Abstract
Study design:
Prospective controlled before-and-after study.
Objectives:
To investigate the effects of antimuscarinic treatment of neurogenic lower urinary tract dysfunction on the cognition of individuals with spinal cord injury (SCI) during the early post-acute phase.
Setting:
Single SCI rehabilitation center.
Methods:
Patients with acute traumatic SCI admitted for primary rehabilitation from 2011 to 2015 were screened for study enrollment. Study participants underwent baseline neuropsychological assessments prior to their first urodynamic evaluation (6–8 weeks after SCI). Individuals suffering from neurogenic detrusor overactivity received antimuscarinic treatment, and those not requiring antimuscarinic treatment constituted the control group. The neuropsychological follow-up assessment was carried out 3 months after the baseline assessment. The effects of group and time on the neuropsychological parameters were investigated.
Results:
The data of 29 individuals were evaluated (control group 19, antimuscarinic group 10). The group had a significant (P≤0.033) effect on immediate recall, attention ability and perseveration. In the control group, individuals performed significantly (P≤0.05) better in immediate recall both at baseline (percentile rank 40, 95% CI 21–86 versus 17, 95% CI 4–74) and follow-up (percentile rank 40, 95% CI 27–74 versus 16, 95% CI 2–74). The time had a significant (P≤0.04) effect on attention ability, processing speed, word fluency and visuospatial performance. The individuals in both groups performed better at the follow-up compared to the baseline assessment.
Conclusion:
Even though, we did not observe cognitive deterioration in the investigated, cognitively intact SCI individuals during the first 3 treatment months, the concerns regarding deleterious effects of antimuscarinics on cognition remain.
Similar content being viewed by others
Introduction
The majority of individuals with spinal cord injury (SCI) suffer from neurogenic lower urinary tract dysfunction (NLUTD). Individuals with suprasacral SCI may incur neurogenic detrusor overactivity and detrusor-sphincter dyssynergia.1 These alterations may lead to incontinence, urinary retention and elevated bladder pressure during urine storage and voiding. Subsequently, increased intravesical pressure and/or vesicoureteral reflux may threaten the upper urinary tract. The focus of bladder management is therefore on achieving adequate drainage (for example, intermittent catheterization) as well as low bladder pressure during urine storage and voiding using antimuscarinic drugs.1, 2, 3 The efficacy of antimuscarinic drugs for the long-term treatment of NLUTD is well established.1 However, SCI individuals often require higher doses of antimuscarinic drugs than those with idiopathic detrusor overactivity, which may lead to more or more severe adverse effects.4
Muscarinic receptors are widespread throughout the body, and thus, antimuscarinic treatment may give rise to a variety of adverse effects. Peripheral effects include constipation, dry mouth, dry eyes and tachycardia, whereas central effects include agitation, confusion, delirium and cognitive dysfunction.5, 6, 7, 8 Particularly older individuals have an increased risk of cognitive deterioration with antimuscarinic treatment.7, 8 The antimuscarinic treatment for NLUTD in SCI individuals starts at a relatively young age and commonly lasts life-long. Thus, cognitive deterioration in SCI individuals with antimuscarinic treatment is a concern. However, the data on the effects of antimuscarinic treatment on cognitive function in individuals with NLUTD are very limited.9, 10
The aim of the present study was thus to investigate the effects of antimuscarinic treatment of NLUTD on the cognition of individuals with SCI during the early post-acute phase. The following hypothesis was tested: the neuropsychological performance of SCI individuals with NLUTD decreases significantly under antimuscarinic treatment during the early post-acute phase (until 3 months after injury).
Materials and methods
This prospective cohort study had been approved by the competent ethics committee (KEK LU 11060) and registered with ClinicalTrials.gov (NCT01600404). All applicable institutional and governmental regulations were followed. All study participants gave written informed consent, and all data were encrypted and kept confidential. No financial or other support had been received for this study.
Study participants and protocol
The admission list of a single SCI rehabilitation center (150 in-patients) was screened weekly from 01 November 2011 to 30 June 2015 for patients with acute traumatic SCI being admitted for primary rehabilitation within 8 weeks after SCI. In order to exclude other factors affecting the results of the neuropsychological assessments, the following exclusion criteria were applied: age younger than 18 or older than 65 years, diagnosed head or brain injury, dementia or impaired cognitive function, psychological disorders, abuse of substances with central nervous effect and antimuscarinic treatment. In order to ensure the fitness of the investigated individuals to undergo the neuropsychological assessments, the following exclusion criteria were applied: neurological lesion level above C5 (5th cervical level according to the International Standards for Neurological Classification of Spinal Cord Injury), severe comorbidities, color blindness or impaired vision and insufficient German language skills. Furthermore, upon receipt of written consent, study participant completed the Beck Depression Inventory II (BDI-II) and a pain questionnaire (modified International Spinal Cord Injury Pain Basic Data Set) (secondary outcome measures). Study participants suffering from moderate to severe depression (BDI-II score >19) or pain (average pain during last 7 days >5/10) were excluded.
Enrolled study participants underwent the baseline neuropsychological assessment prior to their first urodynamic evaluation, which usually takes place 6–8 weeks after SCI. Study participants suffering from neurogenic detrusor overactivity or detrusor-sphincter dyssynergia were started on antimuscarinic treatment according to the guidelines of the European Association of Urology.1 Individuals not requiring antimuscarinic treatment constituted the control group. The neuropsychological follow-up assessment was carried out 3 months after the baseline assessment.
Patient characteristics, the type of NLUTD, the current bladder evacuation method and the administration of drugs with antimuscarinic properties were collected from the medical records (secondary outcome measures). Using the composite reference rating scale (low, moderate, high) collated by Salahudeen et al.,11 the antimuscarinic effect of concomitant medication (indications other than NLUTD) during the course of the study was assessed (antimuscarinic burden) (secondary outcome measure).
Neuropsychological assessment
The neuropsychological assessments were performed using the following tests (primary outcome measures): California verbal learning test, d2 test of attention, stroop test, thurstone word fluency test, visuospatial performance subtests from the Hamburg-Wechsler-Adult-Intelligence-Test and the Gestalt closure test from the Kaufmann Assessment Battery as well as the divided attention subtest from the test battery of Zimmermann and Fimm. The divided attention test was performed on a computer. The assessors were blinded regarding the group allocation of the study participants. If available, parallel test versions were used for the follow-up assessments. All used tests were validated German versions. The percentile rank cutoff value for normal performance in the neuropsychological assessments was set at 16.12
Statistical analyses
A sample size of 20 in each group was determined to be sufficient to detect a difference of >1 s.d. in the neuropsychological test parameters between the two groups in the presence of a 50% variance with a power of 80% and a significance level of 5%. A change of >1 s.d. is considered a clinically relevant change in neuropsychological testing.12, 13
According to international recommendations, injury level and severity were classified into four groups: (1) American Spinal Injury Association Impairment Scale (AIS) D, (2) AIS A-C paraplegia, (3) AIS A-C low-level tetraplegia (C8-C5) and (4) AIS A-C high-level tetraplegia (C4-C1).14 Based on their education level, the study participants were categorized into three groups using the International Standard Classification of Education (ISCED): (1) ISCED level 2 (lower secondary education), (2) ISCED level 3 (upper secondary education) and (3) ISCED level 4–8 (tertiary education). The antimuscarinic burden of concomitant medication was determined for each study participant by calculating the sum of the composite rating scales (low=1, moderate=2, high=3).11
The group assignment was also blinded for the statistical analyses. The median and 95% confidence intervals or proportions were calculated. The differences in the proportions between the groups were tested using Fisher’s exact test. The effects of group assignment (antimuscarinics/control) and time (baseline/follow-up 3 months) on the neuropsychological parameters were investigated using the method described by Brunner et al.15 for the nonparametric (rank-based) analysis of longitudinal data in factorial designs. The Wilcoxon rank-sum test was used to investigate the differences between the groups at the specific time points. The statistical analyses were performed using the R software environment (version 3.3.0, Copyright 2016, The R Foundation for Statistical Computing) and the package.nparLD’.16 A P-value of ⩽0.05 was considered significant.
Results
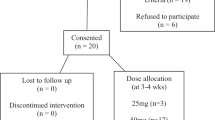
A total of 250 patients with acute traumatic SCI who were admitted for primary rehabilitation were screened for study enrollment. In 191 patients, at least one exclusion criterion was present (Table 1). Written informed consent was denied by 27 patients, and thus, 32 patients were enrolled in the study. The recruitment of study participants was stopped before the full number was enrolled, because of the low recruitment rate. One study participant did not complete the neuropsychological follow-up assessment because of severe pain (control group), and one participant in each group was excluded as a result of mild traumatic brain injury, which had been diagnosed after study enrollment. Thus, the data of 29 individuals were evaluated (control group 19, antimuscarinic group 10). Their characteristics are presented in Table 2. There was no significant (P⩾0.09) difference between the groups regarding gender distribution, age, SCI severity, education level, antimuscarinic burden, BDI-II score, pain and evaluation time points.
The individuals in the antimuscarinic group were treated with solifenacin (10 mg once daily, n=7), fesoterodine (8 mg once daily, n=2) or darifenacin (15 mg once daily, n=1) as a result of neurogenic detrusor overactivity (n=10). The type and dose of antimuscarinic mediation did not change during the course of the study. The concomitant medication with antimuscarinic effects is presented in Table 3. All individuals in the antimuscarinic group used intermittent catheterization for bladder evacuation; whereas in the control group, 11 individuals emptied the bladder with intermittent catheterization and 8 spontaneously.
The median percentile ranks of the assessed neuropsychological parameters were all higher than or equal to 16 (cutoff value for normal performance) in both groups and at both assessment time points (Table 4). The group assignment (antimuscarinics/control) had a significant (P⩽0.033) effect on immediate recall during verbal learning testing, attention ability and perseveration during word fluency testing (non-parametric variance analysis). Post hoc analyses revealed that the individuals in the control group performed significantly (P⩽0.05) better in the immediate recall testing compared to those in the antimuscarinic group both at baseline and follow-up (Table 4). There were no other significant differences in the neuropsychological parameters between the two groups.
The individuals in both groups performed better at the follow-up compared to the baseline assessment. The time (baseline/follow-up 3 months) had a significant (P⩽0.04) effect on attention ability, processing speed, word fluency and visuospatial performance (non-parametric variance analysis) (Table 4).
Discussion
We have investigated the effects of antimuscarinic treatment of NLUTD on the cognition of individuals with SCI during the early post-acute phase. The hypothesis, that the neuropsychological test results of the investigated SCI individuals with NLUTD decrease significantly under antimuscarinic treatment, was rejected. The median percentile ranks of the assessed neuropsychological parameters were all higher than or equal to the cutoff value (that is, 16) for normal performance. The individuals in both groups performed better at the follow-up compared to the baseline assessment.
Non-parametric variance analysis revealed that the group assignment had a significant effect on immediate recall during verbal learning testing, attention ability and perseveration during word fluency testing. However, only immediate recall performance was significantly different between the two groups. The individuals in the control group performed better in immediate recall both at baseline and follow-up. In the antimuscarinic group, the poor performance of one individual had a great effect on the California verbal learning test results due to the small sample size (n=10). Sakakibara et al.9 investigated the effect of imidafenacin on cognitive function in patients with a mean age of 70 years, suffering from neurogenic overactive bladder. They did not observe any changes in cognitive function 3 months after the administration of imidafenacin. Similarly, Veenboer et al.10 did not detect differences between the behavior of children with neurogenic bladder and long-term antimuscarinic treatment and that of children without antimuscarinic medication. However, in older individuals with non-neurogenic overactive bladder, oxybutynin was reported to cause cognitive deterioration.17, 18, 19 The memory deterioration caused by oxybutynin was comparable to brain aging of 10 years,18 whereas darifenacin, solifenacin and fesoterodine showed no effect on cognitive function.18, 19, 20, 21 In the present study, solifenacin, fesoterodine or darifenacin were used to treat neurogenic detrusor overactivity. The differences in cognitive function seem to be due to differences in the binding affinity to muscarinic receptors between the antimuscarinic drugs and the ability to cross the blood-brain barrier.5, 22, 23 The M3 and M2 receptors are involved in bladder contraction and relaxation, respectively, whereas M1 receptors in the brain play an important role in cognition.23 In general though, cognitive deterioration does not seem to be characteristic for antimuscarinic drugs used to treat NLUTD. However, in older individuals with a great antimuscarinic burden due to poly-medication, the detrimental effect of antimuscarinic treatment on cognitive function is well documented.7, 8 In the present study, the antimuscarinic burden of the evaluated individuals was low to moderate.
The evaluated individuals generally performed better in the follow-up tests compared to the baseline assessment. This was observed in both the control and the antimuscarinic group. The time interval between the two assessments was 3 months, and parallel test versions were used, if available. However, practice effects are not completely avoidable in longitudinal neuropsychological assessments.24 After 3 months, study participants most likely did not remember the questions or specific tasks and their original answers, however they were familiar with the tests at the follow-up assessment. Even if the neuropsychological tests are completely different between two assessment time points, individuals acquire knowledge of how to respond to tests and thus, perform better during the subsequent assessment (test sophistication).25
Even modest cognitive impairment has a negative effect on rehabilitation outcomes.26, 27 Cognitive impairment interferes with a patient’s ability to compensate for restrictions or to acquire new skills and thus, rehabilitation strategies and measures need to be adjusted or intensified accordingly. In SCI patients during primary rehabilitation, cognitive impairment may result from brain injury, psychological stress or disorders, pain or neurodegenerative processes in the brain.28, 29, 30, 31 Furthermore, poly-medication due to pain, depression, spasticity and NLUTD, which is common in SCI patients, may increase the antimuscarinic burden and lead to cognitive impairment, especially in older patients.7, 8 Older individuals are more vulnerable to cognitive deterioration due to antimuscarinic bladder treatment, because of the decrease in cholinergic activity in the brain with increasing age.23 In previous investigations, the effects of antimuscarinic bladder treatment on cognition have been investigated mainly in older individuals.9, 22, 23 However, no age-stratified analyses have been performed. Based on the present results, cognitive deterioration does not seem to be an issue in the treatment of NLUTD in younger patients with currently used antimuscarinic drugs during primary rehabilitation,4 if the antimuscarinic burden of the concomitant medication is low. Nevertheless, the possibility of cognitive deterioration in the wake of antimuscarinic medication in SCI individuals should not be excluded from consideration. The antimuscarinic treatment of NLUTD in affected SCI individuals lasts life-long, and thus, the long-term effect of antimuscarinic treatment of NLUTD on cognition needs to be investigated in future studies.
The non-randomized treatment assignment pertains to the limitations of the present study and may have resulted in a selection bias. However, the baseline patient characteristics showed no relevant differences between the two evaluated groups. We decided to stop the recruitment of study participants before the full number was enrolled, because of the low recruitment rate. The low recruitment rate was the result of the stringent exclusion criteria, which had been applied to exclude other relevant factors affecting the results of the neuropsychological assessments apart from the AM treatment of NLUTD. Thus, the statistical power was too low for some differences in the neuropsychological assessments between groups or time points to reach significance. However, the outcome parameters demonstrated a general trend of improvement over time, and our concern was cognitive impairment as a result of the antimuscarinic treatment of NLUTD. Furthermore, differences in the baseline characteristics between the two groups may have not been statistically significant as a result of the small sample size. However, the results showed no relevant differences between the groups. The post hoc tests were not corrected for type 1 errors, which may have resulted in false positive results. However, our concern was cognitive impairment, and there was a general trend of cognitive improvement at follow-up. A further limitation of the present study is that the majority of study participants in both groups were treated with concomitant medication with antimuscarinic effects. However, there was no difference in the number of concomitant drugs with antimuscarinic effects or the antimuscarinic burden between the two investigated groups. The concomitant medication with antimuscarinic effects as well as the antimuscarinic treatment of NLUTD did not have a negative effect on the cognitive abilities of the evaluated individuals. Furthermore, we have investigated the effects of antimuscarinic treatment of NLUTD on the cognition of individuals with SCI during the first 3 months of treatment. Thus, we cannot draw any conclusions regarding the long-term effect of antimuscarinic treatment of NLUTD on cognition. However in previous studies, negative effects of antimuscarinic medication on cognition were observed within 3 weeks of treatment.17, 18, 19, 20
Conclusions
Even though, we did not observe cognitive deterioration in the investigated, cognitively intact SCI individuals with antimuscarinic treatment of NLUTD during the first 3 months of treatment, the concerns regarding deleterious effects of antimuscarinics on cognition remain and need to be investigated in future studies.
Data Archiving
There were no data to deposit.
References
Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol. 2016; 69: 324–333.
Burns AS, Rivas DA, Ditunno JF . The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine 2001; 26: S129–S136.
Wyndaele JJ . The management of neurogenic lower urinary tract dysfunction after spinal cord injury. Nat Rev Urol 2016; 13: 705–714.
Kessler TM, Bachmann LM, Minder C, Lohrer D, Umbehr M, Schunemann HJ et al. Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach. PLoS ONE 2011; 6: e16718.
Chancellor M, Boone T . Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neurosci Ther 2012; 18: 167–174.
Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res 2016; 28: 25–35.
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73: 721–732.
Ruxton K, Woodman RJ, Mangoni AA . Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol 2015; 80: 209–220.
Sakakibara R, Tateno F, Yano M, Takahashi O, Sugiyama M, Ogata T et al. Imidafenacin on bladder and cognitive function in neurologic OAB patients. Clin Auton Res 2013; 23: 189–195.
Veenboer PW, Huisman J, Chrzan RJ, Kuijper CF, Dik P, de Kort LM et al. Behavioral effects of long-term antimuscarinic use in patients with spinal dysraphism: a case control study. J Urol 2013; 190: 2228–2232.
Salahudeen MS, Duffull SB, Nishtala PS . Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015; 15: 31.
Heaton RK, Miller SW, Taylor MJ, Grant I . Revised Comprehensive Norms for an Expanded Halstead Reitan6 Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Inc: Lutz. 2004.
Binder LM, Iverson GL, Brooks BL . To err is human: ‘abnormal’ neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol 2009; 24: 31–46.
DeVivo MJ, Biering-Sorensen F, New P, Chen Y . Standardization of data analysis and reporting of results from the International Spinal Cord Injury Core Data Set. Spinal Cord 2011; 49: 596–599.
Brunner E, Domhof S, Langer F . Nonparametric Analysis Of Longitudinal Data In Factorial Experiments. Wiley: New York. 2002.
Noguchi K, Gel YR, Brunner E, Konietschke F . nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Soft 2012; 50: 1–23.
Katz IR, Sands LP, Bilker W, DiFilippo S, Boyce A, D'Angelo K . Identification of medications that cause cognitive impairment in older people: the case of oxybutynin chloride. J Am Geriatr Soc 1998; 46: 8–13.
Kay G, Crook T, Rekeda L, Lima R, Ebinger U, Arguinzoniz M et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006; 50: 317–326.
Wesnes KA, Edgar C, Tretter RN, Bolodeoku J . Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10 mg. Expert Opin Drug Saf 2009; 8: 615–626.
Lipton RB, Kolodner K, Wesnes K . Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol 2005; 173: 493–498.
Kay GG, Maruff P, Scholfield D, Malhotra B, Whelan L, Darekar A et al. Evaluation of cognitive function in healthy older subjects treated with fesoterodine. Postgrad Med 2012; 124: 7–15.
Wagg A, Verdejo C, Molander U . Review of cognitive impairment with antimuscarinic agents in elderly patients with overactive bladder. Int J Clin Pract 2010; 64: 1279–1286.
Abrams P, Andersson KE . Muscarinic receptor antagonists for overactive bladder. BJU Int 2007; 100: 987–1006.
Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol 2005; 20: 517–529.
Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H . Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 2010; 11: 118.
Whyte E, Skidmore E, Aizenstein H, Ricker J, Butters M . Cognitive impairment in acquired brain injury: a predictor of rehabilitation outcomes and an opportunity for novel interventions. PM R 2011; 3: S45–S51.
Heruti RJ, Lusky A, Dankner R, Ring H, Dolgopiat M, Barell V et al. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch Phys Med Rehabil 2002; 83: 742–749.
Craig A, Guest R, Tran Y, Middleton J . Cognitive impairment and mood states after spinal cord injury. J Neurotrauma 2017; 34: 1156–1163.
Nicholson Perry K, Nicholas MK, Middleton J . Spinal cord injury-related pain in rehabilitation: a cross-sectional study of relationships with cognitions, mood and physical function. Eur J Pain 2009; 13: 511–517.
Wilmot CB, Cope DN, Hall KM, Acker M . Occult head injury: its incidence in spinal cord injury. Arch Phys Med Rehabil 1985; 66: 227–231.
Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T et al. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 2014; 34: 989–1006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Krebs, J., Scheel-Sailer, A., Oertli, R. et al. The effects of antimuscarinic treatment on the cognition of spinal cord injured individuals with neurogenic lower urinary tract dysfunction: a prospective controlled before-and-after study. Spinal Cord 56, 22–27 (2018). https://doi.org/10.1038/sc.2017.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2017.94
This article is cited by
-
A systematic review of neurocognitive dysfunction with overactive bladder medications
International Urogynecology Journal (2021)
-
Multidimensional review of cognitive impairment after spinal cord injury
Acta Neurologica Belgica (2021)
-
A prospective cohort study investigating contributors to mild cognitive impairment in adults with spinal cord injury: study protocol
BMC Neurology (2020)