Key Points
-
Details recommendations from the NICE guidelines on prophylaxis against infective endocarditis which relate to dental practice.
-
Discusses the clinical and cost-effectiveness evidence relating to these guidelines.
Abstract
The National Institute for Health and Clinical Excellence (NICE) has developed a guideline on 'Prophylaxis against infective endocarditis'. This paper details the recommendations from these guidelines which relate to dental practice and discusses the clinical and cost-effectiveness evidence pertaining to them. This is taken from the full NICE guideline, which also includes guidance relating to non-dental procedures (http://www.nice.org.uk/CG064).
Similar content being viewed by others
Background
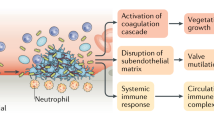
Infective endocarditis (IE) is a rare condition, with an annual incidence of fewer than 10 per 100,000 cases in the normal population. Despite advances in diagnosis and treatment, IE remains a life-threatening disease with significant mortality and morbidity.1 It has been accepted clinical practice to use antibiotic prophylaxis prior to dental procedures with the aim of preventing the development of infective endocarditis in those who are considered to be at risk. The logic for this has been that while many cases of IE are not caused by interventional procedures,2 there have been cases of infective endocarditis which have been associated with patients having a prior interventional procedure, notably following dental procedures. The rationale for prophylaxis against IE is: infective endocarditis usually follows bacteraemia, certain interventional procedures cause bacteraemia with organisms that can cause endocarditis, these bacteria are usually sensitive to antibiotics; therefore, antibiotics should be given to patients with predisposing heart disease before procedures that may cause bacteraemia.3
However, while the argument for using prophylaxis has a rational basis, the evidence base has relied heavily on extrapolation from animal models of the disease4 and the applicability of these models to humans has been questioned. The efficacy of prophylaxis and proof of a link between dental procedures and the development of infective endocarditis has never been firmly demonstrated and clinical practice has been dictated by clinical guidelines based on expert opinion. Recent guidelines by the British Society for Antimicrobial Chemotherapy5 and the American Heart Association6 have highlighted the lack of clear evidence in this area and have also challenged existing dogma by highlighting the prevalence of bacteraemias that arise from everyday activities such as tooth brushing.
NICE process
NICE recommendations are based on systematic reviews of best available evidence; guideline development groups use consensus techniques to develop recommendations using the available evidence. Where there is minimal research evidence, the guideline development group use their opinions about what constitutes best practice.
With a serious rare condition like IE, research using the experimental study designs is difficult and the evidence base consists of observational (predominantly case-control) studies.
Recommendations
Healthcare professionals should regard people with the following cardiac conditions as being at risk of developing infective endocarditis:
-
Acquired valvular heart disease with stenosis or regurgitation
-
Valve replacement
-
Structural congenital heart disease, including surgically corrected or palliated structural conditions, but excluding isolated atrial septal defect, fully repaired ventricular septal defect or fully repaired patent ductus arteriosus, and closure devices that are judged to be endothelialised
-
Previous infective endocarditis
-
Hypertrophic cardiomyopathy.
Healthcare professionals should offer people at risk of infective endocarditis clear and consistent information about prevention, including:
-
The benefits and risks of antibiotic prophylaxis and an explanation of why antibiotic prophylaxis is no longer routinely recommended
-
The importance of maintaining good oral health
-
Symptoms that may indicate infective endocarditis and when to seek expert advice
-
The risks of undergoing invasive procedures, including non-medical procedures such as body piercing or tattooing.
Antibiotic prophylaxis against infective endocarditis is not recommended for people undergoing dental procedures.
Chlorhexidine mouthwash should not be offered as prophylaxis against infective endocarditis to people at risk of infective endocarditis undergoing dental procedures.
Any episodes of infection in people at risk of infective endocarditis should be investigated and treated promptly to reduce the risk of endocarditis developing.
Evaluation of the evidence on clinical effectiveness
This section includes a brief summary of the relevant clinical effectiveness evidence; for the full evaluation see the NICE guideline (http://www.nice.org.uk/CG064).
Consideration of the evidence on dental procedures and the development of infective endocarditis identified that there is an inconsistent association between recent dental interventional procedures and infective endocarditis. This can be exemplified with one study noting that endocarditis due to α-haemolytic streptococci in those with native valve endocarditis appeared to be associated with known heart disease, natural dentition and recent dental procedures (endocarditis occurred 4.9 times more often in those with all three of these factors compared with those without any),7 while a case-control study identified that any dental procedure (including dental extraction) showed no increased risk with cases compared with controls.8
Studies which considered bacteraemia related to dental procedures (usually at one or two time points following the procedures) identified bacteraemia following many dental procedures (though, in a number of studies bacteraemia was also identified prior to procedures). However, studies which also considered tooth brushing also identified bacteraemia, often considered to be to be of similar or greater intensity to that found following dental procedures.9,10,11,12,13,14,15 It was also noted that cases of infective endocarditis have been documented which have arisen following dental procedures for which antibiotic prophylaxis had been given.7
It was concluded that it was biologically implausible that a single dental procedure would lead to a greater risk of infective endocarditis than regular tooth brushing. Appropriate and prompt antibiotic treatment of oral infections is recommended, since when an oral or tissue infection is present it was considered that repetitive, or continuous bacteraemias from the site would be occurring. Also, if an antibiotic is being prescribed this would cover the oral flora involved and therefore cover any potential infective endocarditis causing organisms form this site.
Evaluation of the evidence on cost-effectiveness
This section includes a brief summary of the relevant cost-effectiveness evidence; for the full evaluation see the NICE guideline.
Published health economics literature
A literature review was conducted to identify cost-effectiveness evidence on antimicrobial prophylaxis against IE in individuals with a predisposing cardiac condition undergoing interventional procedures. A total of five relevant studies were identified that considered both costs and outcomes.16,17,18,19,20 These studies provided contradictory evidence on the cost-effectiveness of antibiotic prophylaxis for at-risk patients undergoing interventional procedures. However, it has been commonly observed that penicillin could result in many more deaths (at least in the short term) secondary to anaphylaxis compared with a strategy of no prophylaxis. In addition, the cost-effectiveness of antibiotic prophylaxis appears to also critically depend on the baseline risk of developing IE. It is not apparent if any of the economic evaluations took into account the recurring risk of IE and the additional future costs of antibiotic prophylaxis.
De novo economic evaluation
Given the lack of up-to-date, UK-relevant analyses, it was considered useful to undertake a de novo analysis. A very simple model was developed to explore the cost-effectiveness of antibiotic prophylaxis for infective endocarditis in adults with predisposing cardiac conditions undergoing dental procedures.
In the model, nine antibiotic prophylaxis options were compared against a strategy of no antibiotic prophylaxis. The prophylactic options explored were those set out in the British National Formulary 54th edition (BNF 54) because they represent current UK practice at the time the guideline was developed. All antibiotic strategies were assumed to be of equal effectiveness. Full details of the process are available in the appendices of the full NICE guideline (http://www.nice.org.uk/CG064).
The model suggests that prophylactic antibiotic strategies are highly cost-ineffective under all scenarios explored in the present analysis unless highly optimistic assumptions are made with regard to a number of parameters, chiefly the risk of developing IE following a dental procedure. Even when optimistic assumptions are made with regard to antibiotic efficacy and the risk of developing IE following a dental procedure, the risk of antibiotic side effects (particularly with respect to amoxicillin-containing strategies) can potentially increase the incremental cost-effectiveness ratios markedly and even lead to greater deaths through fatal anaphylaxis than a strategy of no antibiotic prophylaxis.
Conclusion
In summary, this guideline recommends that antibiotic prophylaxis to prevent IE should not be given to people at risk of IE undergoing dental procedures. The basis to support this recommendation is:
-
There is no consistent association between having an interventional procedure, dental or non-dental, and the development of IE
-
Regular tooth brushing almost certainly presents a greater risk of IE than a single dental procedure because of repetitive exposure to bacteraemia with oral flora
-
The clinical effectiveness of antibiotic prophylaxis is not proven
-
Antibiotic prophylaxis against IE for dental procedures may lead to a greater number of deaths through fatal anaphylaxis than a strategy of no antibiotic prophylaxis, and is not cost-effective.
References
Prendergast B D . The changing face of infective endocarditis. Heart 2006; 92: 879–885.
Brincat M, Savarrio L, Saunders W et al. Endodontics and infective endocarditis – is antimicrobial chemoprophylaxis required? Int Endod J 2006; 39: 671–682.
Durack D T . Prevention of infective endocarditis. N Engl J Med 1995; 332: 38–44.
Pallasch T J . Antibiotic prophylaxis: problems in paradise. Dent Clin North Am 2003; 47: 665–679.
Gould F K, Elliott T S J, Foweraker J et al. Guidelines for the prevention of endocarditis: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2006; 57: 1035–1042.
Wilson W, Taubert K, Gewitz M et al. Prevention of infective endocarditis. Guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116: 1736–1754.
van der Meer J T, Thompson J, Valkenburg H A, Michel M F . Epidemiology of bacterial endocarditis in The Netherlands. II. Antecedent procedures and use of prophylaxis. Arch Intern Med 1992; 152: 1869–1873.
Lacassin F, Hoen B, Leport C et al. Procedures associated with infective endocarditis in adults. A case control study. Eur Heart J 1995; 16: 1968–1974.
Lucas V, Roberts G J . Odontogenic bacteremia following tooth cleaning procedures in children. Pediatr Dent 2000; 22: 96–100.
Lucas V S, Omar J, Vieira A, Roberts G J . The relationship between odontogenic bacteraemia and orthodontic treatment procedures. Eur J Orthod 2002; 24: 293–301.
Roberts G J, Holzel H S, Sury M R, Simmons N A, Gardner P, Longhurst P . Dental bacteremia in children. Pediatr Cardiol 1997; 18: 24–27.
Roberts G J, Simmons N B, Longhurst P, Hewitt P B . Bacteraemia following local anaesthetic injections in children. Br Dent J 1998; 185: 295–298.
Roberts G J, Gardner P, Longhurst P, Black A E, Lucas V S . Intensity of bacteraemia associated with conservative dental procedures in children. Br Dent J 2000; 188: 95–98.
Roberts G J, Jaffray E C, Spratt D A et al. Duration, prevalence and intensity of bacteraemia after dental extractions in children. Heart 2006; 92: 1274–1277.
Peterson L J, Peacock R . The incidence of bacteremia in pediatric patients following tooth extraction. Circulation 1976; 53: 676–679.
Gould I M, Buckingham J K . Cost-effectiveness of prophylaxis in dental practice to prevent infective endocarditis. Br Heart J 1993; 70: 79–83.
Agha Z, Lofgren R P, VanRuiswyk J V . Is antibiotic prophylaxis for bacterial endocarditis cost-effective? Med Decis Making 2005; 25: 308–320.
Caviness A C, Cantor S B, Allen C H, Ward M A . A cost-effectiveness analysis of bacterial endocarditis prophylaxis for febrile children who have cardiac lesions and undergo urinary catheterization in the emergency department. Pediatrics 2004; 113: 1291–1296.
Devereux R B, Frary C J, Kramer-Fox R, Roberts R B, Ruchlin H S . Cost-effectiveness of infective endocarditis prophylaxis for mitral valve prolapse with or without a mitral regurgitant murmur. Am J Cardiol 1994; 74: 1024–1029.
Clemens J D, Ransohoff D F . A quantitative assessment of pre-dental antibiotic prophylaxis for patients with mitral-valve prolapse. J Chronic Dis 1984; 37: 531–544.
Acknowledgements
This article is written by the authors on behalf of the Guideline Development Group, which comprised: Nicholas Brooks, Nick Cooley, Deborah Franklin, Martin Fulford, John Gibb, Anne Keatley-Clarke, Danny Keenan, Richard Oliver, Kathy Orr, Suzannah Power, Jonathan Sandoe and David Wray (chair). The NICE Short Guidelines Technical Team comprised: Lynda Ayiku, Emma Banks, Michael Heath, Roberta Richey, Francis Ruiz and Tim Stokes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wray, D., Ruiz, F., Richey, R. et al. Prophylaxis against infective endocarditis for dental procedures – summary of the NICE guideline. Br Dent J 204, 555–557 (2008). https://doi.org/10.1038/sj.bdj.2008.404
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2008.404
This article is cited by
-
Antibiotic prophylaxis in oral healthcare – the agreement between Swedish recommendations and evidence
British Dental Journal (2010)
-
How confident are general dental practitioners in their decision to administer antibiotic prophylaxis? A questionnaire study
BMC Medical Informatics and Decision Making (2008)
-
Top ten downloaded BDJ papers, January-August 2008
British Dental Journal (2008)
-
Post-NICE 2008: antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia
British Dental Journal (2008)