Key Points
-
Reviews advances in regenerative endodontics.
-
Suggests regenerative endodontics as it is currently performed represents more of a reparative than regenerative therapeutic strategy.
Abstract
Background Significant advances in our understanding of the biological processes involved in tooth development and repair at the cellular and molecular levels have underpinned the newly emerging area of regenerative endodontics. Development of treatment protocols based on exploiting the natural wound healing properties of the dental pulp and applying tissue engineering principles has allowed reporting of case series showing preservation of tissue vitality and apexogenesis.
Aim To review current case series reporting regenerative endodontics.
Results Current treatment approaches tend to stimulate more reparative than regenerative responses in respect of the new tissue generated, which often does not closely resemble the physiological structure of dentine-pulp. However, despite these biological limitations, such techniques appear to offer significant promise for improved treatment outcomes.
Conclusions Improved biological outcomes will likely emerge from the many experimental studies being reported and will further contribute to improvements in clinical treatment protocols.
Similar content being viewed by others
Introduction
Dentistry has long been a pioneer of regenerative medicine with the use of calcium hydroxide to stimulate tissue repair after pulp exposure1,2 and more recently with newer agents including mineral trioxide aggregate (MTA)3 and tri-calcium silicate (Biodentine).4 Lack of clarity on the mechanisms of action of these various agents has led to their application being somewhat empirical, however, they have contributed significantly to the preservation of pulp vitality in diseased teeth. Significant research advances in the broader fields of biology and tissue engineering over the last two decades have been paralleled by developments in pulp biology, which have underpinned our mechanistic understanding of the responses of the dental tissues to injury, wound healing and clinical intervention. This has provided a platform from which novel therapeutic strategies have emerged, which aim to promote tissue repair and regeneration following pulpal disease and preserve tooth vitality. The concept of regenerative endodontics5 is broad and encompasses a wide variety of strategies to clinically translate our biological understanding of pulp regeneration into improved patient management approaches. Thus, these strategies range from focus on clinical protocols to harness the natural wound healing capacity of the pulp, through interventions to promote revascularisation of an empty root canal and optimise the actions of canal irrigants to use of stem cells for tissue engineering. Clearly some of these approaches show promise for shorter term translation while others are longer term clinical goals. Nevertheless, the over-riding emphasis on preservation of tooth vitality will be beneficial to the practice of endodontics. This article seeks to outline some of the key biological advances underpinning these new strategies in regenerative endodontics and to highlight the opportunities for clinical translation.
Pulp biology and its underpinning of regenerative endodontics
By definition, wound healing is a pathological process and tissue events may not be under the same regulatory control as the physiological processes of tissue development and homeostasis. Nevertheless, these physiological processes have been extensively investigated to provide a blueprint to understand the molecular and cellular events involved in pulp repair and regeneration. Many parallels appear to exist in these processes, albeit with altered regulatory control.6
In considering the responses of the dentine-pulp complex to injury, whether of traumatic or carious origin, it is important to distinguish initially the extent of this injury since it has significant implications for subsequent cellular events. When the injury is in its earlier stages, or relatively low grade in nature, the pulp responds by upregulation of the secretory activity of the odontoblasts at the site of injury resulting in focal deposition of a 'reactionary' variant of tertiary dentine at the pulp-dentine interface.7 This response may be regarded as both defensive by increasing the barrier to ongoing carious bacterial attack and regenerative in restoring the structural integrity of the tissue. Since the reactionary dentinogenesis results from the activity of existing primary odontoblasts, the new dentine deposited has a regular tubular structure showing tubular continuity with the existing primary/secondary dentine matrices. As a consequence, the new tissue will be expected to behave identically to the existing tissue. These responses are typical of an early carious lesion or perhaps significant wear to the tooth surface. With further progression of disease and the stimulus from the injury becoming more intense, cellular events in the pulp will likely change significantly. Local death of some of the primary odontoblasts at the site of injury will ensue and the inflammatory responses within the pulp will intensify. If the tissue conditions are conducive to repair and regeneration, that is, the inflammation has not become uncontrolled and the pulp is not grossly infected, a typical wound healing response will be initiated in which the pulp will strive to repair and regenerate itself by recruiting and differentiating new cells. The odontoblast, however, is a unique cell in the body in many respects. It is a post-mitotic cell meaning that it is unable to divide and give rise to progeny. Furthermore, this absence of turnover of odontoblasts leads to its continued presence for the life of the tooth in the absence of injury. Clearly, the odontoblast is a very versatile cell with its alternating phases of activity and quiescence throughout life. The consequence of the odontoblast's post-mitotic nature is that following cell death, any new generation of odontoblast-like cells must arise by recruitment of stem/progenitor cells and signalling of their differentiation. When this occurs, the new generation of odontoblast-like cells focally secretes a 'reparative' variant of tertiary dentine at the injury site.7 Reparative dentinogenesis constitutes the process for dentine bridge formation at sites of pulp exposure after pulp capping.
Reparative dentine can show considerable heterogeneity in its structure, the appearance of which can range from a normal regular tubular structure to a very disorganised poorly tubular or atubular structure. This reflects the pathological nature of reparative dentinogenesis, which lacks the regulatory control seen during physiological dentinogenesis. As a consequence, the stem/progenitor cells recruited for odontoblast-like cell differentiation may have varied derivations and thus the formative cells for reparative dentine may be somewhat heterogeneous in nature. Reparative dentinogenesis is clearly a more complex process than reactionary dentinogenesis and is characterised by several key events:
-
Recruitment and proliferation of stem/progenitor cells to the site of injury
-
Signalling of differentiation of odontoblast-like cells from the recruited stem/progenitor cells
-
Upregulation of secretory activity in the new generation of odontoblast-like cells.
Stem/progenitor cell recruitment
Despite considerable debate, there is still lack of consensus as to which cell population(s) are the progenitors of the new generation of odontoblast-like cells for reparative dentinogenesis and dentine regeneration. A number of locally resident cell populations have the potential to give rise to odontoblast-like cells including the undifferentiated mesenchymal cells in the cell rich layer of the mature pulp derived from the developmental stages of odontogenesis8 and the various pulp stem cell populations more recently reported. These pulp stem cells include dental pulp stem cells (DPSCs),9 stem cells from the apical part of the papilla (SCAPs),10 stem cells from human exfoliated deciduous teeth (SHEDs)11 and side-population pulp stem cells.12 Despite characteristic cell surface marker profiles, all of these cells appear to be mesenchymal stem cell (MSC) derived and have varied localisations ranging from perivascular niches to the apical papilla of the tooth. There has been considerable interest in the identification of these cells not only for their potential application in dental regeneration but also much broader clinical applications in regenerative medicine and tissue engineering. Currently, MSCs are most commonly isolated from bone marrow for regenerative medicine, however, pulp offers the opportunity to isolate such cells less invasively, especially in the case of SHED cells from exfoliated teeth. Indeed, commercial stem cell banks have now emerged for storage of these cells. Although resident pulp stem cells represent a possible source of odontoblast-like cell progenitors, MSC recruitment from outside the tooth (possibly bone marrow) to sites of injury in the pulp occurs.13 Once recruited, these cells are exposed to the niche environment of the pulp, which may give rise to their specific phenotypic characteristics. Cell recruitment from outside the pulp may be advantageous to rapidly providing the significant stem cell numbers required post-injury.
Despite many reports proposing stem cell transplantation for pulp regeneration, there is still considerable debate as to whether such cell-based approaches are necessary when cell-free approaches may be feasible and avoid the significant challenges of introducing cell transplantation protocols into general dental practice. Some of the constituents of dentine matrix promote cell recruitment in the pulp14 and their release during carious dissolution of the tooth may contribute to MSC recruitment to the pulp through the vasculature during natural wound healing by promoting such processes through cell homing.15
Odontoblast-like cell differentiation
During tooth development, reciprocal epithelial-mesenchymal interactions in the tooth germ are responsible for signalling cellular events, including odontoblast differentiation, the latter of which is triggered by enamel organ-derived molecular signals (including growth factors of the TGF-b superfamily). The absence of enamel epithelium in the mature tooth requires an alternative signalling source for odontoblast-like cell differentiation during dentine-pulp regeneration. While dentine has traditionally been regarded as a relatively inert tissue comprising largely of the structural protein collagen and mineral, it is now clear that it contains a variety of bioactive molecules with potent cell signalling functions (reviewed in Smith et al.).16 Among these bioactive molecules are a variety of growth factors and cytokines, many of which have been implicated in signalling developmental events including odontoblast differentiation. Carious dissolution of dentine matrix will release many of these bioactive molecules and their diffusion to the pulp can lead to signalling of odontoblast-like cell differentiation. The presence of these bioactive signalling molecules in dentine explains the ability of dentine chips to stimulate dentine bridge formation17 and the demonstration of their stimulation of reparative dentinogenesis (reviewed in Smith et al.)16 offers exciting opportunities for clinical application. Sequestration of these bioactive molecules in a fossilised state in dentine provides an exquisite self-repair mechanism for the tooth. It is likely that clinical exploitation of these molecules will target protocols to release endogenous stores within the dentine (see below) rather than rely upon their application within new materials.
Odontoblast-like cell secretory activity
Achieving successful dentine-pulp regeneration clinically relies upon regulation of cell secretory activity if pulp canal obliteration and loss of tooth vitality is to be avoided.18 Much of the research focus on tooth development and pulp regeneration has been concerned with the triggers to switch-on odontoblast differentiation, but subsequent regulation of secretory activity and the signals downregulating odontoblast activity after primary dentinogenesis have received little attention. Transcriptome analyses of young and mature odontoblasts have revealed modulation in expression of a number of genes associated with changes in cellular activity, including downregulation of the p38-MAPK pathway.19 P38-MAPK signalling may be key to the regulation of odontoblast activity since it is stimulated again on odontoblast20 and pulp cell21 activation during exposure to bacterial products and bioactive components of dentine matrix during tertiary dentinogenesis and regenerative events in the pulp. This pathway provides a key clinical target for regulation of dentinogenic activity and the availability of inhibitory agents may allow pharmaceutical control of tissue events.
Regenerative endodontics as a clinical treatment strategy
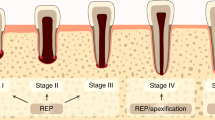
A number of single case and case series reports of regenerative endodontics are now emerging in the literature,22,23,24,25 which highlight the potential for such treatment strategies. These reports focus on use of treatment protocols, which build on our knowledge of the biology of the dentine-pulp complex in health and disease and aim to preserve tissue vitality. The approaches range from adoption of protocols that strive to create a conducive tissue environment for natural wound healing processes to ensue in the pulp, to those which seek to exploit the basic principles of tissue engineering to promote pulp regeneration. A common theme for all of these approaches, however, is focus on those aspects widely recognised to be primary determinants of treatment outcome. Thus, control of infection and inflammation is a key consideration in all of these approaches, as in any endodontic management plan. In contrast to more traditional root canal treatment approaches, there is greater emphasis on more gentle infection and inflammation control measures to minimise tissue damage. These control measures may pose a slightly higher risk in terms of their efficacy by virtue of their need to preserve host tissue vitality and thus, case selection for adoption of regenerative endodontics is of significant importance. Regenerative endodontics will likely be the treatment of choice for those cases where infection and inflammation are clinically judged to be not too extensive. This highlights the critical challenge facing endodontics of the constraints imposed by current diagnostic techniques.26,27 The lack of robust diagnostic markers hampers treatment planning and can lead to treatment protocols being more empirical and not exploiting our biological understanding of the pulp optimally. The majority of the clinical reports of regenerative endodontics have focused on treatment of immature teeth where stimulation of completion of root growth is a particular benefit to the treatment approach. Currently, the evidence base for use of regenerative endodontics for permanent teeth is very limited and conventional treatment approaches may be preferable for such teeth at this stage.
Infection and inflammation control measures
The complexity of the pulpal microbiome28 poses significant challenges to infection control in endodontics, which are exacerbated by the lack of specificity of most disinfection agents to bacterial rather than host cells. A wide variety of disinfection and irrigation protocols are commonly used with little consensus on their use. Sodium hypochlorite and ethylenediaminetetraacetic acid (EDTA) are widely used in canal preparation and their order of application has marked effects on tissue damage. Application of sodium hypochlorite followed by EDTA allows preservation of dentine structure, while the converse order of application leads to significant dentine damage due to reduction in the protective effects of the mineral against the oxidising effects of hypochlorite.29 Hypochorite is a powerful oxidising agent and the broad range of concentrations that it is used at can have significant implications for further pulpal injury. Generally, lower concentrations are preferable if used in the context of regenerative endodontics.
The rich reservoir of growth factors and other bioactive molecules sequestered in a fossilised state within the dentine matrix offers exciting opportunities for exploitation to drive regenerative events in pulp.16 While carious demineralisation of dentine will initiate release of some of these molecules, various irrigants and etchants can also locally release and expose these molecules30,31,32,33 and stimulate dentinogenic events.34
Release of bioactive molecules may also be stimulated by pulp-capping agents, including calcium hydroxide and mineral trioxide aggregate (MTA).35,36 Such effects may also favour stem cell recruitment through cell homing15 as a consequence of local release of dentine matrix components at sites of injury allowing chemotactic attraction of pulp stem cells.14 Careful attention to canal irrigation and disinfection protocols will be paramount to successful treatment outcomes in regenerative endodontics and considerable scope remains for optimisation of these protocols.
The extent of surgical intervention during canal preparation may also be a determinant of treatment outcome in terms of preservation of tissue vitality. Pulpotomy is commonly used in the management of the infected pulp, although difficulties in the evaluation of the depth of penetration of infection and inflammation can result in more radical surgery than necessary. The observation that pulp is frequently only inflamed to a depth of two mm37 led to the proposal of limiting surgical intervention to a more superficial partial pulpotomy.38 Adoption of a more conservative approach to pulpotomy likely contributed to the successful outcomes reported recently for use of regenerative endodontics in a case-series.24
Clearly, there is a strong relationship between canal preparation procedures and treatment outcomes for regenerative endodontics. Careful attention to infection control (for example, rubber dam, disinfection protocols etc), the extent of surgical excision of inflamed tissue and case selection in the context of preservation of tissue vitality are central features of regenerative endodontics.
Natural pulp wound healing versus tissue engineering approaches
The scope of regenerative endodontics ranges from procedures aimed at promoting natural wound healing events in the pulp to application of tissue engineering principles to treatment protocols. At this stage, there is insufficient evidence that either is superior, but advances in pulp biology will likely lead to clinical introduction of tissue engineering techniques, either for the pulp or the whole tooth in the future. There is already proof-of-principle at the laboratory level that such techniques are feasible39 and such studies offer exciting future possibilities for novel treatment modalities in endodontics. Currently, there are attempts to apply tissue engineering principles within regenerative endodontics.22 In tissue engineering, cells (often stem or progenitor cells) are combined with scaffolds and appropriate signalling molecules to construct tissues resembling their physiological counterparts. Although application of cells for regenerative endodontic procedures has been restricted to experimental studies, there have been attempts to introduce scaffolds through blood clot formation – termed 'revascularisation' procedures.22 This emulates the role of the blood clot as a scaffold for tissue regeneration in natural soft-tissue wound healing. While encouraging results have been observed with this approach, there is insufficient evidence to determine whether it is better than other regenerative endodontic approaches. This perhaps highlights the challenges of haemostasis during endodontic procedures and the problems of controlling tissue events in a carefully defined manner within revascularisation procedures.
Regeneration or repair – semantics or clinical reality?
Most case reports/case series in regenerative endodontics show examples of revascularisation of the pulp space where there is a pre-existing endodontic lesion. A number of these reports demonstrate radiographically completion of apexogenesis with increased root dentinal wall thickness and reduction in volume of the root canal space implying treatment success. Where histological analysis of teeth has been possible after treatment by a simple revascularisation procedure40 or by application of platelet-rich plasma,41 deposition of a mineralised layer on the radicular walls was observed, which appeared to be of periodontal rather than pulpal origin. This suggests that the new tissue was not of dentinogenic origin and emphasises the limitations of radiography for characterising new mineralised tissue formation in endodontics. Nevertheless, effective apical closure is a key goal of apexification techniques42 and whether this is achieved with a dentinogenic or a periodontal structure might be of lesser importance
It is important, however, to return to the objectives when trying to assess treatment success. If the objective is to induce healing of the periapical tissues, stimulate bone regeneration, and render the patient free from any signs or symptoms, then current regenerative treatments can be regarded a clinical success (although perhaps a biological failure). Filling the root canal space with a vital biological tissue has the significant advantage of providing an immuno-competent root canal filling with defensive capabilities for any future bacterial exposure like normal pulp tissue However, if the objective is restitutio ad integrum, that is, to regenerate a physiological-like pulp tissue, then these current treatments should be regarded as failures. Nevertheless, reports of some of the experimental studies involving recruitment of stem/progenitor cells with dentinogenic potentiality to sites of pulp injury suggest that true pulp-dentine regeneration may be a clinical reality in the future.
Conclusions
Regenerative endodontics offers a number of exciting opportunities for preservation of pulp vitality following episodes of trauma and disease and the many biological advances have helped to underpin the development of this approach. From a semantics viewpoint, regenerative endodontics as it is currently performed represents more of a reparative than regenerative therapeutic strategy. Treatment outcomes from techniques, such as revascularisation procedures, generally give rise to a vital biological tissue, albeit not necessarily representative of the physiological structure of pulp. Despite these current limitations, such treatment strategies still offer significant clinical benefits, especially for immature teeth. True pulp regeneration will emerge as a viable clinical treatment strategy from the many recent advances being reported at the experimental level. Such approaches will likely target recruitment of specific stem/progenitor cell populations and exploit endogenous signalling molecules sequestered in dentine to regenerate dentine-pulp tissue with physiological characteristics.
References
Hermann B . Ein weiterer Beitrag zur Frage der Pulpenbehandlung. Zahnärztl Rundsch 1928; 37: 1327–1376.
Zander H A . Reaction of the pulp to calcium hydroxide. J Dent Res 1939; 18: 373–379.
Nair P N, Duncan H F, Pitt Ford T R, Luder H U . Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with Mineral Trioxide Aggregate: a randomized controlled trial. Int Endod J 2009; 42: 422–444.
Zanini M, Sautier J M, Berdal A, Simon S . Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod 2012; 38: 1220–1226.
Hargreaves K M, Diogenes A, Teixeira F B . Treatment options: biological basis of regenerative endodontic procedures. Paediatr Dent 2012; 35: 129–140.
Smith A J, Lesot H . Induction and regulation of crown dentinogenesis – embryonic events as a template for dental tissue repair. Crit Rev Oral Biol Med 2001; 12: 425–437.
Smith A J, Cassidy N, Perry H, Begue-Kirn C, Ruch J V, Lesot H . Reactionary dentinogenesis. Int J Dev Biol 1995; 39: 273–280.
Fitzgerald M, Chiego D J, Heys D R . Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 1990; 35: 707–715.
Gronthos S, Brahim J, Li W et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81: 531–535.
Sonoyama W, Liu Y, Yamaza T et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 2008; 34: 166–171.
Miura M, Gronthos S, Zhao M et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 2003; 100: 5807–5812.
Iohara K, Zheng L, Wake H et al. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells 2008; 26: 2408–2418.
Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe P T . Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA 2011; 108: 6503–6508.
Smith J G, Smith A J, Shelton R M, Cooper P R . Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exper Cell Res 2012; 318: 2397–2406.
Kim J Y, Xin X, Moioli E K et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 2010; 16: 3023–3031.
Smith A J, Scheven B A, Takahashi Y, Ferracane J, Shelton R M, Cooper P R . Dentine as a bioactive extracellular matrix. Arch Oral Biol 2012; 57: 109–121.
Anneroth G, Bang G . The effect of allogeneic demineralized dentin as a pulp capping agent in Java monkeys. Odontol Revy 1972; 23: 315–328.
McCabe P S, Dummer P M . Pulp canal obliteration: an endodontic diagnosis and treatment challenge. Int Endod J 2012; 45: 177–197.
Simon S, Smith A J, Lumley P J et al. Molecular characterization of young and mature odontoblasts. Bone 2009; 45: 693–703.
Simon S, Smith A J, Berdal A, Lumley P J, Cooper P R . The MAPK pathway is involved in odontoblast stimulation via p38 phosphorylation. J Endod 2010; 36: 256–259.
Botero T M, Son J S, Vodopyanov D, Hasegawa M, Shelburne C E, Nör JE . MAPK signalling is required for LPS-induced VEGF in pulp stem cells. J Dent Res 2010; 89: 264–269.
Trope J . Regenerative potential of dental pulp. J Endod 2008; 34: S13–17.
Shimizu E, Ricucci D, Albert J et al. Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J Endod 2013; 39: 1078–1083.
Simon S, Perard M, Charpentier E et al. Should pulp chamber pulpotomy be seen as a permanent treatment? Preliminary findings of a pilot study. Int Endod J 2012; 46: 79–87.
Diogenes A, Henry M A, Teixeira F B, Hargreaves K M . An update on clinical regenerative endodontics. Endod Topics 2013; 28: 2–23.
Mejàre I A, Axelsson S, Davidson T et al. Diagnosis of the condition of the dental pulp: a systematic review. Int Endod J 2012; 45: 597–613.
Petersson A, Axelsson S, Davidson T et al. Radiological diagnosis of periapical bone tissue lesions in endodontics: a systematic review. Int Endod J 2012; 45: 783–801.
Hsiao W W, Li K L, Liu Z, Jones C, Fraser-Liggett C M, Fouad A F . Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 2012; 13: 345.
Haapasalo M, Shen Y, Qian W et al. Irrigation in endodontics. Dent Clin North Am 2010; 54: 291–312.
Smith A J, Smith G . Solubilisation of TGF-B1 by dentine conditioning agents. J Dent Res 1998; 77: 1034.
Zhao S, Sloan A J, Murray P E et al. Ultrastructural localisation of TGF-b exposure in dentine by chemical treatment. Histochem J 2000; 32: 489–494.
Galler K M, Hartgerink J D, Cavender A C, Schmalz G, D'Souza R N . A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A 2012; 18: 176–184.
Ferracane J L, Cooper P R, Smith A J . Dentin Matrix Component Solubilization by Solutions of pH Relevant to Self-etching Dental Adhesives. J Adhes Dent 2013; 15: 407–412.
Murray P E, Smith A J, Garcia-Godoy F, Lumley P J . Comparison of operative procedure variables on pulpal viability in an ex vivo model. Int Endod J 2008; 41: 389–400.
Graham L, Cooper P R, Cassidy N, Nor J E, Sloan A J, Smith A J . The effect of calcium hydroxide on solubilisation of bi-active dentin matrix components. Biomater 2006; 27: 2865–2873.
Tomson P L, Grover L M, Lumley P J, Sloan A J, Smith A J, Cooper P R . Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent 2007; 35: 636–642.
Mjor I A, Tronstad L . Experimentally induced pulpitis. Oral Surg Oral Med Oral Path Oral Radiol Endod 1972; 34: 102–108.
Cvek M . A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod 1978; 4: 232–237.
Cordeiro M M, Dong Z, Kaneko T et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008; 34: 962–969.
Shimizu E, Jong G, Partridge N, Rosenberg P A, Lin L M . Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod 2012; 38: 1293–1297.
Martin G, Ricucci D, Gibbs J L, Lin L M . Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod 2013; 39: 138–144.
Simon S, Rilliard F, Berdal A, Machtou P . The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J 2007; 40: 186–197.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Simon, S., Smith, A. Regenerative endodontics. Br Dent J 216, E13 (2014). https://doi.org/10.1038/sj.bdj.2014.243
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2014.243
This article is cited by
-
Do hypoxia and L-mimosine modulate sclerostin and dickkopf-1 production in human dental pulp-derived cells? Insights from monolayer, spheroid and tooth slice cultures
BMC Oral Health (2018)
-
Tooth Repair and Regeneration
Current Oral Health Reports (2018)
-
What BDJ readers were reading spring 2014
British Dental Journal (2014)