Abstract
Introduction:
Spinal dural arteriovenous fistulae (sdAVF) are rare lesions. Patients typically present with slowly progressive myelopathy that is often mistaken for degenerative cervical or lumbar stenosis. On spinal magnetic resonance imaging (MRI), multisegmental T2 hyperintensities along with associated flow voids are pathognomonic of sdAVF. However, diagnosis can be difficult. Definitive diagnosis and localization is achieved with complete spinal angiography. Treatment options include open surgical ligation, endovascular embolization or multimodality treatment. The purpose of this study is to present a series of cases to aid in the assessment, diagnosis and treatment of this unusual pathology.
Case presentation:
We present 10 cases of sdAVF treated at our center over an 8-year period. Seventy percent of patients were male. The mean age of presentation was 62.6 years. The most common lesion was a dorsal dural AVF with single feeder. All patients underwent open surgical ligation, six having preoperative coil embolization of the radicular artery to allow for intraoperative localization of the fistula. Eight patients showed improvement following treatment as graded by the Nurick system. Two patients failed to improve. None of the patients worsened. One patient had a radiation burn from the spinal angiogram requiring secondary closure and one patient had a pseudomeningocele at the site of surgery that resolved.
Discussion:
The successful treatment of sdAVF requires a detailed understanding of clinical presentation and imaging findings to allow for precise treatment. Owing to the rarity of the condition, clinicians must continue to share their experiences to advance our knowledge.
Similar content being viewed by others
Introduction
Vascular lesions of the spine are rare, accounting for only 1–2% of vascular neurological pathologies.1,2,3,4 They are divided into arteriovenous fistulae and arteriovenous malformations. They are further classified based on pathophysiology, neuroimaging and neuroanatomy.5,6 Spinal intradural arteriovenous fistulae (sdAVF) are consistently the most common representing up to 80% of lesions identified.3,7,8,9
sdAVF tend to be located dorsally in the low thoracic and lumbar area with 80% occurring between T6 and L2.10 They represent an abnormal connection between a spinal radicular artery and vein, typically at the dural sleeve of the dorsal nerve root. Arterialization of the medullary vein leads to venous congestion in the coronal venous plexus of the spinal cord. As venous pressure increases there is decreased tissue perfusion along with vascular steal, ischemia and in some cases hemorrhage.5,6, 11,12,13
Patients with sdAVF often present with non-specific clinical features that are related to progressive myelopathy. Frequently they are misdiagnosed as having other more common spinal pathologies, which can lead to a significant delay in diagnosis, treatment and poorer prognosis. The average patient will present 1–3 years before the diagnosis is made.14
The fundamental component to diagnosing sdAVF is understanding the radiological features found on MRI and spinal angiography. Although T1 weighted MRI is variable and non-specific, multisegmental spinal cord hyperintensity on T2-weighted images with associated subarachnoid flow voids are pathognomonic of the condition. Spinal angiography confirms and localizes the fistula allowing for definitive management. Open ligation of these lesions has been shown to be 98% successful with endovascular embolization showing variable long-term results.15
We present our series of cases to further aid in the assessment, diagnosis and timely treatment of this unusual pathology. We also highlight a variant of this rare condition to improve early identification and outcomes.
Case presentation
Our local cerebrovascular center database was searched to identify all patients that were treated at our institution from 2004 to 2011. A retrospective chart review was undertaken to document the patient’s clinical presentation, radiographic findings, treatment, neurological outcome and overall lesion classification.
All patients had detailed pre- and postoperative neurological examinations documented on the chart. The patient’s degree of myelopathy was scored using the Nurick grading system, with a higher score indicating greater severity.16,17 The grading system is described in Table 1.
Our database allowed for each of the patient’s complete imaging to be available. All pertinent images, including radiographs, computed tomography (CT), MRI and spinal angiography, were reviewed. Working diagnoses from the clinicians and radiologists throughout the patients’ clinical course were documented.
All treatment regimes were recorded. We specifically identified initial treatment strategies aimed at the patient’s presenting complaints, prior to diagnosis of the vascular lesion, as well as treatment aimed at the vascular lesion, namely embolization and/or open intradural ligation. Intraoperative images were saved for more recent cases once the technology was available. Following operative intervention, each patient was classified based on their lesion type according to the system proposed by Spetzler (Table 2). Outcome was assessed at follow-up using their final Nurick grade.
Between 2004 and 2011, a total of 10 patients were identified who underwent treatment for a spinal vascular lesion at our center and met our inclusion criteria. Seven of the patients (70%) were male with a mean age of 62.6 years at diagnosis. The most common lesion identified was a dorsal intradural fistula between a radicular artery and vein. Several patients underwent placement of an aneurysm micro-coil in the radicular artery. All patients eventually underwent laminectomy and open ligation of the fistula.
The majority of patients presented with slowly progressive lower extremity weakness, ascending sensory changes and variable bowel and bladder involvement. Two patients presented with a subacute presentation that led to an earlier diagnosis. Two other patients presented with an acute-on-chronic decline leading to more aggressive investigation and diagnosis. Further details of clinical presentation are summarized in Table 3. The delay to diagnosis was quite variable ranging from 4 to 146 weeks (mean 55 weeks). The shortest being a 55-year-old male with a 4-week history of progressive numbness and weakness followed by a 24-h rapid deterioration with the inability to ambulate, urinary retention and loss of anal tone. The diagnosis of the lesion was made on initial MRI. The longest delay was a 65-year-old male with a 30-month history of ascending numbness and mild weakness in his lower extremities. Most of our patients had interim diagnosis; two with Brown–Sequard syndrome, one each of progressive multiple sclerosis, acute demyelinating polyneuropathy, cervical radiculopathy, tumor and lumbar spinal stenosis.
All patients eventually had an MRI showing typical features of sdAVF: long multisegment central cord T2 hyperintensities ±dorsal subarachnoid flow voids. Two patients who were initially misdiagnosed had features suggestive of sdAVF when the initial images were reviewed retrospectively. Angiography confirmed and localized the lesion in all patients except for one who required an aortic angiogram and eventually a repeat spinal angiogram to identify a large paraspinal arteriovenous malformation with venous drainage into the spinal canal mimicking an sdAVF. Nine lesions were confirmed as dorsal intradural arteriovenous fistulae with a single feeder (Type Ia). One was eventually diagnosed as an arteriovenous malformation as described above. The majority of the lesions were found in the thoracolumbar region; seven from T6-L2, two above T6 (T1 and T4) and one below L2 (L5). Of note, the three cases outside of T6-L2 were all initially diagnosed with other ailments and one even underwent an unsuccessful lumbar spinal decompression.
Among the 10 patients, six initially underwent endovascular embolization with micro-coils at the time of the spinal angiogram. This was performed to mark the feeding artery and facilitate localization during surgical intervention. One patient had partial embolization due to the anatomy of the feeding vessel and the location of the fistulae. The nine patients with sdAVF underwent successful surgical ligation via laminectomy. The patient with the paraspinal arteriovenous malformation underwent initial attempted surgical ligation as this was thought to be an sdAVF. This was unsuccessful and he subsequently required two endovascular embolization procedures that led to angiographic cure.
Follow-up (median interval of 12 months; range 6–53) showed eight patients improved their Nurick grade; one marked improvement and seven mild-moderate improvements. Two patients showed no improvement at their last follow-up and no patients had neurological worsening. Of note, the one patient who showed marked improvement presented with acute onset was diagnosed on initial presentation and underwent open ligation 3 days later. The two that did not improve had a prolonged (>13 months) and atypical presentation. See Table 4 for details.
Case example
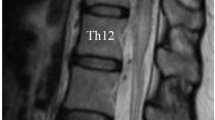
A 55-year-old male presented to physiotherapy with a 3-month history of severe right lower extremity pain, weakness and sensory changes in the buttock and right foot. Ambulation, prolonged standing and valsalva aggravated his pain. He developed voiding difficulties 1-month before assessment. Working diagnosis was neurogenic claudication and referral was made to a spine specialist. Initial MRI showed a T1 hypointense and T2 hyperintense lesion at T12 with expansion and leftward displacement of the conus. Preliminary differential included a primary tumor suggestive of ependymoma or nerve sheath tumor (Figure 1).
One month later, he underwent T10-L2 bilateral laminectomy with biopsy and partial resection of the intramedullary spinal cord tumor. Exposure revealed an intradural arterialized vessel which was protected. The cord lesion was difficult to identify without a discrete tissue plane. Frozen section showed low cellularity. Motor and sensory evoked potentials decreased intraoperative and surgical intervention was discontinued. Final pathology reported necrotic spinal cord parenchyma and increased vascularity with fibrous thickening suggestive of vascular anomaly. Referral was made to neurology and our centers endovascular specialist.
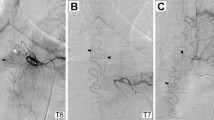
Postoperatively he had significant clinical decline—bilateral lower extremity weakness, bilateral ‘stocking and glove’ and perianal hypoesthesia, urinary incontinence and erectile dysfunction. Following rehabilitation, his strength recovered on the left side and he could ambulate with assistance. His urinary incontinence resolved. A magnetic resonance angiogram 6-months postoperative showed some decrease in the T2 hyperintensity and serpiginous T2 structures from L1-3 that were reported as redundant nerve roots. On review on the initial MRI, subtle dorsal flow voids were noted along with the T2 hyperintensity. Spinal angiography confirmed a thoracic sdAVF at T6. Multiple coils were placed in the T6 intercostal artery for operative localization (Figure 2).
One week following the angiogram, the patient underwent laminectomy with isolation of the sdAVF and ligation (Figure 3). Postoperative MRI showed no residual enhancement of the conus or central cord. The patient developed a radiation burn at the site of the surgery that was initially managed conservatively but required a secondary closure. At the last documented follow-up, he was ambulatory with assistance and had normal bowel and bladder function.
Discussion
Pathophysiology
Spinal vascular lesions, although rare, must be included in the differential for patients with non-specific features of myelopathy. Understanding the key features around patient demographics, presentation and diagnostic features will help improve the diagnosis and management of these patients. The features identified in our case series largely correlate with the findings reported in other studies. Seven (70%) of our patients were male with a mean age at diagnosis of 62.2, the youngest being 51 years. Several studies support these demographics; the general rule being nearly 80% male predominance with diagnosis in the early sixth decade.14,18,19,20,21,22,23 Diagnosis is extremely uncommon in the younger population and is rarely discovered before the age of 30.14,22,24,25 Most studies report one or two patients in their second or third decade.14,19,22
There are no specific characteristics for the clinical presentation of sdAVF. The majority of patients present with myelopathic symptoms and occasionally radiculopathy. This is largely based on the pathophysiology of the lesion. In sdAVF, the fistulae are located lateral to the intervertebral foramen, near the nerve root where there is an intradural connection between the radicular artery and medullary vein. Although many theories exist, it is generally accepted that low-flow arterialization of the medullary veins leads to venous hypertension that is then transmitted to the intradural coronal venous plexus.25,26,27 With increasing pressure, venous engorgement progresses, largely in a caudal to cranial direction, placing pressure on the spinal cord and decreasing the arteriovenous pressure gradient. Cord edema and eventual ischemia/infarction ensues.28 Naturally, this is typically associated with slowly progressive ascending spinal cord pathology.
Clinical presentation and misdiagnosis
As in our series, only a small number of patients present acutely and those that do are often diagnosed sooner than their counterparts.28 The majority progress gradually over time with only 5% presenting acutely and about one-quarter with an acute-on-chronic decline.25 The average delay to diagnosis in our series was 55 weeks. This is correlated with several studies reporting average delays of 1–3 years with as many as 10–34% of patients have taken even longer than 3 years.11,14,18,28,29
Given the age of presentation, many patients will have associated clinical and imaging findings, such as spinal stenosis, disc deterioration, previous surgery and even trauma, that can confuse the diagnosis.19 Other studies have shown mis-attribution to degenerative disc disease, tumors, Guillain–Barré syndrome, amyotrophic lateral sclerosis, peripheral vascular disease and diabetic neuropathy.14,25,27 In our series, we had interval diagnoses of Brown–Séquard syndrome (2), progressive multiple sclerosis (1), acute demyelinating polyneuropathy (1), tumor (1) cervical radiculopathy (1) and lumbar spinal stenosis (1). The patient thought to have a tumor underwent open biopsy and partial resection as described in the case above. The patient diagnosed with lumbar stenosis underwent a two-level laminectomy without clinical improvement and a further 6-month delay to diagnosis of sdAVF.
The concern with such a delay to diagnosis is that many patients develop severe neurological changes including spastic paraplegia, flaccid paralysis and eventually loss of sphincter control.29 These patients tend to show slower and less complete recovery following successful treatment.14 In our series, those who had longer delays to diagnosis and significant preoperative disability (Nurick grade 3–5) improved the least.
Most patients will present with lower extremity sensory disturbances (paresthesia and hypoesthesia) that are often patchy and ill-defined. These changes can be unilateral or bilateral and are often asymmetric.27 Lower extremity weakness is present in varying degrees followed by non-specific back pain and eventual bladder/bowel dysfunction. Given the location of the fistulae, few patients present initially with radicular symptoms and lower motor neuron findings. In our series, 90% of our patients presented with sensory disturbances, 80% with weakness, 50% with non-specific back pain and 40% with bladder/bowel dysfunction. Only 20% had radicular symptoms and 10% pure lower motor neuron findings. These findings are similar to those reported in larger series.14,18,20
Imaging findings
Diagnosis of sdAVF is based on MRI and angiogram. The typical features on MRI include hyperintense centromedullary edema on T2-weighted images, an increase in cord volume due to edema and the presence serpiginous flow voids that represent dilated perimedullary vessels.1,10,11 These findings typically span an average of 5–7 vertebrae and around 80% will involve the conus.30 The length of the signal enhancement does not correlate with clinical findings and it is rare to have a fistula without the associated long segment T2 hyperintensity.30 All of our patients had suggestive MRI features at the time of diagnosis. Central cord enhancement on T2 was found to span between 3 and 10 vertebrae and 7 of these cases included the conus. Flow voids were less frequently reported. Also in accordance with other studies, most of the sdAVF originated from the thoracolumbar region. It is reported that more than 80% are typically located between T6 and L2.29 Only two of our patients had fistulae above T6 and one below L2. As the majority are type I with a single feeder, it is not surprising that seven of the confirmed sdAVF had a single segmental arterial feeding artery and only two demonstrated multiple feeders.
All patients underwent angiography for confirmation and localization. Two patients required repeat angiography for diagnosis and one required multiple attempts due to difficulty identifying the lesion and concern over contrast load.
Treatment
Patients with a confirmed sdAVF require treatment to prevent further neurological deterioration. Although endovascular techniques, such as glue embolization, can initially appear to occlude the fistula, recurrence rates are high.29 To be effective and durable, the venous side of the fistula must be occluded. In our opinion, this exposes that patient to an increased risk of venous thrombosis and subsequent neurologic deterioration.
However, we have been utilizing an endovascular technique to assist with surgical localization. At the conclusion of the angiogram, a platinum radiopaque micro-coil is placed in either the proximal radicular artery or the segmental artery. This allows for simple fluoroscopic identification of the coil after the patient was positioned prone in the operating room. Benefits include reduction in operating time and requirement for a smaller laminectomy. Finally, we maintain that surgical ligation is the preferred treatment modality because of high rates of long-term cure.
Conclusion
All physicians who are involved in the management and diagnosis of patients with spinal disorders must have an excellent understanding of sdAVF. Misdiagnosis and delay in diagnosis is common. A timely diagnosis and treatment likely leads to improved neurological outcomes in select patients.
References
Krings T, Lasjaunias PL, Hans FJ, Mull M, Nijenhuis RJ, Alvares H et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am 2007; 17: 57–72.
Kendall BE, Logue V . Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology 1977; 13: 181–189.
Rashad S, Mohamed AB, Waseem A, Tamer H . Management of spinal dural arterio-venous fistulas. Report of 12 cases and review of literature. Clin Neurol Neurosurg 2014; 125: 81–86.
Merland JJ, Riche MC, Chiras J . Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol 1980; 7: 271–320.
Kim LJ, Spetzler RF . Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery 2006; 59(Suppl 3): S195–S201.
Spetzler RF, Detwiler PW, Riina HA, Porter RW . Modified classification of spinal cord vascular lesions. J Neurosurg 2002; 96: 145–156.
Takai K, Taniguchi M . Comparative analysis of spinal extradural arteriovenous fistulas with or without intradural venous drainage: a systematic literature review. Neurosurg Focus 2012; 32: E8.
Nishio A, Ohata K, Takami T, Nishikawa M, Hara M . Atypical spinal dural arteriovenous fistula with supply from the lateral sacral artery. J Clin Neurosci 2007; 14: 65–68.
Nogueria RG, Dabus G, Rabinov JD, Ogilvy CS, Hirsch JA, Pryor JC . Onyx embolization for the treatment of spinal dural arteriovenous fistulae: initial experience with long-term follow-up: technical case report. Neurosurgery 2009; 64: E197–E198.
Krings T, Geibprasert S . Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 2009; 30: 639–648.
Amanieu C, Hermier M, Peyron N, Chabrol A, Deiana G, Manera L . Spinal dural arteriovenous fistula. Diagn Interv Imaging 2014; 95: 897–902.
Jeng T, Chen DYT, Hsu HL, Huang YL, Chen CJ, Tseng YC . Spinal dural arteriovenous fistula: Imaging features and its mimics. Korean J Radiol 2015; 16: 1119–1131.
Ramanathan D, Levitt MR, Sekhar LN, Kim LJ, Hallam DK, Ghodke B . Management of spinal epidural arteriovenous fistulas: interventional techniques and results. J Neurointerv Surg 2014; 6: 144–149.
Gemmete JJ, Chaudhary N, Elias AE, Toma AK, Pandey AS, Parker RA et al. Spinal dural arteriovenous fistulas: clinical experience with endovascular treatment as a primary therapy at 2 academic referral centers. AJNR Am J Neuroradiol 2013; 34: 1974–1979.
Chibbaro S, Gory B, Marsella M, Tigan L, Herbrecht A, Orabi M et al. Surgical management of spinal dural arteriovenous fistulas. J Clin Neurosci 2015; 22: 180–183.
Kalsi-Ryan S, Singh A, Massicotte EM, Arnold PM, Brodke DS, Norvell DC et al. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013; 38: S111–S122.
Nurick S . The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain 1972; 95: 87–100.
Muralidharan R, Saladino A, Lanzino G, Atkinson JL, Rabinstein AA . The clinical and radiological presentation of spinal dural arteriovenous fistula. Spine (Phila Pa 1976) 2011; 36: E1641–E1647.
Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery 2008; 62: 159–167.
Jellema K, Canta LR, Tijssen CC, van Rooij WJ, Koudstaal PJ, van Gijn J . Spinal dural arteriovenous fistulas: clinical features in 80 patients. J Neurol Neurosurg Psychiatry 2003; 74: 1438–1440.
Behrens S, Thron A . Long-term follow-up and outcome in patients treated for spinal dural arteriovenous fistula. J Neurol 1999; 246: 181–185.
Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC . Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and follow-up in 49 patients. Stroke 2002; 33: 1578–1583.
Donghai W, Ning Y, Peng Z, Shuo X, Xueen L, Peng A et al. The diagnosis of spinal dural arteriovenous fistulas. Spine (Phila Pa 1976) 2013; 38: 546–553.
Nachanakian A, Helou AE, Chedid GA, Alaywan M . Spinal dural arteriovenous fistulas – treatable cause of myelopathy. IntechOpen 2013; chpt 4: 65–84. Retrieved from http://dx.doi.org/10.5772/56725.
Fugate JE, Lanzino G, Rabinstein AA . Clinical presentation and prognostic factors of spinal dural arteriovenous fistulas: an overview. Neurosurg Focus 2012; 32: E17.
Aminoff MJ, Barnard RO, Logue V . The pathophysiology of spinal vascular malformations. J Neurol Sci 1974; 23: 255–263.
Marcus J, Schwarz J, Singh IP, Sigounas D, Knopman J, Gobin YP et al. Spinal dural arteriovenous fistulas: a review. Curr Atheroscler Rep 2013; 15: 335.
Rubin MN, Rabinstein AA . Vascular diseases of the spinal cord. Neurol Clin 2013; 31: 153–181.
Xiangqian Q, Liquan L, Kaiwei H, Zheng X, Qiyong M, Huairui C et al. Analysis of the embolization spinal dural arteriovenous fistula and surgical treatments on 52 cases of the patients. Int J Clin Exp Med 2014; 7: 3062–3071.
Morris JM . Imaging of dural arteriovenous fistula. Radiol Clin North Am 2012; 50: 823–839.
Acknowledgements
There was no sponsorship or funding for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fox, S., Hnenny, L., Ahmed, U. et al. Spinal dural arteriovenous fistula: a case series and review of imaging findings. Spinal Cord Ser Cases 3, 17024 (2017). https://doi.org/10.1038/scsandc.2017.24
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.24
This article is cited by
-
Concomitant spinal dural arteriovenous fistula and nodular fasciitis in an adolescent: case report
BMC Pediatrics (2022)
-
A 72-year-old man with fatigable leg weakness: think out of the box
Acta Neurologica Belgica (2022)