Abstract
Introduction:
More than 30 years ago, functional electrical stimulation (FES) was developed as an orthotic system to be used for rehabilitation for SCI patients. In the present case report, FES-assisted training was combined with continuous low-frequency stimulation of the pelvic somatic nerves in a SCI patient.
Case Presentation:
We report on unexpected findings in a 41-year-old man with chronic complete flaccid paraplegia, since he was 18 years old, who underwent spinal stem cell therapy and a laparoscopic implantation of neuroprosthesis (LION procedure) in the pelvic lumbosacral nerves. The patient had complete flaccid sensomotoric paraplegia T12 as a result of a motor vehicle accident in 1998. In June 2011, he underwent a laparoscopic implantation of stimulation electrodes to the sciatic and femoral nerves for continuous low-frequency electrical stimulation and functional electrical stimulation of the pelvic nerves. Neither intraoperative direct stimulation of the pelvic nerves nor postoperative stimulation induced any sensation or muscle reactions. After 2 years of passive continuous low-frequency stimulation, the patient developed progressive recovery of electrically assisted voluntary motor functions below the lesions: he was first able to extend the right knee and 6 months later, the left. He is currently capable of voluntary weight-bearing standing and walking (with voluntary knee movements) about 50 m with open cuff crutches and drop foot braces.
Discussion:
Our findings suggest that continuous low-frequency pelvic nerve stimulation in combination with FES-assisted training might induce changes that affect both the upper and the lower motor neuron and allow supra- and infra-spinal inputs to engage residual spinal and peripheral pathways.
Similar content being viewed by others
Introduction
In the last several decades, acute and long-term survival rates for persons with spinal cord injury (SCI) have improved dramatically. The functional outcome has been more static, and shared evidence from several research disciplines may point the way forward. The procedure of functional electrical stimulation (FES) is an approach that was developed more than 30 years ago as an orthotic system for SCI patients. The primary goal of FES is to induce immediate contraction of the skeletal muscles to achieve functional movements. Recent case studies suggest that electrical stimulation may also be used for restoring voluntary control of locomotion in motor-complete paraplegia.1–3 Here we report on an unexpected clinical improvement in a chronic complete flaccid SCI man who presents enough recovery of voluntary hip flexion and knee extension for voluntary-assisted standing and stepping after spinal stem cell therapy and continuous low-frequency stimulation of the lumbosacral nerves. To the best of our knowledge, this is the first report of such an observation in literature.
Materials and methods
We report about a single chronic SCI 41-year-old patient who was hit by a motor vehicle in 1998. In April 2010, the patient underwent a posterior microsurgical application of autologous adult stem cells (5 830 000 CD34+ HCP stem cells with a viability of 85.73%) to the spinal cord lesion and was placed under general anesthesia during the application. In October of that year, he was reinjected with 1 586 000 CD34+ stem cells with a viability of 97.81% via lumbar puncture after he had been given a local anesthetic. Both applications were done extra muros. The patient had been presented to our department as candidate for participation in a clinical study that focused on laparoscopic implantation of neuroprothesis on the pelvic nerves for functional recovery. This study was approved by the Ethics Committee of the University of Zurich before the recruitment of patients was begun. Previous neurologic examination by independent investigators confirmed the established diagnosis of complete flaccid sensorimotor SCI level T12, AIS A, without any zone of partial preservation (ZPP), with severe muscle atrophy. The ASIA Lower Extremity Motor Score was 0 and the ASIA sensory score for both light touch and pin-prick sensation was 76. Despite different intensive re-education programs and the strong motivation of the patient, not any improvement in the ASIA sensorimotor scores was observed after the time of the primary injury. Because of the type of flaccid of the lesion (severe damage or even complete destruction of the lower motor neurons), we explained to the patient that stimulation of the pelvic nerves would not be able to induce any skeletal muscle activation and rejected him for the study. The patient insisted strongly and asked explicitly for the procedure.
Operative technique
After the patient signed informed consent form for surgery and permissions (for case details, personal information and images), he underwent the LION procedure in June 2011 in accordance with the technique described previously:4 The procedure consisted of the implantation, by laparoscopy, of fine wire (designed for spinal cord stimulation or for sacral nerve root stimulation) in direct contact to the endopelvic portion of the somatic pelvic nerves for electrical stimulation or neuromodulation. An epidural spinal cord stimulation unit (Restore ADVANCED, Medtronic, Minneapolis, MN, USA) and four 4-arrays fine wire electrodes were implanted to the sciatic, pudendal and femoral nerves according to our surgical technique described previously. The procedure was performed under general anaesthesia. Laparoscopic exposure of the endopelvic portion of both the sciatic and of the pudendal nerves was obtained by passing laterally to the external iliac vessels through the lumbosacral space and gentle detachment of the inter-iliac lymph-fat-tissue from the pelvic sidewall to avoid lymphocele. Multiple channel electrodes enabled stimulation of both nerves with only one lead electrode. Exposure of the femoral nerves was also obtained by a transperitoneal approach behind the major psoas muscle. Before implantation, the electrical stimulability of the nerves was tested by direct intraoperative laparoscopic electrical stimulation. Four lead electrodes were implanted and attached by suturing them to perineural tissues. The attached electrodes were passed through the pelvic wall and finally connected to a pulse generator implanted subcutaneously into the anterior abdominal wall.
Results
Laparoscopic implantation of electrode leads to the sciatic and femoral nerves did not present any difficulties. Intraoperative stimulation of the nerves5 did not induce any skeletal muscle reaction at all. The duration of the procedure was 104 min. No pre- or postoperative complications occurred. The patient was discharged on the second postoperative day. Postoperative pelvic nerve stimulation failed to induce any muscles reaction at all, even with a current of 10 V. Continuous bilateral sciatic and femoral nerve stimulation was started with a current arbitrarily fixed at 5 Hz/60μs/2 V. In addition to the continuous low-frequency stimulation, three further programs for femoral nerve stimulation were installed, one for each leg separately and one for both legs together. These stimulation parameters were also fixed arbitrarily at 150 Hz/60μs/5 V. The patient quickly became familiar with the electric stimulation unit and could switch between the different programs. Behind permanent low-frequency stimulation of the nerves, the patient started with 5 V-stimulation electrical stimulation 3–5 times per week at home. To eventually improve the control and harmony of possible neural connections, we advised the patient to concentrate on possible movements with the use of mental imagery when the ‘training programs’ were activated.
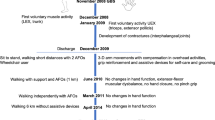
After 6 months, no changes had occurred: stimulation of the pelvic nerves failed to induce any sensory or motor reactions. One and half year after implantation, the patient started to develop some visible electrically induced contraction of the knee extensors. At the 2-year follow-up, the patient became capable of electrically assisted voluntary (fixed current at 1.5 V/60μs/15 Hz) full extension of the right knee against gravity and some resistance, and contraction of the left quadriceps is enough for minimal extension of the knee against gravity. At this point, he started with daily at-home FES-exercises that consisted of four sets of 10 electrically induced knee extensions at a 5:5 s (work/rest ratio) with a 3 min recovery interval between sets, repeated several times throughout the day. Knee extension was performed with the subject seated in a chair. Current from the stimulator was manually increased in 2–3 s intervals to evoke full knee extension followed by eccentric actions. At the same time, the patient started with voluntary extension of the knees: using the remoter; he fixed the current at a level that enabled only 45° extension of the knees (1 V/60 μs/15 Hz), and completed the full extension of the knees by himself, in front of a mirror, without increasing the electrical stimulation’s parameters.
After a period of 2.5 years, he became capable of complete voluntary extension of the knees against resistance (from the null position to full extension) assisted by constant femoral nerve stimulation fixed at 0.5 V/60 μs/15 Hz (Figure 1). He also started with assisted training for standing and finally walking, first using a bar table, then a walker. The configuration of the pacemaker was programed such that modulation of the stimulation current could be controlled by the patient to obtain optimum efferent patterns for standing and walking at home.
Temporal changes in the patients’ ASIA Lower Extremity Motor Scores (LEMS)6 are reported in Table 1.
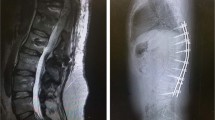
At the time of writing this manuscript (36 months post-OP), the patient gained a control of voluntary extension of both knees enough for standing and walking for 30 meters using minimal stimulation (10 Hz/60 μs/0.3 V right-0.5 V left) without a blockade of the knee in extension (active voluntary extension while stepping), with drop foot braces and open cuff crutches (Figure 2). The motor changes of the patient are even more surprising if one considers the actual MRI scan of the spinal cord (Figure 3).
Discussion
We reported previously on unexpected sensory and locomotor recovery in four paraplegics with SCI.3 The findings at that time suggested that FES-assisted locomotor training and continuous low-frequency pelvic nerve stimulation in SCI patients may induce changes that affect the central pattern generator and allow supra- and infraspinal inputs to engage residual spinal pathways. In this paper, we report about a chronic (18 years after SCI) complete paraplegic man who achieved some progressive recovery of voluntary motor functions in the lower extremities after continuous low-frequency pelvic nerve stimulation and FES-assisted reeducation. The particularity in this patient is that he was not affected by a spastic paraplegia, but presented a T12 complete-flaccid paraplegia. It is well known that nerve stimulation in SCI people really only works for upper motor neuron injuries where there is already some kind of spasticity present; low motor neurons are then more or less still intact and can be excited by electrical stimulation. Stimulation of the pelvic nerves enable such patients some re-education with FES-controlled eccentric muscle contractions. In lower motor neuron injuries, the axon hillock is injured so, potentially, no action can be created and electrical stimulation is unable to induce muscle contraction. The improvement observed in our patient may indicate not only some reconnection or reactivation of neuronal pathways between both the spinal levels, above and below the SCI (that is, between the upper to the lower motor neuron) but also some reconnection of the spinal cord to the peripheral skeletal muscles. This, in turn, supposes some regeneration or a kind of reactivation of the lower motor neuron with axonal growth down to the muscles. The fact that no recovery had occurred in 18 years, despite constant reeducation represents a clinical argument in favor of a potential effect of continuous low-frequency stimulation of the affected/destroyed lower-motor neurons. Also, no other improvement had occurred after stem cell transplantation and probably, at time of the LION procedure, the transplanted cells were already dead. Apparently, FES-assisted reeducation did not have a primary role in the early recovery since it could be applied only after 2 years of continuous low-frequency stimulation to the sciatic and the femoral nerves.
Continuous low-frequency stimulation could correspond to a permanent ‘reeducation of afferent fibers.’ Low-frequency stimulation might produce afferent stimuli without the need for active participation of the patient. In support, histological analysis and measurement of regeneration showed that low-frequency stimulation had a more successful outcome than high-frequency stimulation on the regeneration of damaged nerves.7 Continuous stimulation creates, in turn, a ‘continuous need for a reaction/adaptation process to new challenges’ that may also support a process of regeneration or reconnection. Electrical stimulation promotes not just the direction of axonal growth.8 It is also an attractive guiding cue for promoting axonal regeneration in central and peripheral nerve injury repair.9,10 It has been demonstrated that axonal regeneration and remyelination of the regenerated axons in large nerve defects are significantly enhanced by electrical stimulation also when the stimulation is applied to conductive scaffold; both motor and sensory functional recovery is significantly improved and muscle atrophy is partially reversed by stimulation localized at the conductive scaffold.11
Nevertheless, strong-will, high motivation and active participation of the patient are sine qua non-conditions that produce strong ‘efferent stimuli’ down to the level of SCI.
The effect of the presented therapeutic concept of functional recovery was only investigated by clinical observations, but clinical outcome is, in fact, the end point for the affected people and the highest level of evidence of every research project. Such spectacular motor recovery may enable us to postulate the hypotheses that continuous low-frequency stimulation of the lower motor neurons has the possibility of providing a neural plasticity that obviously may result in some cure of the patient’s flaccid SCI.
References
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011; 377: 1938–1947.
van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012; 336: 1182–1185.
Possover M . Recovery of sensory and supraspinal control of leg movement in people with chronic paraplegia: a case series. Arch Phys Med Rehabil 2014; 95: 610–614.
Possover M, Schurch B, Henle KP . New pelvic nerves stimulation strategy for recovery bladder functions and locomotion in complete paraplegics. Neurourol Urodyn 2010; 29: 1433–1438.
Possover M . Laparoscopic exposure and electrostimulation of the somatic and autonomous pelvic nerves: a new method for implantation of neuroprosthesis in paralysed patients? Minim Invasive Ther Allied Technol 2004; 1: 87–90.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221.
Lu MC, Ho CY, Hsu SF, Lee HC, Lin JH, Yao CH et al. Effects of electrical stimulation at different frequencies on regeneration of transacted peripheral nerve. Neurorehabil Neural Repair 2008; 22: 367–373.
McCaig CD, Sangster L, Stewart R . Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev Dyn 2000; 217: 299–308.
Al-Majed AA, Neumann CM, Brushart TM, Gordon T . Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 2000; 20: 2602–2608.
Chang YJ, Hsu CM, Lin CH, Lu MS, Chen L . Electrical stimulation promotes nerve growth factor-induced neurite outgrowth and signaling. Biochim Biophys Acta 2013; 1830: 4130–4136.
Huang J, Lu L, Hu X, Ye Z, Peng Y, Yan X et al. Electrical stimulation accelerates motor functional recovery in the rat model of 15 mm sciatic nerve gap bridged by scaffolds with longitudinally oriented micro-channels. Neurorehab Neural Re 2010; 24: 736–745.
Acknowledgements
We thank the research patient whose dedication, motivation and perseverance made these findings possible.
Author information
Authors and Affiliations
Contributions
MP and AF designed the study. MP did the surgical implantation of electrodes, collected the data and developed the figures. MP and AF designed the data collection system, supervised the study, interpreted the data and wrote the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
MP has a US patent for the LION procedure. AF declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Possover, M., Forman, A. Recovery of supraspinal control of leg movement in a chronic complete flaccid paraplegic man after continuous low-frequency pelvic nerve stimulation and FES-assisted training. Spinal Cord Ser Cases 3, 16034 (2017). https://doi.org/10.1038/scsandc.2016.34
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2016.34