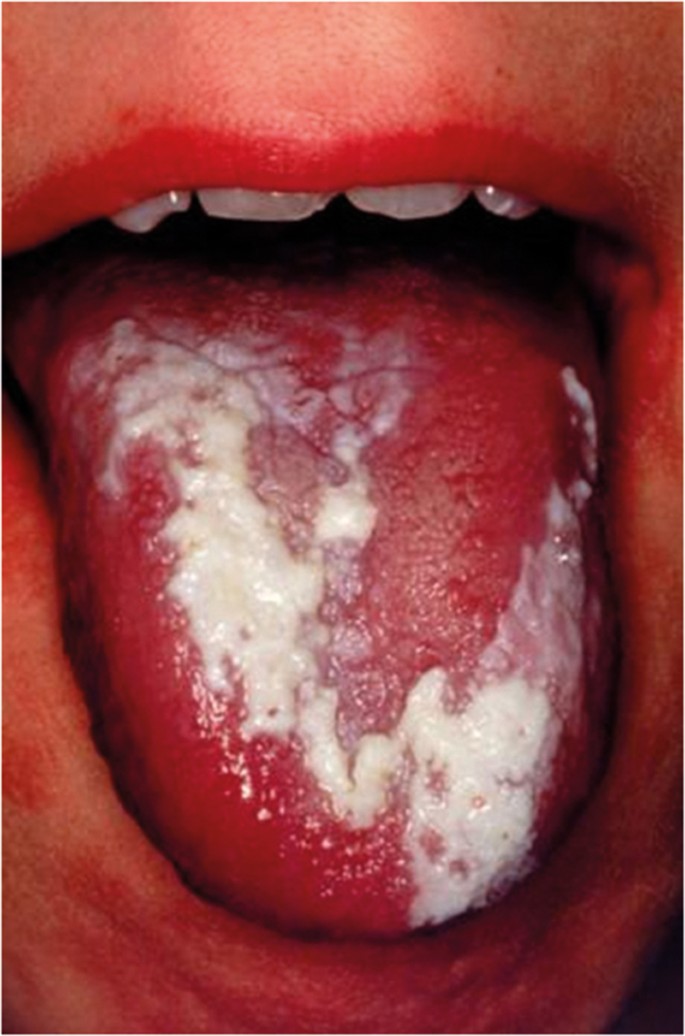
The classical appearance of mouth cancer is a solitary deep ulcer with rolled margins on the lateral margin of the tongue (Fig. 1). Palpation of the margin of the ulcer will reveal firm, often described as indurated, tissues. Unfortunately however, other than induration, mouth cancer has no pathognomonic presenting feature and involves a spectrum of mucosal changes in addition to ulceration including swelling (Fig. 2), erythema (Fig. 3) or a speckled red/white patch (Fig. 4).4
Examination of the patient
A number of educational videos showing methods of clinical examination of the mouth and neck are freely available on the Internet via providers such as YouTube or Google video. However, the three minute 'Oral Cancer Recognition Toolkit' video developed by Cancer Research UK and the British Dental Association is particularly useful for dental professionals.5
Extra-oral examination
The soft tissues of the neck should be palpated on both sides using the tips of the fingers to detect any abnormal tissue swelling or enlargement of the lymph nodes. The patient's neck should be flexed and the examination start in the submental region moving back to the submandibular region, then down the jugular chain to the supra-clavicular fossa.
Intra-oral examination
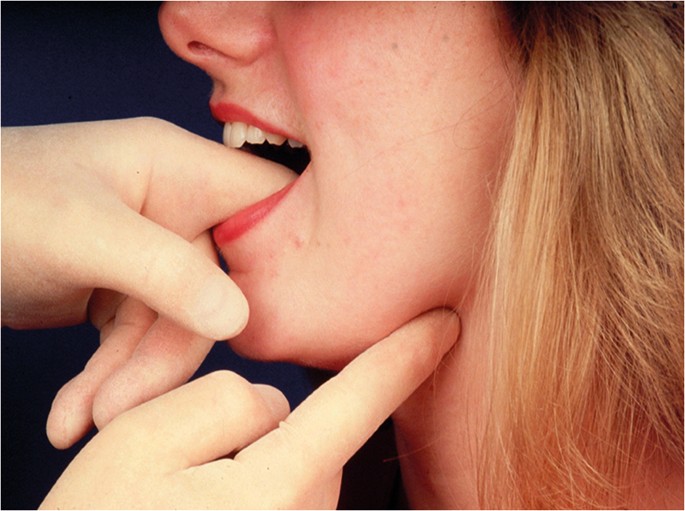
There is no correct order for examining the oral soft tissues as long as that at the completion of the examination the entire mouth and the oropharynx, including the tonsils has been assessed. Good lighting is obviously essential. Any mucosal abnormality noted should be palpated to determine consistency of the soft tissue (Fig. 5). As a general rule, induration reflects the presence of a benign or malignant neoplasm while softness to palpation represents a non-neoplastic inflammatory condition. In addition, mouth cancer is often painless until well advanced while inflammatory conditions are usually painful from the outset.
Mucosal biopsy
A scalpel biopsy is the gold standard investigation for the diagnosis of any mucosal abnormality. The biopsy may be excisional or incisional depending on the size of the abnormality detected. Small areas of mucosal change can be removed with a surrounding margin of clinically normal mucosa. There is divided opinion on the complete removal of a small mucosal abnormality that is found to be cancer since it may subsequently cause problems in identifying the primary site. However, the vast majority of small localised mucosa lesions are not squamous cell carcinoma and in the cases where they are modern imaging techniques and clinical examination will indicate the diagnostic surgical site. In the case of larger areas of change, a clinical decision has to be made as to what tissue to remove. As a rule the most suspicious looking area of mucosa should be included in the biopsy material. The tissue specimen should therefore include any aspects of ulceration, induration, erosion and erythema along with some apparently normal adjacent mucosa.
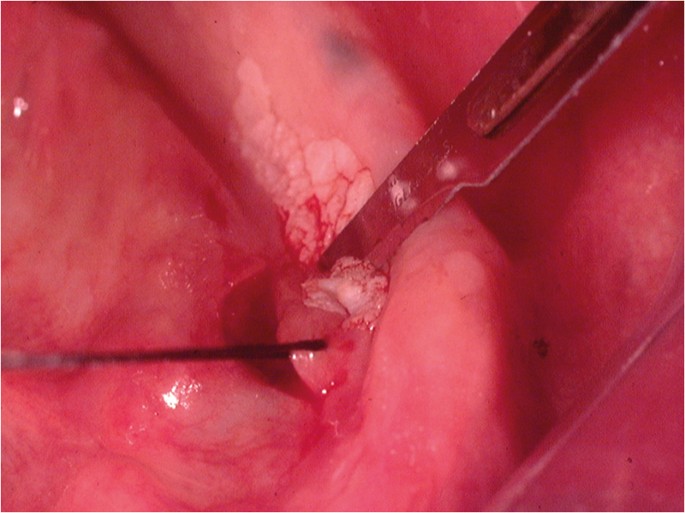
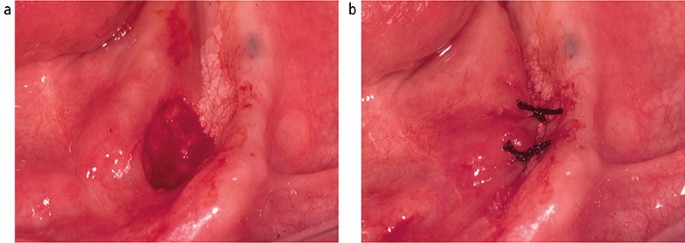
Following the placement of local anaesthesia, an incisional mucosal biopsy should involve the taking of an ellipse of tissue from the affected site. It is helpful to firstly place a suture in the tissue to be removed.6 This is preferable to the use of toothed-tissue tweezers since these may become dislodged and frequent re-application can cause crush damage to the biopsy material. A number-15 blade with a rounded tip is the scalpel of choice for the majority forms of oral surgery. The lower incision is made first to prevent bleeding potentially obscuring the site of the upper incision (Fig. 6). The tissue can then be removed as a sheet of mucosa. Closure of the surgical defect can be made with simple interrupted sutures using either a resorbable or non-resorbable material (Fig. 7). A wide range of suture materials are available and their use depends of the type of surgery being undertaken. Although black silk, a non-resorbable material, has historically been used for intra-oral soft tissue closure, resorbable polyglactin 910 (a copolymer of 90% glycolide and 10% L-lactide), is now the preferred material. Sutures are available on a variety of needles which differ in cross section, size and length. A 19 mm curved reverse cutting needle, which is triangular in cross section, is the needle of choice for closure of the oral mucosa.
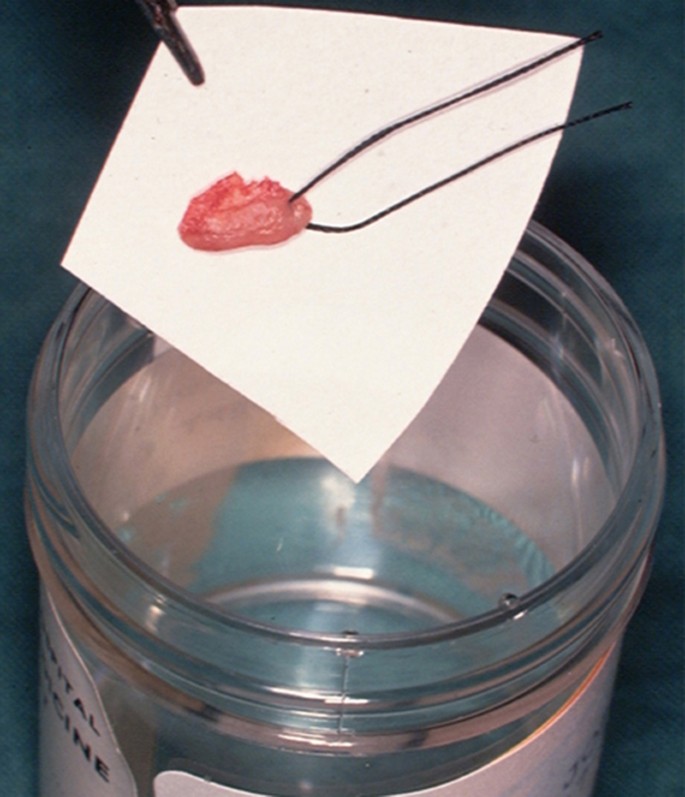
The biopsy tissue removed can be supported on filter paper before placement in a pre-labelled specimen pot containing 10% neutral buffered formalin to minimise the impact of shrinkage and distortion during fixation (Fig. 8). This step is important since curling up of the tissue can cause cross sectioning that may be mistaken for epithelial invasion in tissue sections when examined microscopically in two dimensions.
Punch biopsy
Punch biopsy, which removes a cylindrical specimen of tissue of between 0.4–0.8 mm in diameter, has been promoted as a simple and quick method of sampling the oral mucosa.7 However, since the amount of tissue removed is relatively small there is potential to not obtain sufficient material when assessing the epithelium for the presence of dysplasia or invasion. On this basis some oral and maxillofacial pathologists have expressed a preference for a scalpel biopsy rather than punch biopsy (personal communication).
Potentially malignant conditions
A primary mouth cancer can either arise in what was previously clinically normal tissue or develop within a pre-existing mucosal abnormally. Such pre-existing conditions that are associated with an increased incidence of undergoing malignant transformation are referred to as being oral potentially malignant disorders (OPMDs) (Box 1).
Leukoplakia
The most frequently recognised OPMD is leukoplakia (Fig. 9), which was first defined by the World Health Organisation (WHO) in 1978 as 'a white patch or plaque that cannot be characterised clinically or pathologically as any other disease'.8 The term has subsequently been refined following various international workshops and is now used to describe 'white plaques of questionable risk having excluded (other) known diseases and disorders that carry no increased risk for cancer'.9 It is essential to remember that leukoplakia is a clinical term and not a diagnosis. A biopsy is required to exclude known mucosal disorders. Although often associated with the presence of epithelial dysplasia, leukoplakia itself has no specific histopathological features. The reported malignant transformation rate for oral leukoplakia has ranged from low levels of 0.13% in India10 and 2.6% in the UK11 to high levels of 17.5% in USA.12 These findings are undoubtedly influenced by the geographical site, population studied, number of patients and length of follow-up observation period. The global malignant transformation rate of leukoplakia is generally accepted to be 1.36% per year.13
There is a rare form of leukoplakia, termed proliferative verrucous carcinoma (PVL), which has a reported transformation rate as high as 70%.14 The presentation of PVL is characterised by an initial white patch that develops into multiple areas of exophytic/wart-like changes within the mucosa. The aetiology of PVL is unknown and treatment difficult due to progressive recurrence following surgical removal.
Erythroplakia
Oral erythroplakia (Fig. 10) is defined as 'a fiery red patch that cannot be characterised clinically or pathologically as any other definable lesion'.15 Erythroplakia, in a similar way to leukoplakia has no specific histopatholigical features. The term is used clinically to record the presence of an erythematous mucosal abnormality that does not have a clinical appearance characteristic of known red patches, such as denture-associated candidosis or median rhomboid glossitis. Oral erythroplakia has the highest transformation rate of all of the OPMDs, being reported as between 14–50%.16 Erythroleukoplakia is an alternative clinical term that can be used when the mucosa has a speckled red and white appearance. Importantly erythroleukoplakia is associated with a high risk of malignant change.
Lichen planus
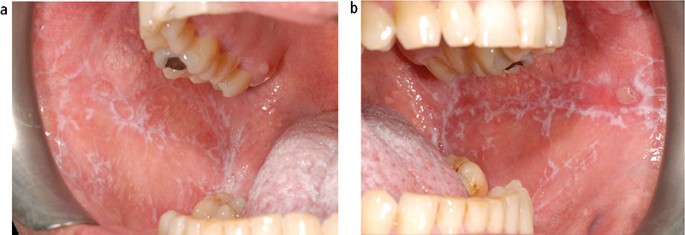
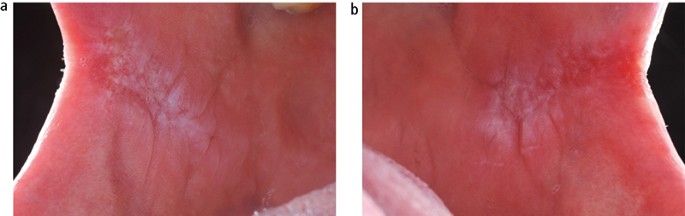
The aetiology of oral lichen planus (OLP) is unknown but does involve immune system since a primary histopathological feature is a sub-epithelial band of T-lymphocytes, indicating a cell-mediated reaction. OLP is one of the most frequently occurring mucosal conditions in the population, with a reported prevalence of between 0.5–2.2%.17 Different types of OLP have been described, including reticular, atrophic, erosive, plaque-like and bullous, based on the appearance of the mucosa. However, actual typing in an individual patient is often difficult since different types may be present simultaneously and also change during the course of disease over months. Overall, the most characteristic feature is the presence of bilateral white striations in the buccal mucosa (Fig. 11). The reported malignant transformation for OLP worldwide has varied between 0.4–6.4% depending on population studied and length of follow-up period. Two historical UK-based studies revealed yearly transformation rates of 0.07% and 0.27%.18,19 A recent systematic review revealed an increased transformation risk in females with red clinical forms on the tongue.20
Submucous fibrosis
Submucous fibrosis is a chronic disorder of the upper alimentary tract, which presents most obviously within the mouth as vertical fibrous bands in the buccal mucosa that limit mouth opening. The patient will also complain of an overall burning sensation within the mouth, and the buccal mucosa may appear white or erythematous (Fig. 12). Oral submucous fibrosis (OSF) has a strong association with the social habit of chewing areca (betel) nut, which is popular in populations living in and originating from the Indian subcontinent and surrounding countries. Alkaloids within the nut stimulate fibroblast proliferation. The malignant transformation rate from a long-term, follow-up study of OSF in an Indian population was reported as 7.6%.21
Palatal keratosis in reverse smokers
Reverse smoking, in which the lit end of a cigar or cigarette is placed in the mouth, is popular in the rural populations of the Amazon, New Guinea and Indian subcontinent. The physical irritation from heat and tobacco smoke induces hyper-keratinisation and erythamatous changes within palatal mucosa. A high incidence of cancer in the hard palate, which is a relatively rare site in non-reverse smokers, has been associated with this habit.22
Other OPMDs
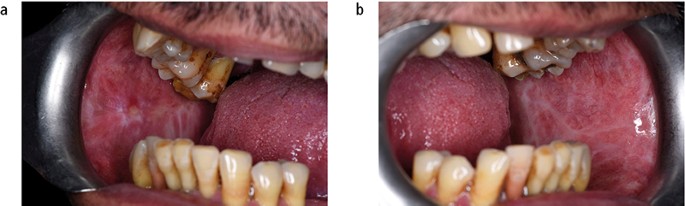
Actinic keratosis is associated with exposure to ultraviolet light and characteristically affects the vermilion lower lip presenting as palpable white plaques. Regular review is required and the development of palpable induration would indicate the need for biopsy to exclude transformation into either a squamous cell carcinoma or basal cell carcinoma (Fig. 13). The actual transformation rate of actinic keratosis is unknown. Discoid lupus erythematous, which can on occasion produce oral signs that resemble oral lichen planus or erythroplakia, has been reported to transform into squamous cell carcinoma. Dyskeratosis congenita, an example of inherited OMPD, is a bone marrow disorder that is associated with oral white patches (Fig. 14) which transform into mouth cancer and cause death in young adulthood.23
Although not included in the WHO list of OPMDs, chronic hyperplastic candidosis (CHC) is recognised as having the potential to undergo malignant transformation.24 CHC characteristically presents as bilateral adherent white patches in the buccal commissure regions of the mouth (Fig. 15) or dorsum of tongue. This type of oral candidosis is seen almost exclusively in smokers. Although the provision of systemic antifungal therapy will produce a dramatic clinical improvement, CHC will recur if the patient does not stop the tobacco habit. All patients with CHC should be provided with smoking cessation advice.
Detection aids
As mentioned above, mouth cancer has a wide spectrum of clinical presentation with no pathognomonic feature. It has been demonstrated that dentists, dental hygienists and dental therapists are able to confidently recognise mucosal abnormalities and have relatively high accuracy for the clinical detection of mouth cancer or OMPD.25 However, it is generally accepted that even while the most experienced clinician may have strong suspicion of the presence of squamous cell carcinoma, there can never be 100% certainty until the diagnosis is confirmed on the basis of histopathological findings following gold standard mucosal biopsy. Occasionally a highly suspicious clinical area of mucosa change is found to be an essentially benign abnormality and conversely a benign-looking mucosal abnormality can be found to contain a carcinoma. The lack of specific clinical signs for mouth cancer probably in part accounts for the delay in a patient seeking an opinion of dentist or doctor in primary care and hence the high percentage of patients who are not diagnosed till the tumour has advanced to Stage III or Stage IV.1
A range of adjunctive clinical aids have been and are continuing to be developed commercially to increase the detection of potential mouth cancer at the chair side.26 The technology involved includes the use of nuclear dyes, tissue reflectance light visualisation and fluorescence imaging, all of which aim to increase the clinician's ability to detect an area of mucosal abnormality that may represent cancer or OMPDs. The primary aim of such diagnostic adjuncts is to increase the proportion of mouth cancers that are detected in primary care while at Stage I or Stage II. The promotion of these diagnostic aids not only emphasises the importance within the dental profession to thoroughly assess the oral mucosa but when used in the clinic also raises awareness of the potential of mouth cancer in patients. However, the subjective interpretation of the tests coupled with relatively good sensitivity but poor specificity, makes their use problematic. There is limited evidence to support the use of light-based techniques in primary care.27,28 At the present time further research is required to develop the technology to achieve a tool that could be used as an effective screen for mouth cancer within the general population. It is not possible to present details of all the available diagnostic adjuncts here (for reviews, see Macey et al.29 and Lingen et al.30). Some of the more widely known systems are described below.
Vital tissue staining
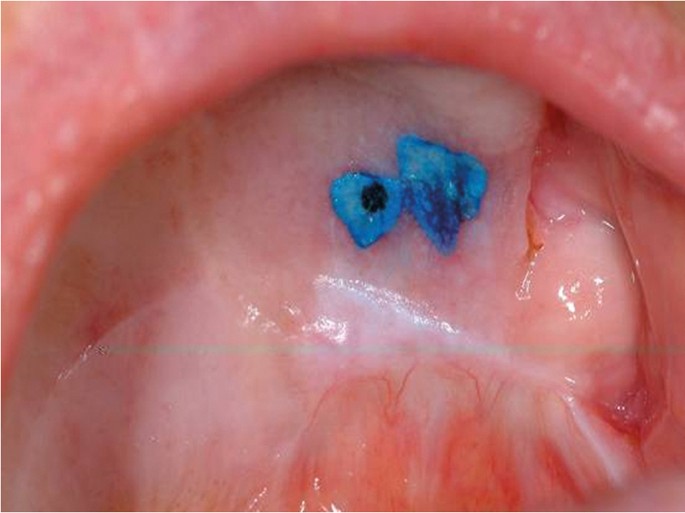
The use of vital tissue staining in cancer detection is based on the assumption that a nuclear dye will be taken up in the epithelial cells where there is a high density of nuclear material, such as in malignancy, and therefore have a different appearance to normal mucosa. This methodology was originally used for cervical screening in women. Toluidine blue (tolonium chloride) is a metachromatic dye has been used widely in this context. Retention of the dye, seen as dark blue, following application and de-staining with acetic acid is considered positive (Fig. 16). However, it is not cancer specific and false positives due to uptake by benign inflammatory and ulcerative of the oral mucosa are frequent. Equally, failure of the dye to penetrate keratinised mucosa can lead to false negatives. A Cochrane review of the use of the toluidine blue test for detection of oral carcinoma or OPMDs in 14 studies reported an overall sensitivity of 0.84 and specificity of 0.70 for both conditions.29
Tissue reflectance light visualisation
The use of tissue reflectance or chemiluminescence to detect cancer is based on the theory that the increased nuclear to cytoplasmic ratio of malignant epithelial cells results in increased light reflectance when compared to normal mucosa. ViziLite Plus (Panadent, Orpington, UK) and MicroLux DL (Panadent, Orpington, UK) are two cancer detection systems that employ this approach. The systems differ in their light sources with ViziLite Plus using a disposable stick (490–510 nm wavelengths) and MicroLux DL using a battery operated device (410–710 nm). Abnormal mucosa appears 'aceto-white' when compared to normal tissues which are light blue. Both systems have been found to brighten areas of leukoplakia, which had already been detected using conventional examination. Unfortunately, due to the lack of surgical biopsy information in the studies evaluating these two systems it is not possible to determine sensitivity and specificity figures.
Fluorescence imaging
It has been discovered that cancer cells have different autofluorescence emission patterns when compared to normal tissues. The Visually Enhanced Lesion Scope (VELscope, LED Dental Inc, White Rock, British Columbia, Canada) is a handheld device that shines high-intensity blue excitation light (400–460 nm wavelengths) onto the oral mucosa. Normal tissues fluoresce green while malignant cells appear relatively dark. Unfortunately, some normal tissue or benign conditions may also remain dark. A Cochrane review of twelve studies of the use of VELscope reported an overall sensitivity of 0.91 and specificity of 0.58 for oral carcinoma or OPMDs.29
Brush cytology
Exfoliative cytology has been investigated as a possible mouth cancer detection system. OralCDx (OralCDx Laboratories, Inc) is an example of this technology that uses a special designed brush to collect cells from an identified mucosal abnormality within the mouth. The sample is then sent for computerised analysis for the presence of abnormal cells and reported as negative, atypical or positive. All atypical and positive results require a tissue biopsy. OralCDx has been found to have a sensitivity of 0.92 and specificity of 0.94 to detect dysplasia or squamous cell carcinoma.31 More recently cytological methods have been combined with molecular markers in an attempt to improve their prognostic value.
Salivary diagnostics
Molecular biology has enabled the ability to detect biomarkers (peptides, proteins, DNA, mRNAs and miRNAs) in saliva and as such provide a non-invasive diagnostic test and screening tool for human disease. The potential to use saliva to detect the presence of cancer has been researched in relation to tumours of the ovary, breast, pancreas and lung. In addition, a number of studies have been undertaken to look at biomarkers for oral squamous cell carcinoma.32,33,34,35 At the present time further work is required to identify specific biomarkers within saliva that can be reliably used to detect different forms of human cancer and other forms of disease. Salivary diagnostic technology has huge potential for the future and could become a routine aspect of dental primary care.
Referral
As previously described, the outcome of mouth cancer is significantly improved if the tumour is detected and treated when it is less than 2 cm in diameter with no local metastasis. On this basis any suspected cancer patient should be referred for specialist assessment rapidly. Terminology used in this situation is urgent suspected cancer (USC) with an expectation, in the UK, that a patient referred a USC will be seen within 14 days (2 week wait, 2WW) or 10 working days(10 day rule).1 The 2WW system for cancer, including head and neck cancer, was introduced in the UK in 2000 and there is little doubt that it has been valued by patients since it speeds up the overall management. An audit of 2WW rule in the oral and maxillofacial surgery department at the Newcastle General Hospital and then subsequently in the oral surgery and oral medicine departments at Newcastle Dental Hospital revealed positive head and neck cancer detection rates of 11% and 7% respectively in the cohorts of patients examined.36 A similar study at the oral medicine department in King's College, London, reported that 8% of urgent referrals were found to have oral cancer.37 Both audits concluded that further education of referring practitioners and refinement of the referral guidelines were required to ensure a more efficient service.
A systematic review of the impact of the 2WW rule for head and neck cancer between 2000–14 concluded that the conversion rate (proportion of 2WW referrals who were diagnosed as having cancer) was falling, and the detection rate (proportion of diagnosed cancers referred under 2WW rule) was rising due to increased number of referrals. In addition the impact of the 2WW rule on outcome, particularly survival, was not clear.38
Methods of referral from primary care to specialist secondary care vary widely. Historically, referrals have been made in the form of a written letter with inevitable risk of delay within a postal system. This has in part been overcome by the use of a fax machines or a secure NHS email address. Personal email accounts must not be used due to the high risk of breach of patient confidentiality. However, free written communication via any of these methods may be problematic in that the clinical information provided by the primary care practitioner may be insufficient to enable the receiving specialist to identify the need for a 2WW appointment. The inclusion of a question on a preformed referral forms 'Do you think that this patient may have a cancer?' or a 'Yes/No' tick-box for USC is helpful but again not foolproof. Details of risk factors for the patient, in particular any tobacco, betel nut or alcohol habits, are important to include.
Electronic record management and referral systems within managed clinical networks (MCNs) are being introduced and these have a range of advantages, including an assurance of rapid and safe delivery of the referral request. In addition, some electronic systems ensure that all relevant information is provided by the referring practitioner since the referral will not be accepted until this is completed online. Individual practitioners must be aware of their local referral system.
In the UK, the National Institute for Health and Clinical Excellence (NICE)39 and Healthcare Improvement Scotland (HIS)40 have published referral guidelines to help clinicians decide which patients should be refereed as USC. These guidelines focus on the presenting clinical symptoms and recommend the need for a USC referral for any individual with:
-
Unexplained ulceration in the oral cavity lasting more than three weeks
-
A persistent and unexplained lump in the neck
-
A lump on the lip or in the oral cavity consistent with oral cancer
-
A red or red and white patch in the oral cavity consistent erythroplakia or erythroleukoplakia.
There has been discussion in relation to the recommendation that a doctor should refer a patient to a dentist for assessment within two weeks if the doctor thinks that there is 'a lump on the lip or in the oral cavity consistent with oral cancer or a red or red and white patch in the oral cavity consistent erythroplakia or erythroleukoplakia'. Concern has been expressed that due to the lack of a clear pathway between the doctor and the dentist this advice exposes the patient to unnecessary delay in diagnosis.41,42
Communication with the patient
If a soft tissue examination reveals an abnormality and it is felt that a referral is indicated then what should the patient be told? It is probably best to inform the patient that changes in the mouth are seen frequently and the vast majority are innocent but it is best to get it double checked by a specialist. Other information given to the patient and/or carer could include:
-
Where there are being referred
-
How long they may have to wait
-
Who will see them
-
What types of test may be done.43
This communication has to be done sensitively and obviously the amount of information given decided on an individual patient basis.
Interestingly, it has been reported that some dentists have a reluctance to discuss mouth cancer with their patients due to a lack of confidence to answer patients' questions.44 There would appear to be a need for specific training and guidance for the dental team on how to raise the issue of mouth cancer during routine examination and also how to communicate any issues without causing unnecessary anxiety. This aspect of mouth cancer could be a subject for future undergraduate and postgraduate education.
Conclusion
The clinical presentation of mouth cancer is highly variable. Regular and thorough examination of the soft tissues to detect any abnormality is an essential aspect of dental primary care. If any mucosal change found is thought to possibly represent cancer, then the patient needs to be referred appropriately for a specialist evaluation. All dental professionals should be aware of mouth cancer and feel comfortable about discussing the subject with their patients.
References
Cancer Research UK Website. Available at www.cancerresearchuk.org/health (accessed August 2018).
Sciubba J J . Oral cancer. The importance of early diagnosis and treatment, Am J Clin Dermatol 2001; 2: 239–251.
Ong T K, Murphy C, Smith A B, Kanatas A N, Mitchell D A . Survival after surgery for oral cancer: a 30-year experience. Br J Oral Maxillofac Surg 2017; 55: 911–916.
Lewis M A O, Lamey P-J . A clinical guide to oral medicine. 3rd ed. London: British Dental Journal, 2011.
Oral cancer recognition toolkit. Available at www.doctors.net.uk/eclientopen/cruk/oral_cancer_toolkit_2015_open/ (accessed 19 October 2018).
Oliver R J, Sloan P, Pemberton M N . Oral biopsies: methods and applications. Br Dent J 2004; 196: 329–333.
Lynch D P, Morris F M . The oral mucosa punch biopsy. J Am Dent Assoc 1990; 121: 145–149.
Kramer I R H, Lucas R B, Pindborg J J, World Health Organization Collaborating Centre for Oral Precancerous Lesions: Definition of leukoplakia and related lesions. An aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol 1978; 46: 518–539.
Warnakulasuriya S, Johnson N W, van der Waal I . Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007; 36: 575–580.
Silverman S, Bhargava, K, Smith L W, Malaowalla A M . Malignant transformation and natural history of oral leukoplakia in 57: 518 industrial workers of Gujarat, India. Cancer 1976; 38: 1790–1795.
Warnakulasuriya S, Kovacevic T, Madden P et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med 2011; 40: 677–683.
Silverman S, Gorsky M, Lozada F . Oral Leukoplakia and malignant transformation: a follow up study of 257 patients. Cancer 1984; 53: 563–568.
Petti S . Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol 2003; 39: 770–780.
Munde A, Karle R . Proliferative verrucous leukoplakia: An update. J Can Res Ther 2016; 12: 469–473.
Pinborg J J, Reichart Smith C J, van der Waal I, Sobin L H and pathologists in nine countries. World Health Organization international histological classification of tumours, histological typing of cancer and precancer of the oral mucosa. 2nd ed. Berlin: Springer, 1997.
Reichart P A, Philipsen H P . Oral erythroplakia – a review. Oral Oncol 2005; 41: 551–561.
De Rossi S S, Ciarrocca K . Oral lichen planus and lichenoid mucositis. Dent Clin North Am 2014; 58: 299–313.
Rajentheran R, McLean N R, Kelly C G, Reed M F, Nolan A . Malignant transformation of oral lichen planus. Eur J Surg Oncol 1999; 25: 520–523.
Ingafou M, Leao J C, Porter S R, Scully C . Oral lichen planus: a retrospective study of 690 British patients. Oral Dis 2006; 12: 463–468.
Giuliani M, Troiano, G, Cordaro M et al. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis 2018; 1111/odi.12885 [Epub ahead of print].
Murti P R, Bhonsle R B, Pindborg J J, Daffary D K, Gupta P C, Mehta F S . Malignant transformation rate in oral submucous fibrosis over 17 period. Community Dent Oral Epidemiol 1985; 13: 340–341.
Gupta P C, Mehta F S, Daftary D K et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow up study of Indian villagers. Community Dent Oral Epidemiol 1980; 8: 287–333.
Ogden G R, Connor E, Chisholm D M . Dyskeratosis congenita: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1988; 65: 586–591.
Williams D W, Bartie K L, Potts A J C, Wilson M J, Fardy M J, Lewis M A O . Strain persistence of invasive Candida albicans in chronic hyperplastic candidosis that underwent malignant change. Gerodontology 2001; 18: 73–78.
Brocklehurst P, Pemberton M N, Macey R, Cotton C, Walsh T, Lewis M A O . Comparative accuracy of different members of the dental team in detecting malignant and non-malignant oral lesions. Br Dent J 2015; 218: 525–529.
Warnakulasuriya S . Diagnostic adjuncts on oral cancer and precancer: an update for practitioners. Br Dent J 2017; 223: 663–666.
Rashid A, Warnakulasuriya S . The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med 2015; 44: 307–328.
Warnakulasuriya S . Translational research in oral oncology – A bridge between basic science and clinical application. Transl Res Oral Oncol 2016; 1: 1–2.
Macey R, Walsh T, Brocklehurst P et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients with clinically evident lesions. Cochrane Database Sys Rev 2015; 5: Cd010276. 10.1002/14651858.CD010276.pub2.
Lingen M W, Tampi M P, Urquhart O et al. Adjuncts for the evaluation of potentially malignant disorders in the oral cavity: Diagnostic test accuracy systematic review and meta-analysisa report of the American Dental Association. J Am Dent Assoc 2017; 148: 797–813.e52.
Scheifele C, Schmidt-Westhausen A M, Dietrich T, Reichart P A . The sensitivity and specificity of the OralCDx technique: evaluation of 103 cases. Oral Oncol 2004; 40: 824–828.
Radhika T, Jeddy N, Nithya S, Muthumeenakshi R M . Salivary biomarkers in oral squamous cell carcinoma – An insight. J Oral Biol Craniofac Res 2016; 6: S51–S54. 10.1016/j.jobcr.2016.07.003.
Bano S, David M P, Indira A P . Salivary biomarkers for oral squamous cell carcinoma: An overview. IJSS Case Rep Rev 2015; 1: 39–45.
Spielmann N, Wong D T . Saliva: diagnostics and therapeutic perspectives. Oral Dis 2011; 17: 345–354.
Cheng Y S, Rees T, Wright J . A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med 2014; 3: 3. 10.1186/2001-1326-3-3.
Miller C C, Hierons R J . Two audits of the diagnosis of oral cancer and the two-week rule following referrals from primary care practitioners in Newcastle. Prim Dent Care 2012; 19: 63–68.
Singh P, Warnakulasuriya S . The two-week wait cancer initiative on oral cancer; the predictive value of urgent referrals to an oral medicine unit. Br Dent J 2006; 201: 717–720.
Langton S, Siau D, Bankhead C . Two-week rule in head and neck cancer 2000–2014: asystematic review. Br J Oral Maxillofac Surg. 2016: 54: 120–131.
National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. 22 June 2015. Available at www.nice.org.uk/guidance/ng12 (accessed August 2018).
Healthcare Improvement Scotland. Scottish referral guidelines for suspected cancer. August 2014. Available at www.healthcareimprovementscotland.org/our_work/cancer_care_improvement/programme_resources/scottish_referral_guidelines.aspx (accessed August 2018).
Grimes D . Patel J, Avery C . New NICE referral guidance for oral cancer: does it risk delay in diagnosis? Br J Oral and Maxillofac Surg 2016; 55: 404–406.
Yeung C A . Referrals to dentists by GPs could delay diagnosis of oral cancer. BMJ 2017; 356: i6784. Available at https://doi.org/10.1136/bmj.i6784 (accessed 19 October 2018).
Kalavrezos N, Scully C . Mouth cancer for clinicians Part 8: Referral. Dent Update 2016; 43: 176–185.
Awojobi O, Newton J T, Scott S E . Pilot study to train dentists to communicate about oral cancer: the impact on dentist's self-confidence reported behaviour, confidence and beliefs. Br Dent J 2016; 220: 71–76.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, M. Mouth cancer: presentation, detection and referral in primary dental care. Br Dent J 225, 833–840 (2018). https://doi.org/10.1038/sj.bdj.2018.931
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2018.931
This article is cited by
-
Oral medicine considerations for the older patient
British Dental Journal (2024)
-
Appropriateness of two-week wait head and neck cancer referrals to a district general hospital
British Dental Journal (2023)
-
Slow to heal or slow to diagnose cancer?
British Dental Journal (2021)
-
An audit to analyse the two-week wait pathway at an oral cancer specialist district general hospital
British Dental Journal (2020)
-
The reconstructive oral cancer patient: what the general dental practitioner needs to know
British Dental Journal (2019)