Abstract
Study design:
A prospective observational study reporting correlation between sCD95L (serum cluster of differentiation 95 ligand) serum levels and remission after traumatic spinal cord injury (SCI).
Objectives:
To describe the correlation between sCD95L serum levels and remission after traumatic SCI in a human protocol compared with animal studies.
Setting:
Rhineland-Palatinate (Rheinland-Pfalz), Germany.
Methods:
We included 45 patients with traumatic SCI. According to their neurological outcome, patients were divided into two groups, patients with (G1, n=26) and without (G2, n=19) remission. Blood was collected on post-admission and according to a fixed scheme, that is, after 4, 9, 12 h, 1, 3 days and 1, 2, 4, 8, 12 weeks.
Results:
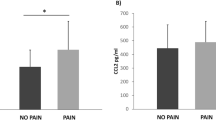
By comparing G1 with G2, we found a correlation between neurological remission and sCD95L serum concentrations. Consistently elevated levels of sCD95L in G1 between 9 h and 1 month after injury show significantly differing values 7 days after injury. This indicates a correlation between patients with clinically documented neurological remission and elevated sCD95L serum concentrations.
Conclusions:
In opposite to animal studies, our patients with neurological remission show on average higher levels of sCD95L compared with patients without. Therefore, spinal cord-injured patients would probably not profit from neutralizing CD95L. Our results present that the transfer of findings from animal studies to humans must always be considered critically. We were able to show that peripheral serum cytokine expression is suitable to state processes after SCI in humans.
Similar content being viewed by others
Introduction
Cluster of differentiation 95 ligand (CD95L) is a well-known transmembrane protein belonging to the tumor necrosis factor family. The receptor CD95 (Apoptosis antigen 1) of the CD95/CD95L system has been shown to be involved in the mediation of apoptosis. Recent studies revealed further contributions of CD95/CD95L to other dynamic systems such as the inflammatory reaction in acute spinal cord injury (SCI).1, 2, 3, 4
Letellier et al.5 observed that injury to the spinal cord triggered surface expression of CD95 ligand on peripheral myeloid cells in animals and humans, which ultimately led to an increased inflammatory response at lesion site. Following their hypothesis, migration of myeloid cells is mediated by CD95L via activation of phosphatidylinositol-3-kinase and Spleen Tyrosine Kinase-Matrix Metallo Proteinase. In conclusion, they claimed that peripheral neutralization of CD95L would have a beneficial effect by inducing a controlled inflammatory response and therefore improving neurological recovery. One major hypothesis was that the peripheral amount of CD95L produced by myeloid cells and not CD95L at lesion site is responsible for multiplication of chemotactic signals leading to an increased inflammatory response after SCI and initiated further tissue damage. To confirm these findings, we previously determined peripheral serum concentrations of sCD95L in patients in a small patient group (n=8) with traumatic SCI and demonstrated the results in the Spinal Cord Journal.6 In a larger cohort population (n=23), we correlated the serum levels of sCD95L to the neurological outcome.7 Our ambition now was to test the hypothesis of Lettelier et al., depending on their animal studies on the basis of a human protocol with an enlarged cohort population.
Materials and methods
Between March 2010 and September 2014, we included 45 patients in our prospective observational study and established a complete database including demographic and clinical parameters such as age, gender, etiology of accident, neurological level of injury, and initial and final AIS scores (AIS, ASIA Impairment Scale, ASIA, American Spinal Injury Association)8 in order to compare laboratory findings with functional neurological outcome.9 We defined neurological remission as improvement of the final AIS score compared with the initially measured AIS score. Finally, we divided patients according to their neurological outcome into two groups: G1, patients with neurological remission (G1=Group 1, improved the final AIS score compared with the initial AIS score), and G2, patients without remission (G2=Group 2, no improvement in the final AIS score compared with the initial AIS score). In our recent prospective observational study on sCD95L in a large population of traumatic spinal cord-injured patients (n=45), venous blood samples were taken on post-admission and according to a fixed scheme, that is, 4, 9, 12 h, 1, 3 days and 1, 2, 4, 8, 12 weeks after SCI. Table 1 shows demographic and clinical characteristics of the subjects.
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 21.0, IBM Corp., Armonk, NY, USA). Correlation analysis was carried out between all variables. Because of the partly non-normal distribution of the data, non-parametric tests were used to compare samples. Statistical analysis for two independent variables (cytokine concentrations of two different AIS groups) was carried out with the Mann–Whitney U-Test. Two dependent variables (cytokine concentrations within one group at different points of time) were compared with the Wilcoxon signed-rank test.9
Our prospective observational study has been approved by the ethics committees of the University of Heidelberg (S-514/2011) and the Landesärztekammer Rheinland-Pfalz (837.188.12/8289-F), Germany. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Results
By analyzing the entire patient collective, we found a significant decrease at 4, 9 and 12 h as well as 1, 3 and 7 days after SCI compared with initial sCD95L levels at admission (on average 1 h after trauma) (P<0.05, Wilcoxon signed-rank test). Thereafter, we measured an increase in sCD95L concentrations 7 days after SCI with a return to initial levels at 6 weeks. There was a peak after 2 months (mean=68.25 pg ml−1) and a slight decrease between 2 and 3 months (Figure 1). Exact serum concentrations are presented in Table 2. By comparing group 1 (G1, n=26) with group 2 (G2, n=19), we found consistently elevated levels of sCD95L in G1 between 9 h and 1 month after injury (Figure 2). Mean values were significantly different 7 days after injury (P<0.05; Mann–Whitney U-test). Figure 3 shows the AIS improvement from initial to final measures.
AIS improvement from initial to final measures. AIS start, ASIA Impairment Scale determined at administration to the hospital; AIS end, ASIA Impairment Scale determined at discharge from the hospital. Numbers indicate the quantity of patients; bold numbers indicate the number of patients ⩾5. Arrows show improvement from starting score to final score.
Discussion
Our study did show an initial decrease in sCD95L, but this was followed by a steady increase above initially measured concentrations. Furthermore, we found that patients with clinically documented neurological remission showed higher sCD95L serum concentrations than patients without remission. These results indicate a correlation between neurological remission and higher levels of peripheral serum levels of sCD95L. On the basis of our results, we are not able to confirm the hypothesis of Letellier et al. High levels of sCD95L in peripheral human blood serum seem to have a positive influence on the outcome after traumatic SCI. Assuming peripheral elevated CD95L would be the main trigger to multiply inflammation at lesion site and therefore increase cell damage as suggested by Lettelier et al., patients with low sCD95L in peripheral blood serum should present themselves with improved neurological outcome. Neutralization of peripheral CD95L would most likely not lead to neurological remission. The complex interaction between pro- and anti-inflammatory cell mediators and their diverse feedback mechanisms often present with major fluctuations due to the efforts of our organism to limit inflammation.10 Recently, we could demonstrate that it is possible to verify biochemical processes after traumatic SCI, depending on the neurological outcome. Further, we were able to demonstrate that pro- and anti-inflammatory cytokines might have a key role by inducing and controlling these feedback loops after SCI.9 However, there is still an ongoing discussion about inflammation and its influence on neuro de- and regeneration. In general, inflammation needs to be controlled in order to avoid additional damage by disproportionate defense mechanisms, but it is also essential for responding to external noxae such as bacterial or viral wound infections, as well as putting important repair mechanisms in motion.10
The observed decline in sCD95L within the first 24 h may perhaps mark a still unknown feedback mechanism or form part of a compensatory counter-regulation of the body to acute injury. Corsini et al.1 demonstrated that the CD95/CD95L system can lead to stem cell survival and neuronal specification in ongoing and injury-induced neurogenesis in the central nervous system. Assuming that this CD95/CD95L injury-induced neurogenesis signal can also be found in the damaged spinal cord, it could explain why there is an increase in sCD95L after SCI in patients with neurological remission. Further research in that area is needed to unravel more precise feedback mechanisms, leading to improved clinical outcome after SCI. A special challenge has always been the question of whether and to what extent findings obtained from animal studies are also valid in humans.11 A recent round table discussion of members of the SCI research community about the role of animal trials in SCI underlined the importance of these studies before human protocols and also emphasized the issue of how data derived from these studies should be interpreted.12 For choosing the right measurement times and relating them to the appropriate times in the human body, further research is much needed. Without this knowledge, conclusions for human therapy derived from animal model observations most likely lead to misinterpretation of dynamic systems. In the field of traumatic SCI, the current literature provides no data of animal models presenting transferable results to humans.12, 13 Most studies investigated the secondary phase after traumatic SCI in animal models. Even though valuable observations in the process leading to the formation of the glial scar14 and depending on these results promising approaches in future therapy of traumatic SCI had been made, clinical studies can provide additional and much needed information. Measurements of blood serum and liquor cytokines for instance seem to differ quite a lot in animal to human protocols. Mestas and Hughes15 reviewed the literature for that matter and found that differences between mouse and human immunology, including the Cluster of Differentiation Superfamily, are especially critical in terms of transferability. The very few human studies analyzed either blood and/or cerebrospinal fluid samples in very short time intervals without correlating them to clinical parameters16 or blood samples were taken at random time points without following to a fixed protocol.17 A systemic analysis including patient follow-up and outcome is missing. Even though Lettelier et al. were able to reveal most complicated signaling pathways and cytokine interactions in an SCI animal model, it is highly critical to transfer these findings to humans without considering correlation between their findings and clinical measures of patients.
Conclusions
In conclusion, we found that the sCD95L serum concentration decreases in the acute phase after traumatic SCI but increases again in the following sub-acute and intermediate phase and even rises above initial levels. Patients with neurological remission show on average higher levels of sCD95L compared with patients without neurological remission, indicating that higher levels of sCD95 after SCI have beneficial effects on functional recovery. Therefore, spinal cord-injured patients would probably not profit from neutralizing CD95L. Our results show that the transfer of findings from animal studies to humans must always be considered critically. The treatment in spinal cord-injured patients is one of the greatest challenges of modern medicine. Only through the consistent observation and analysis of large databases in accordance with patient data can result found in animal trials be validated and further efforts considered targeting specific interacting regulatory pathways.
Data archiving
There were no data to deposit.
References
Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E et al. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell 2009; 5: 178–190.
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008; 13: 235–248.
Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK . Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol 2003; 5: 118–125.
Zuliani C, Kleber S, Klussmann S, Wenger T, Kenzelmann M, Schreglmann N et al. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell Death Differ 2006; 13: 31–40.
Letellier E, Kumar S, Sancho-Martinez I, Krauth S, Funke-Kaiser A, Laudenklos S et al. CD95-ligand on peripheral myeloid cells activates Syk kinase to trigger their recruitment to the inflammatory site. Immunity 2010; 32: 240–252.
Biglari B, Buchler A, Swing T, Biehl E, Roth HJ, Bruckner T et al. Increase in soluble CD95L during subacute phases after human spinal cord injury: a potential therapeutic target. Spinal Cord 2013; 51: 183–187.
Biglari B, Buchler A, Swing T, Child C, Biehl E, Reitzel T et al. Serum sCD95L concentration in patients with spinal cord injury. J Int Med Res 2015; 43: 250–256.
Marino RJ, Ditunno JF Jr, Donovan WH, Maynard F Jr . Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil 1999; 80: 1391–1396.
Moghaddam A, Child C, Bruckner T, Gerner HJ, Daniel V, Biglari B . Posttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol Sci 2015; 16: 7900–7916.
Donnelly DJ, Popovich PG . Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 2008; 209: 378–388.
Kwon BK, Casha S, Hurlbert RJ, Yong VW . Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med 2011; 49: 425–433.
Kwon BK, Streijger F, Hill CE, Anderson AJ, Bacon M, Beattie MS et al. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp Neurol 2015; 269: 154–168.
Kwon BK, Oxland TR, Tetzlaff W . Animal models used in spinal cord regeneration research. Spine 2002; 27: 1504–1510.
Garcia-Alvarez I, Fernandez-Mayoralas A, Moreno-Lillo S, Sanchez-Sierra M, Nieto-Sampedro M, Doncel-Perez E . Inhibition of glial proliferation, promotion of axonal growth and myelin production by synthetic glycolipid: a new approach for spinal cord injury treatment. Restor Neurol Neurosci 2015; 33: 895–910.
Mestas J, Hughes CC . Of mice and not men: differences between mouse and human immunology. J Immunol (Baltimore, Md: 1950) 2004; 172: 2731–2738.
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
Huang W, Vodovotz Y, Kusturiss MB, Barclay D, Greenwald K, Boninger ML et al. Identification of distinct monocyte phenotypes and correlation with circulating cytokine profiles in acute response to spinal cord injury: a pilot study. PMR 2014; 6: 332–341.
Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma 2007; 21 (10 Suppl): S1–S133.
Acknowledgements
Statistical analyses were supported by the Institute for medical Biometrics and Information Technology, University of Heidelberg, Germany. We thank Viola Graeser for performing the ELISA analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Moghaddam, A., Sperl, A., Heller, R. et al. sCD95L in serum after spinal cord injury. Spinal Cord 54, 957–960 (2016). https://doi.org/10.1038/sc.2016.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.44